Abstract
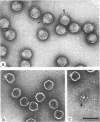
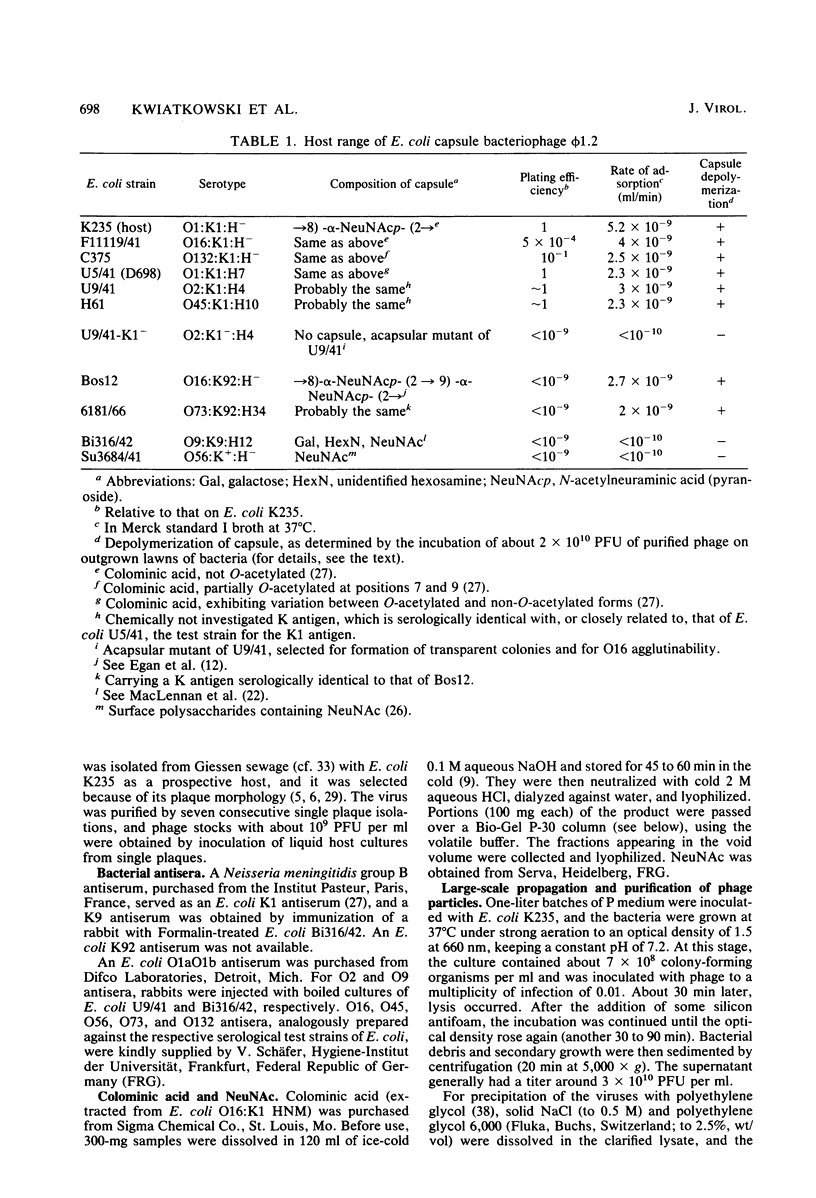
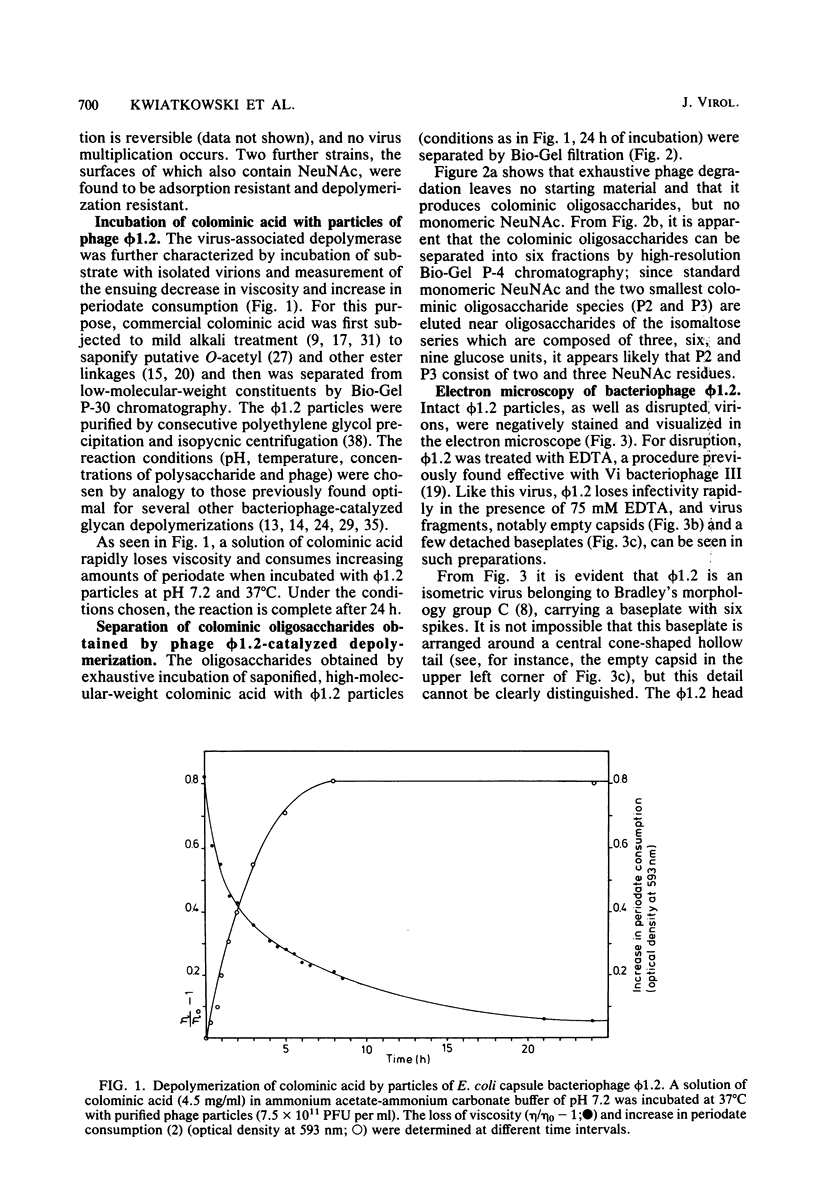
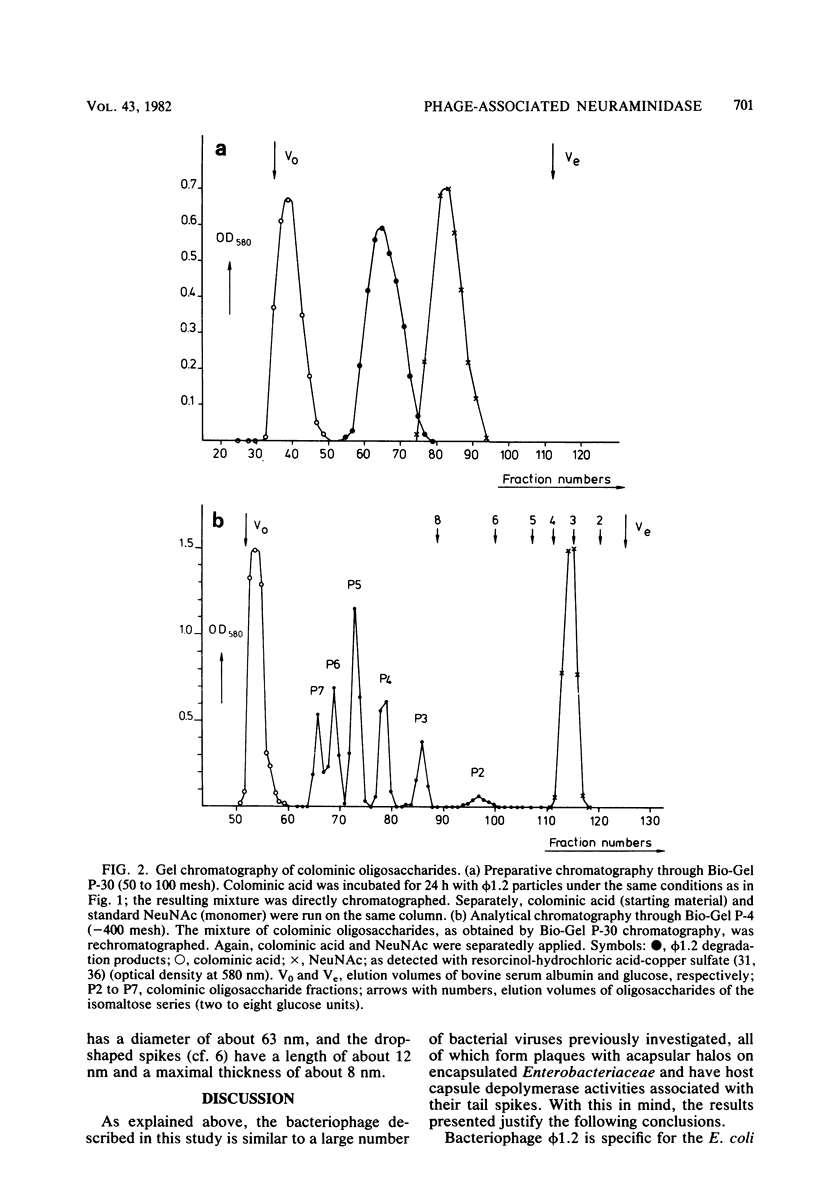
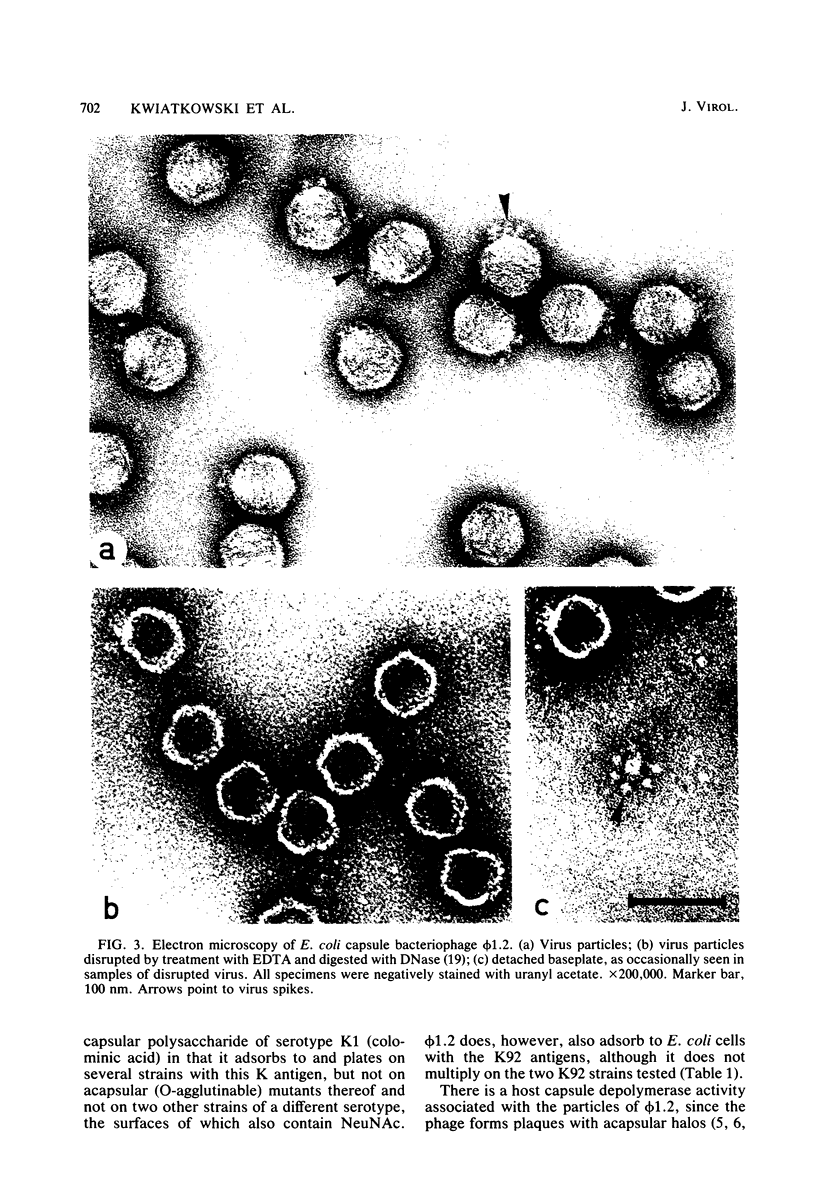
A bacteriophage (phi 1.2) has been isolated for Escherichia coli K235 (O1:K1:H-). phi 1.2 is specific for the host capsular polysaccharide (colominic acid). The phage forms plaques with acapsular halos and thus carries a glycanase activity for colominic acid, a homopolymer of alpha (2 leads to 8)-linked N-acetylneuraminic acid (NeuNAc) residues. Upon incubation with purified phi 1.2 particles, a solution of K1 polysaccharide loses viscosity and consumes increasing amounts of periodate. Also, by gel filtration, the production of colominic oligosaccharides (down to a size of two to three NeuNAc residues) can be demonstrated. No NeuNAc monomers, however, are formed. The capsules of E. coli strains with the K92 antigen, which consists of NeuNAc residues linked by alternating alpha (2 leads to 8) and alpha (2 leads to 9) bonds, are also depolymerized by the phi 1.2 enzyme. Under the electron microscope, phage phi 1.2 is seen to belong to Bradley's morphology group C (D. E. Bradley, Bacteriol. Rev. 31:230-314, 1967); it has an isometric head, carrying a baseplate with six spikes. By analogy to other virus particles with host capsule depolymerase activity, it is probable