Abstract
Rats infected with Trichinella spiralis were examined during the course of infection for various changes in the leucocytic population. In each experiment rats were divided into three groups: Group A, inoculated with Escherichia coli B-5-lipopolysaccharide (LPS) administered four days before each experiment; Group B, infected with Trichinella spiralis; and Group C, untreated controls.
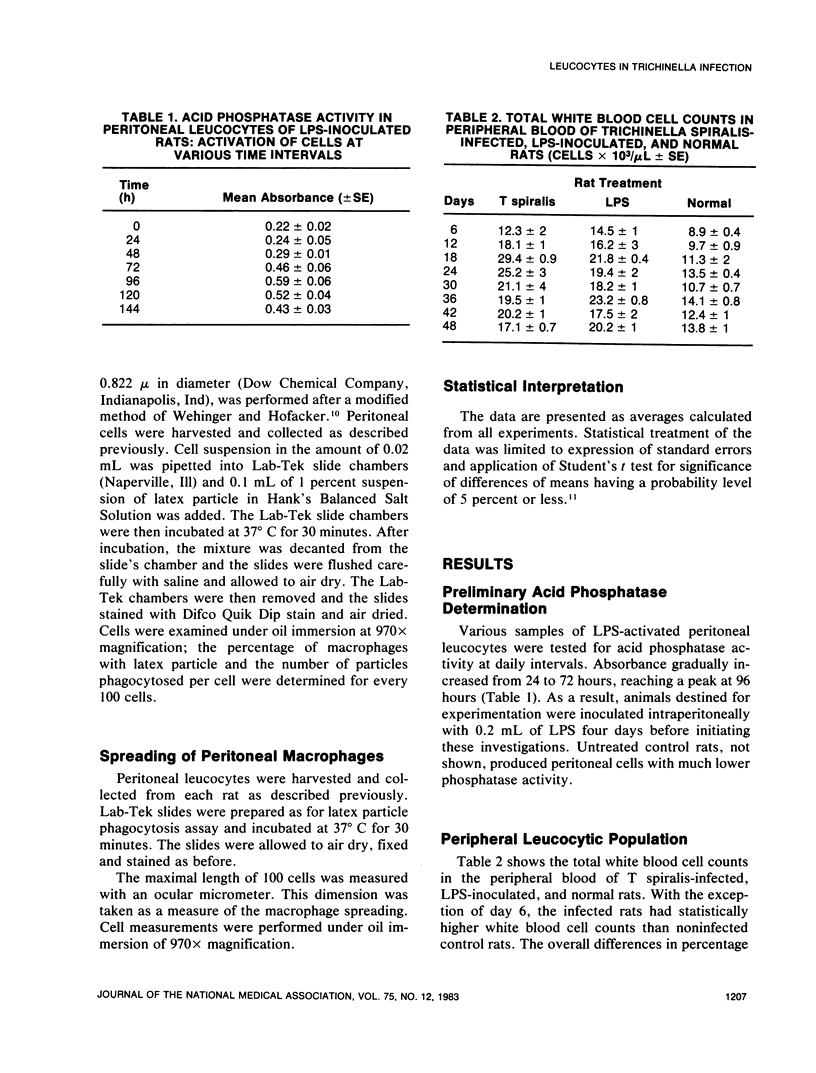
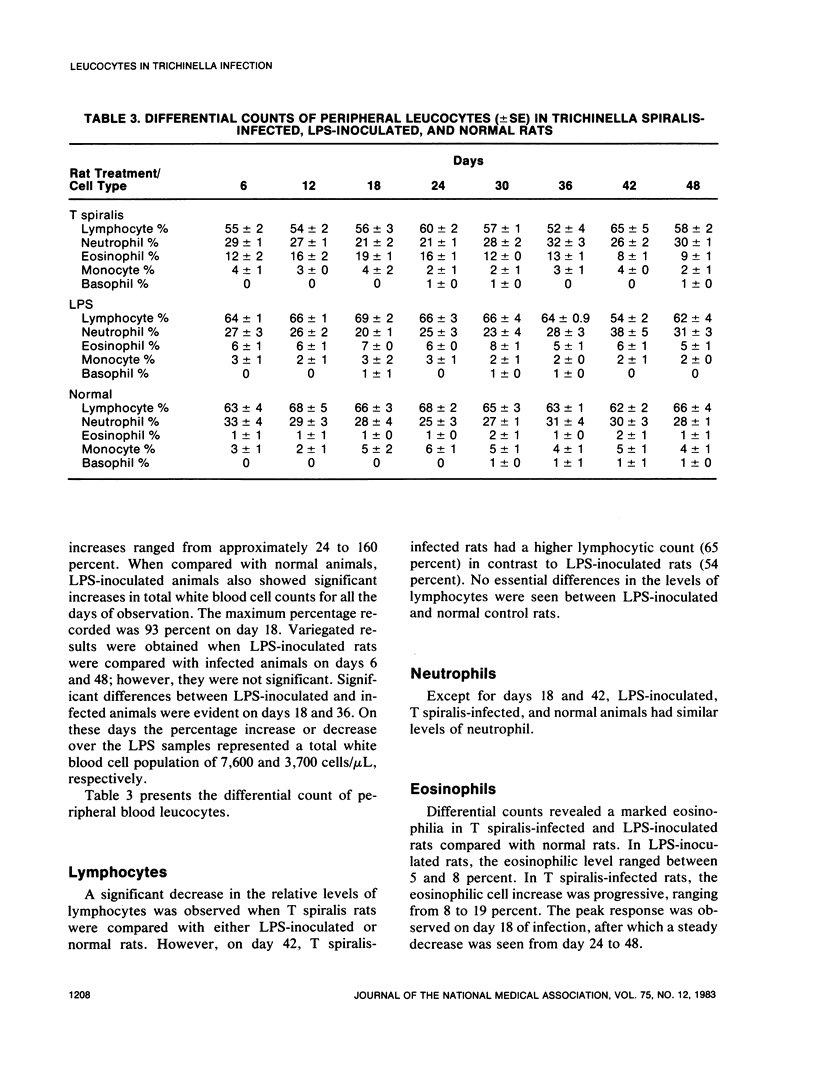
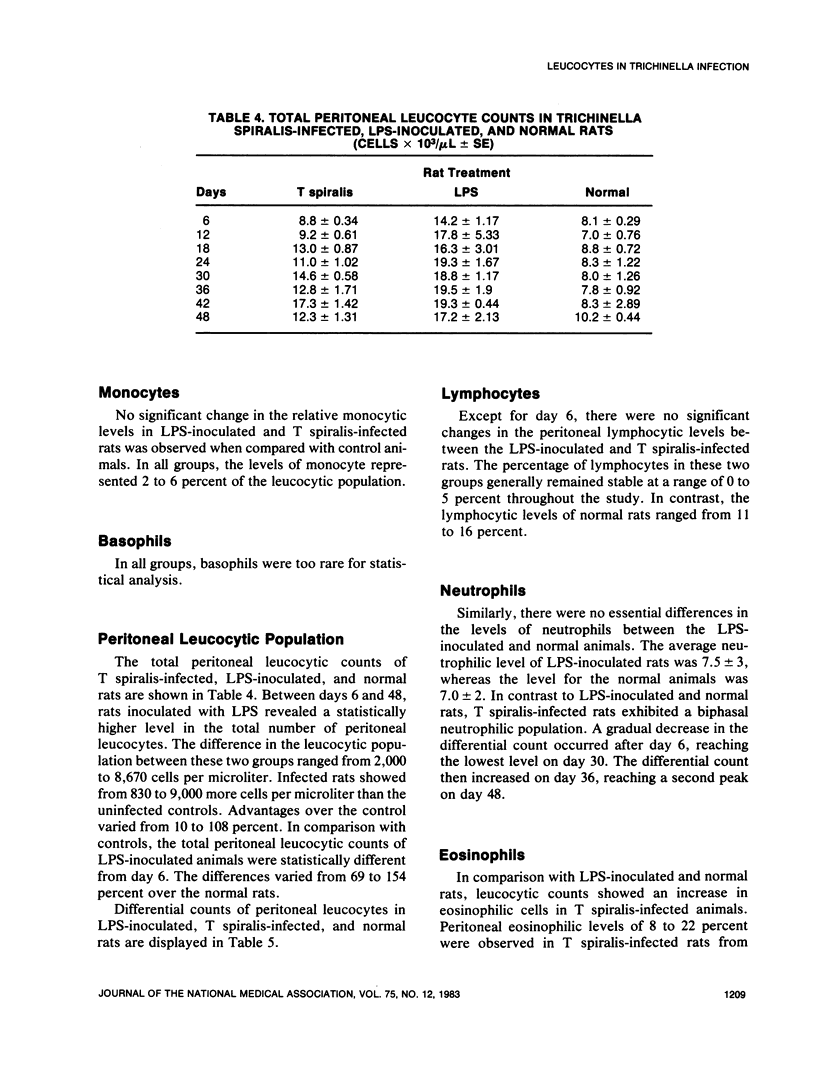
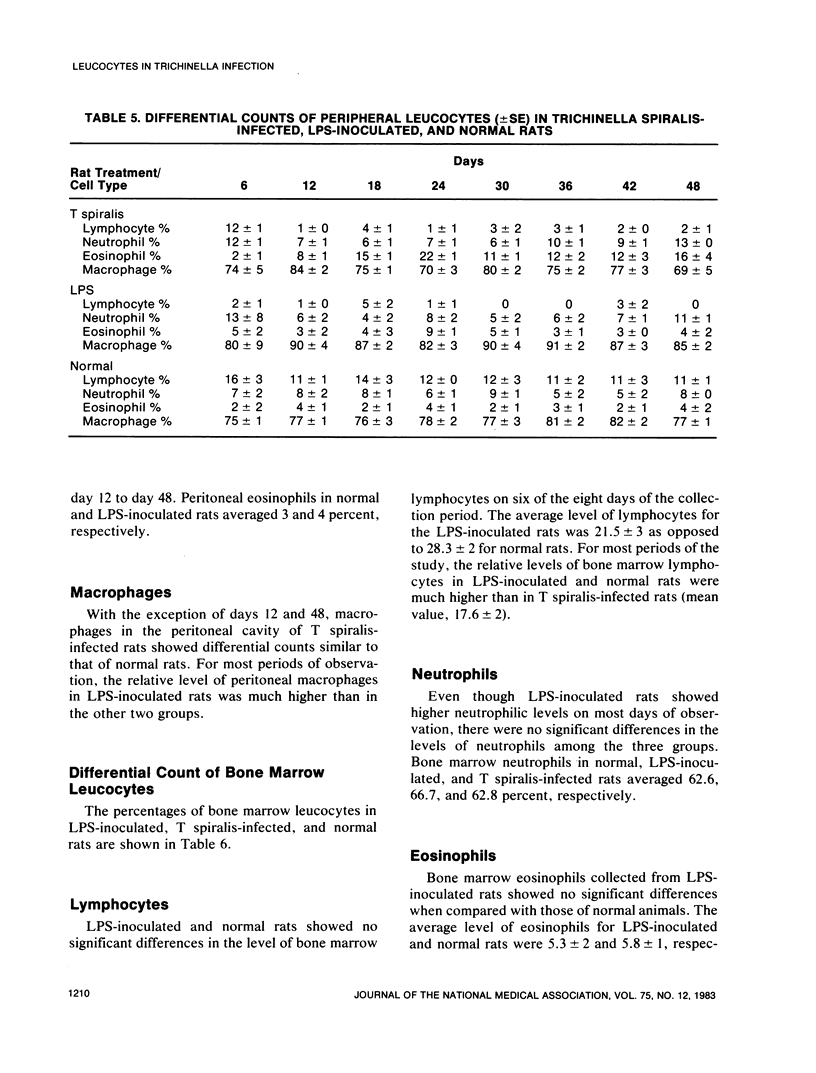
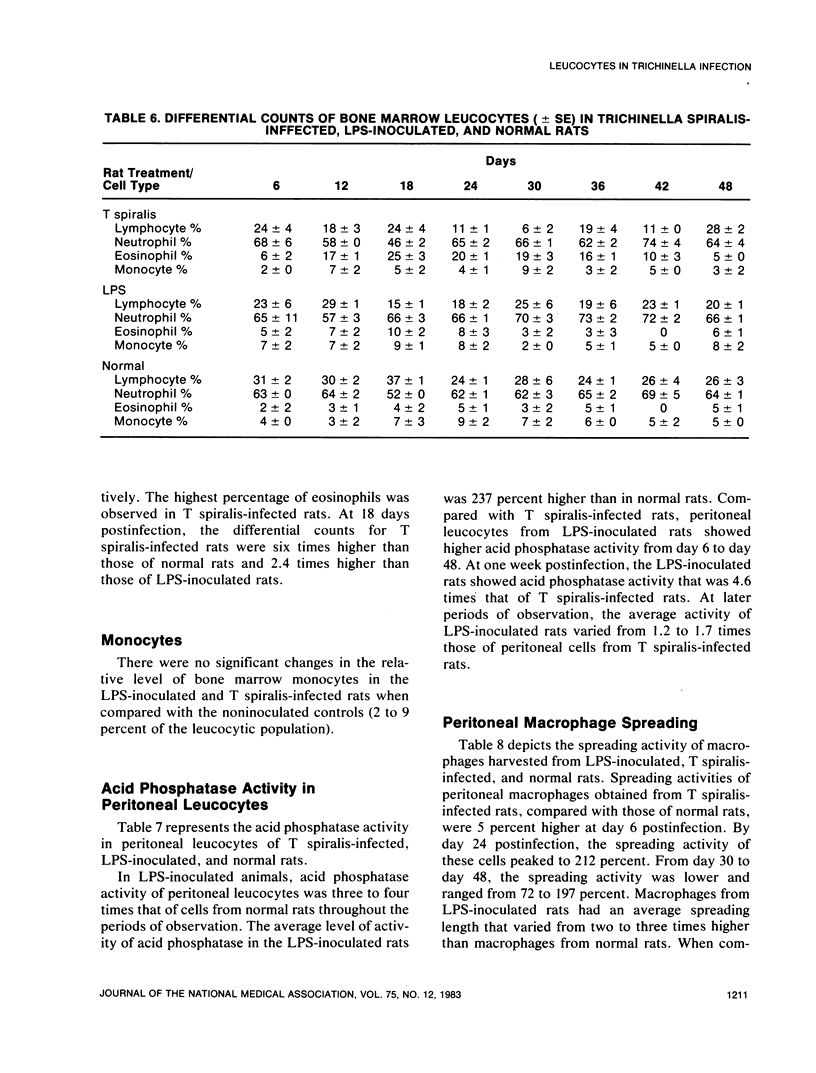
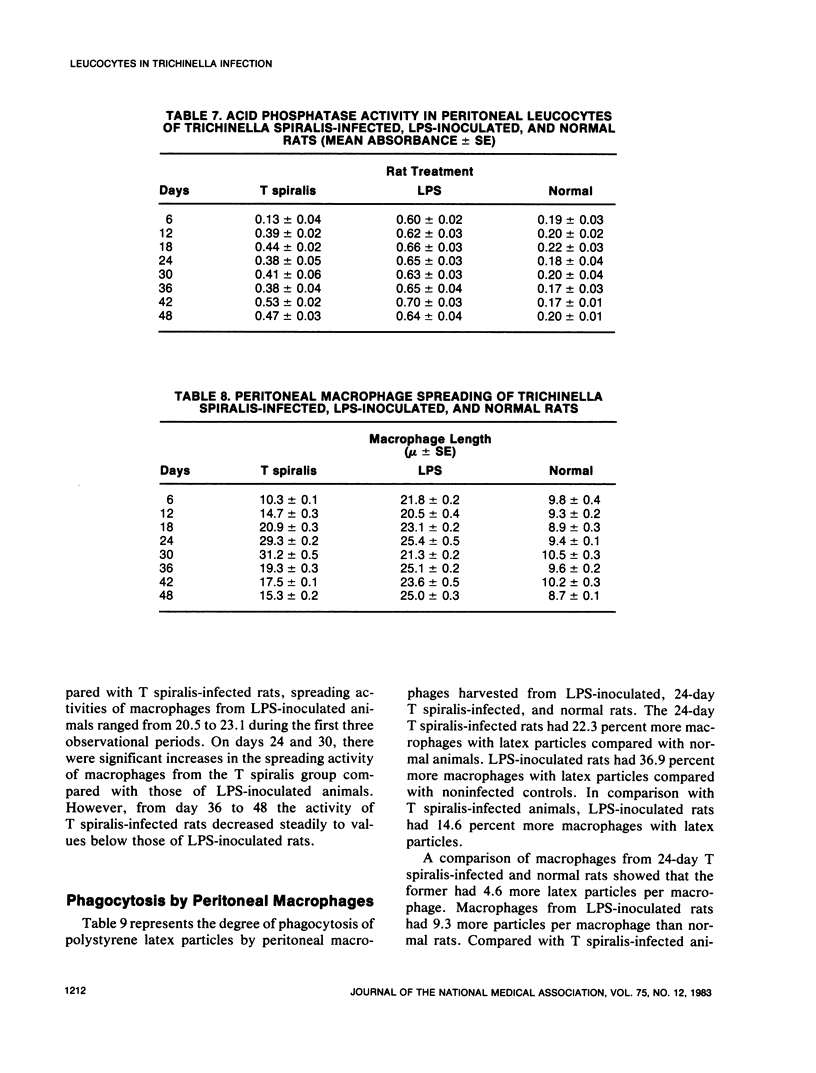
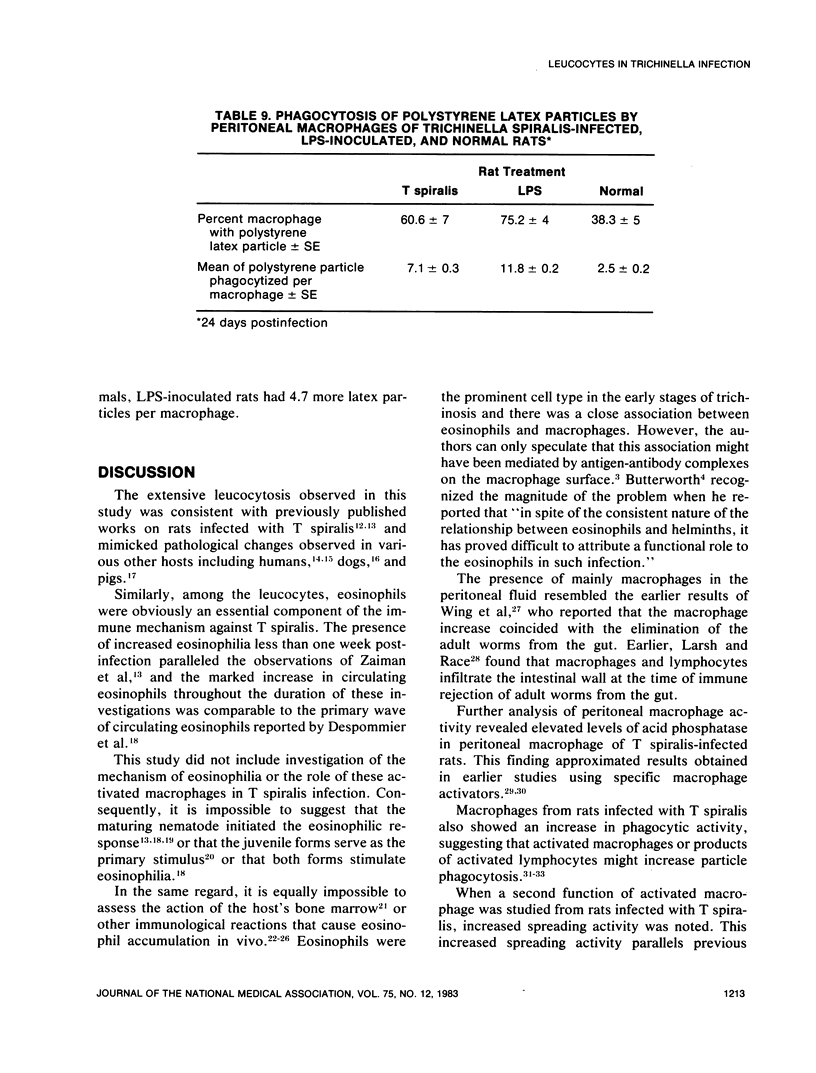
An extensive leucocytosis was observed in the peripheral blood and peritoneal cavity of infected rats. Regardless of the site (peripheral blood, bone marrow, peritoneal cavity), the most obvious change was an increase in eosinophils. Differential counts of peritoneal exudate cells also revealed a significant population of