Abstract
Proteins containing the EF-hand Ca2+-binding motif, such as calmodulin and calcineurin B, function as regulators of various cellular processes. Here we focus on p22, an N-myristoylated, widely expressed EF-hand Ca2+-binding protein conserved throughout evolution, which was shown previously to be required for membrane traffic. Immunofluorescence studies show that p22 distributes along microtubules during interphase and mitosis in various cell lines. Moreover, we report that p22 associates with the microtubule cytoskeleton indirectly via a cytosolic microtubule-binding factor. Gel filtration studies indicate that the p22–microtubule-binding activity behaves as a 70- to 30-kDa globular protein. Our results indicate that p22 associates with microtubules via a novel N-myristoylation–dependent mechanism that does not involve classic microtubule-associated proteins and motor proteins. The association of p22 with microtubules requires the N-myristoylation of p22 but does not involve p22’s Ca2+-binding activity, suggesting that the p22–microtubule association and the role of p22 in membrane traffic are functionally related, because N-myristoylation is required for both events. Therefore, p22 is an excellent candidate for a protein that can mediate interactions between the microtubule cytoskeleton and membrane traffic.
INTRODUCTION
One of the most challenging and least understood aspects of cell biology addresses the question of how the cytoskeleton participates in the delivery of carrier vesicles between organelles and from these to the plasma membrane. Although the role of microtubules in membrane traffic is well documented (Cole and Lippincott-Schwartz, 1995), much of the work in the field has focused on the motor-mediated transport of vesicular carriers along microtubule tracks between distinct membrane-bound organelles and from these to the plasma membrane (Vallee and Sheetz, 1996; Goodson et al., 1997; Bloom and Goldstein, 1998). Less is known, however, about how the morphology and location of organelles as well as the targeting and directionality of vesicle movement depend on an organized microtubule cytoskeleton. Thus, it is important to identify and characterize nonmicrotubule motor molecules that might regulate localized microtubule dynamics and/or couple membrane traffic to the microtubule network to modulate and promote cellular processes like cytokinesis, cell polarity, cell motility, endocytosis, and secretion.
Proteins containing the EF-hand Ca2+-binding motif, such as calmodulin and calcineurin B, function as regulators of various cellular processes that are sensitive to Ca2+ (Nakayama and Kretsinger, 1994; Kawasaki and Kretsinger, 1995; Ikura, 1996; Polans et al., 1996; Schafer and Heizmann, 1996). Here, we investigate the cellular role(s) performed by a novel, widely expressed, and conserved EF-hand Ca2+-binding protein known as p22. Although numerous EF-hand proteins have been identified, only a few have been functionally characterized in a detailed manner. Some, for example recoverin, are thought to be involved in specialized functions of a specific cell type (Polans et al., 1996). Thus, the study of widely expressed and conserved regulatory EF-hand proteins, like calmodulin and p22, is essential for understanding how these proteins coordinate often rapid cellular responses to Ca2+ fluctuations and regulate various general cellular functions.
p22 was isolated by screening a rat liver expression cDNA library with rabbit serum against proteins present in a population of transport vesicles (Sztul et al., 1991; Barroso et al., 1995, 1996). p22 shares extensive amino acid sequence homology with calcineurin B, the regulatory subunit of protein phosphatase 2B (Klee et al., 1988), and less homology with other members of the EF-hand superfamily, such as recoverins, centrins, calmodulins, or cdc31 (Barroso et al., 1996). Recently, two homologues of p22 have been identified, one in humans (99% identity with rat p22) (Lin and Barber, 1996) and another in Caenorhabditis elegans (60% identity with rat p22). Interestingly, there is no yeast homologue for p22. Comparisons between these p22 sequences (rat, human, and C. elegans) and sequences of other members of the EF-hand superfamily suggest that p22 belongs to a separate but related subfamily of the calcineurin B subfamily and has revealed several highly conserved regions (Barroso et al., 1996), as indicated in Figure 1A.