that the phi 1.2 endo-N-acetylneuraminidase activity is associated with these spikes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY G. T., GOEBEL W. F. Colominic acid, a substance of bacterial origin related to sialic acid. Nature. 1957 Jan 26;179(4552):206–206. doi: 10.1038/179206a0. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H., Bayer M. H. Penetration of the polysaccharide capsule of Escherichia coli (Bi161/42) by bacteriophage K29. Virology. 1979 Apr 15;94(1):95–118. doi: 10.1016/0042-6822(79)90441-0. [DOI] [PubMed] [Google Scholar]
- Bessler W., Fehmel F., Freund-Mölbert E., Knüfermann H., Stirm S. Escherichia coli capsule bacteriophages. IV. Free capsule depolymerase 29. J Virol. 1975 Apr;15(4):976–984. doi: 10.1128/jvi.15.4.976-984.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessler W., Freund-Mölbert E., Knüfermann H., Rduolph C., Thurow H., Stirm S. A bacteriophage-induced depolymerase active on Klebsiella K11 capsular polysaccharide. Virology. 1973 Nov;56(1):134–151. doi: 10.1016/0042-6822(73)90293-6. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Jennings H. J., Kenny C. P., Martin A., Smith I. C. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J Biol Chem. 1975 Mar 10;250(5):1926–1932. [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Stenzel J., Buscher H. P., Schauer R. Gas--liquid chromatography of N- and O-acylated neuramic acids. Anal Biochem. 1975 May 12;65(1-2):507–524. doi: 10.1016/0003-2697(75)90536-9. [DOI] [PubMed] [Google Scholar]