macrophages. Acid phosphatase activity, macrophage phagocytosis of polystyrene latex particles, and macrophage spreading revealed that peritoneal exudate cells from T spiralis-infected rats are activated from 6 to 48 days postinfection.
This paper serves to reinforce existing information on the changes and state of the various leucocytic populations during infection with T spiralis and aids in assessing the activity change of macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. R. STUDIES ON TRICHINOSIS, WITH ESPECIAL REFERENCE TO THE INCREASE OF THE EOSINOPHILIC CELLS IN THE BLOOD AND MUSCLE, THE ORIGIN OF THESE CELLS AND THEIR DIAGNOSTIC IMPORTANCE. J Exp Med. 1898 May 1;3(3):315–347. doi: 10.1084/jem.3.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E. The eosinophil and its role in immunity to helminth infection. Curr Top Microbiol Immunol. 1977;77:127–168. doi: 10.1007/978-3-642-66740-4_5. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Dean D. A., Wistar R., Murrell K. D. Combined in vitro effects of rat antibody and neutrophilic leukocytes on schistosomula of Schistosoma mansoni. Am J Trop Med Hyg. 1974 May;23(3):420–428. doi: 10.4269/ajtmh.1974.23.420. [DOI] [PubMed] [Google Scholar]
- Despommier D., Weisbroth S., Fass C. Circulating eosinophils and trichinosis in the rat: the parasitic stage responsible for induction during infection. J Parasitol. 1974 Apr;60(2):280–284. [PubMed] [Google Scholar]
- Douglas S. D., Spicer S. S. Acid phosphatase cytochemistry of phagocytizing leukocytes from patients with chronic granulomatous disease. Infect Immun. 1971 Jan;3(1):179–183. doi: 10.1128/iai.3.1.179-183.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evron R. In vitro phagocytosis of Candida albicans by peritoneal mouse macrophages. Infect Immun. 1980 Jun;28(3):963–971. doi: 10.1128/iai.28.3.963-971.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GURSCH O. F. Effects of digestion and refrigeration on the ability of Trichinella spiralis to infect rats. J Parasitol. 1948 Oct;34(5):394–394. [PubMed] [Google Scholar]