- Drzeniek R. Viral and bacterial neuraminidases. Curr Top Microbiol Immunol. 1972;59:35–74. doi: 10.1007/978-3-642-65444-2_2. [DOI] [PubMed] [Google Scholar]
- Egan W., Liu T. Y., Dorow D., Cohen J. S., Robbins J. D., Gotschlich E. C., Robbins J. B. Structural studies on the sialic acid polysaccharide antigen of Escherichia coli strain Bos-12. Biochemistry. 1977 Aug 9;16(16):3687–3692. doi: 10.1021/bi00635a028. [DOI] [PubMed] [Google Scholar]
- Elsässer-Beile U., Stirm S. Substrate specificity of the glycanase activity associated with particles of Klebsiella bacteriophage no. 6. Carbohydr Res. 1981 Feb 2;88(2):315–322. doi: 10.1016/s0008-6215(00)85544-5. [DOI] [PubMed] [Google Scholar]
- Fehmel F., Feige U., Niemann H., Stirm S. Escherichia coli capsule bacteriophages. VII. Bacteriophage 29-host capsular polysaccharide interactions. J Virol. 1975 Sep;16(3):591–601. doi: 10.1128/jvi.16.3.591-601.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Fraser B. A., Nishimura O., Robbins J. B., Liu T. Y. Lipid on capsular polysaccharides of gram-negative bacteria. J Biol Chem. 1981 Sep 10;256(17):8915–8921. [PubMed] [Google Scholar]
- Gross R. J., Cheasty T., Rowe B. Isolation of bacteriophages specific for the K1 polysaccharide antigen of Escherichia coli. J Clin Microbiol. 1977 Dec;6(6):548–550. doi: 10.1128/jcm.6.6.548-550.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K., Jann B., Orskov F., Orskov I., Westphal O. Immunchemische Untersuchungen an K-Antigenen von Escherichia Coli. II. Das K-Antigen von E. coli 08:K42(A):H-. Biochem Z. 1965 Jun 3;342(1):1–22. [PubMed] [Google Scholar]
- Kwiatkowski B., Beilharz H., Stirm S. Disruption of Vi bacteriophage III and localization of its deacetylase activity. J Gen Virol. 1975 Dec;29(3):267–280. doi: 10.1099/0022-1317-29-3-267. [DOI] [PubMed] [Google Scholar]
- Lifely M. R., Gilbert A. S., Moreno C. Sialic acid polysaccharide antigens of Neisseria meningitidis and Escherichia coli: esterification between adjacent residues. Carbohydr Res. 1981 Aug 1;94(2):193–203. doi: 10.1016/s0008-6215(00)80717-x. [DOI] [PubMed] [Google Scholar]
- MCGUIRE E. J., BINKLEY S. B. THE STRUCTURE AND CHEMISTRY OF COLOMINIC ACID. Biochemistry. 1964 Feb;3:247–251. doi: 10.1021/bi00890a017. [DOI] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Jann B., Jann K. Immunoelectrophoretic patterns of extracts from all Escherichia coli O and K antigen test strains: correlation with pathogenicity. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):142–152. doi: 10.1111/j.1699-0463.1971.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Sutton A., Schneerson R., Lin W., Egan W., Hoff G. E., Robbins J. B. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979 Mar 1;149(3):669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger-Hug D., Stirm S. Comparative study of host capsule depolymerases associated with Klebsiella bacteriophages. Virology. 1981 Aug;113(1):363–378. doi: 10.1016/0042-6822(81)90162-8. [DOI] [PubMed] [Google Scholar]
- Rieger D., Freund-Mölbert E., Stirm S. Escherichia coli capsule bacteriophages. III. Fragments of bacteriophage 29. J Virol. 1975 Apr;15(4):964–975. doi: 10.1128/jvi.15.4.964-975.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph C., Freund-Mölbert E., Stirm S. Fragments of Klebsiella bacteriophage no. 11. Virology. 1975 Mar;64(1):236–246. doi: 10.1016/0042-6822(75)90095-1. [DOI] [PubMed] [Google Scholar]
- Schauer R. Characterization of sialic acids. Methods Enzymol. 1978;50:64–89. doi: 10.1016/0076-6879(78)50008-6. [DOI] [PubMed] [Google Scholar]
- Stirm S., Freund-Mölbert E. Escherichia coli capsule bacteriophages. II. Morphology. J Virol. 1971 Sep;8(3):330–342. doi: 10.1128/jvi.8.3.330-342.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurow H., Niemann H., Stirm S. Bacteriophage-borne enzymes in carbohydrate chemistry. Part I. On the glycanase activity associated with particles of Klebsiella bacteriophage No. 11. Carbohydr Res. 1975 May;41:257–271. doi: 10.1016/s0008-6215(00)87024-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]