- HIRSCH J. G., COHN Z. A. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J Exp Med. 1960 Dec 1;112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth-Scott R. S., Johansson S. G., Bennich H. Antibodies to Toxocara in the sera of visceral larva migrans patients: the significance of raised levels of IgE. Clin Exp Immunol. 1969 Dec;5(6):619–625. [PMC free article] [PubMed] [Google Scholar]
- Ismail M. M., Tanner C. E. Trichinella spiralis: peripheral blood, intestinal, and bone-marrow eosinophilia in rats and its relationship to the inoculating dose of larvae, antibody response and parasitism. Exp Parasitol. 1972 Apr;31(2):262–272. doi: 10.1016/0014-4894(72)90117-8. [DOI] [PubMed] [Google Scholar]
- Job C. K. Lysosomal activity of macrophages in leprosy. Arch Pathol. 1970 Dec;90(6):547–552. [PubMed] [Google Scholar]
- Jones D. G., Kay A. B. The effect of anti-eosinophil serum on skin histamine replenishment following passive cutaneous anaphylaxis in the guinea-pig. Immunology. 1976 Sep;31(3):333–336. [PMC free article] [PubMed] [Google Scholar]
- Larsh J. E., Jr Allergic inflammation as a hypothesis for the expulsion of worms from tissues: a review. Exp Parasitol. 1975 Apr;37(2):251–266. doi: 10.1016/0014-4894(75)90077-6. [DOI] [PubMed] [Google Scholar]
- Matossian-Rogers A. Specificity of the macrophage spreading test with reference to Leishmania antigens and correlation with delayed hypersensitivity. Clin Exp Immunol. 1979 Apr;36(1):38–45. [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Jones E. E., Boetcher D. A. Increased chemotactic responses of macrophages from BCG-infected mice. Cell Immunol. 1975 May;17(1):268–276. doi: 10.1016/s0008-8749(75)80026-8. [DOI] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. II. The role of the T and B lymphocytes. Immunology. 1974 Nov;27(5):825–840. [PMC free article] [PubMed] [Google Scholar]
- Pelley R. P., Karp R., Mahmoud A. A., Warren K. S. Antigen dose response and specificity of production of the lymphokine eosinophil stimulation promoter. J Infect Dis. 1976 Sep;134(3):230–237. doi: 10.1093/infdis/134.3.230. [DOI] [PubMed] [Google Scholar]
- SAITO K., SUTER E. LYSOSOMAL ACID HYDROLASES IN MICE INFECTED WITH BCG. J Exp Med. 1965 May 1;121:727–738. doi: 10.1084/jem.121.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMTER M., KOFOED M. A., PIEPER W. A factor in lungs of anaphylactically shocked guinea pigs which can induce eosinophilia in normal animals. Blood. 1953 Dec;8(12):1078–1090. [PubMed] [Google Scholar]
- Strafuss A. C., Zimmermann W. J. Hematologic changes and clinical signs of trichinosis in pigs. Am J Vet Res. 1967 May;28(124):833–838. [PubMed] [Google Scholar]
- VOLKMAN A., GOWANS J. L. THE PRODUCTION OF MACROPHAGES IN THE RAT. Br J Exp Pathol. 1965 Feb;46:50–61. [PMC free article] [PubMed] [Google Scholar]
- Walls R. S., Hersey P., Quie P. G. Macrophage-eosinophil interactions in the inflammatory response to Trichinella spiralis. Blood. 1974 Jul;44(1):131–136. [PubMed] [Google Scholar]
- Wehinger H., Hofacker M. Latex phagocytosis by polymorphonuclear leukocytes. In vitro and in vivo studies with a simple screening test. Eur J Pediatr. 1976 Sep 1;123(2):125–132. doi: 10.1007/BF00442642. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Krahenbuhl J. L., Remington J. S. Studies of macrophage function during Trichinella spiralis infection in mice. Immunology. 1979 Mar;36(3):479–485. [PMC free article] [PubMed] [Google Scholar]
- ZAIMAN H., HOWARD C. J., DROLETTE B. Eosinophilia in rats infected with Trichinella spiralis. Exp Parasitol. 1962 Aug;12:253–262. doi: 10.1016/0014-4894(62)90073-5. [DOI] [PubMed] [Google Scholar]
- ZAIMAN H., VILLAVERDE H. STUDIES ON THE EOSINOPHILIC RESPONSE OF PARABIOTIC RATS INFECTED WITH TRICHINELLA SPIRALIS. Exp Parasitol. 1964 Feb;15:14–31. doi: 10.1016/0014-4894(64)90004-9. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D. Eosinophil function and disorders. Adv Intern Med. 1974;19:1–25. [PubMed] [Google Scholar]