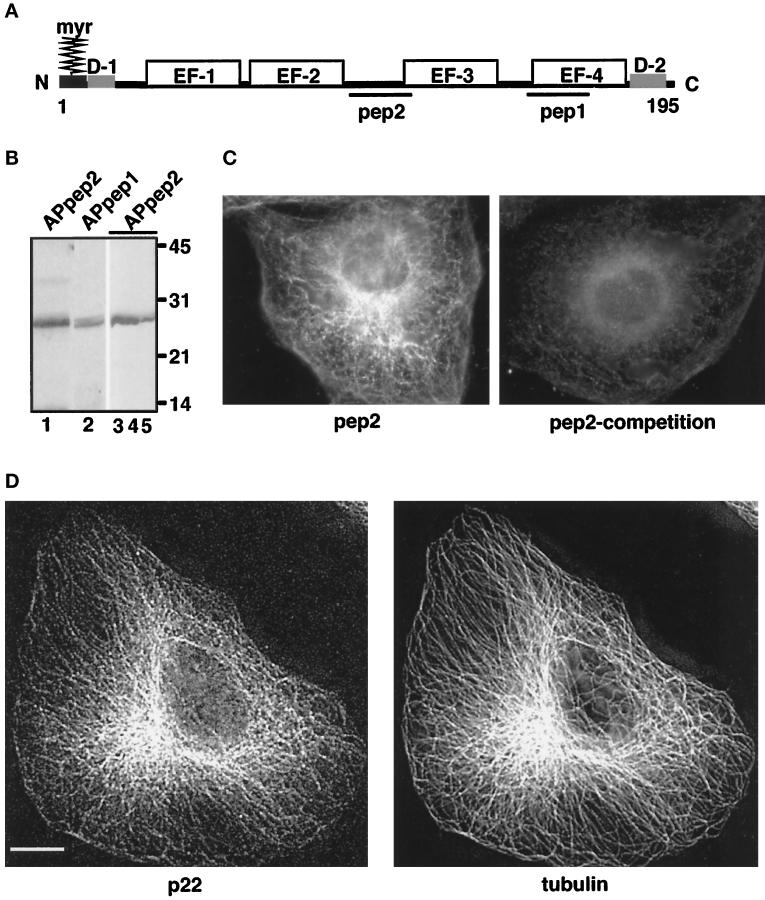
Figure 1.
Characterization of anti-p22 antibodies. (A) p22 conserved domains: myr, N-myristoylation consensus sequence; D-1, N-terminal acidic domain (9-LRDEELEEI-17); D-2, C-terminal domain (184-DVEQKMSIRFLH-195). EF-1, EF-2, EF-3, and EF-4 represent the four EF-hands of p22; pep1 and pep2 represent the sequences used to generate anti-p22 antibodies, APpep1 and APpep2, respectively. The D1 and D2 domains have 90% identity between the rat, human, and C. elegans sequences. (B) APpep2 antibodies recognize recombinant and rat liver p22. Rat liver cytosol (lanes 1 and 2), p22-rec (lane 3), p22-myr (lane 4), and p22-E134A (lane 5) were processed for immunoblotting using APpep2 (lanes 1, 3–5) or APpep1 antibodies (lane 2). A chemiluminescence image is shown. The molecular masses (kDa) are on the right. Note the consistent but retarded gel migration of p22 in relation to its predicted molecular mass (22 kDa). The same phenomenon is observed for rat liver p22 as well as for recombinant p22 and other EF-hand proteins such as recoverin. (C and D) p22 displays a microtubular pattern.NRK cells were processed for immunofluorescence using APpep2 antibodies (pep2) and anti-tubulin antibodies (tubulin). APpep2 antibodies incubated with pep2, before immunofluorescence, show a marked reduction in p22 staining (pep2-competition). Strong colocalization between p22 (p22) and microtubule (tubulin) staining patterns is detected in D. Images were collected by an Integrating CCD camera (PXL, 25°C cooled, Photometrics) in an IX-70 inverted Olympus fluorescence microscope using a 60 or 100× oil objective (W. M. Keck Center for Cellular Imaging, University of Virginia, Charlottesville, VA). Image processing was performed using Inovision ISee software Version 3.8 and Adobe Photoshop. Digital deconvolution was used to obtain images shown in D. Digital deconvolution was applied to 30 optical sections collected using DeltaVision Version 2.10 and a step of 0.4 μm. A representative optical section is shown. Bar, 10 μm.
Previously, we have shown that p22 is N-myristoylated, binds Ca2+, and undergoes conformational changes upon binding of physiological concentrations of Ca2+ (Barroso et al., 1996). We have used a well established transport assay, which reconstitutes the targeting and fusion of donor membrane vesicles with the acceptor apical plasma membrane (Sztul, 1992; Sztul et al., 1993), to demonstrate that N-myristoylation and Ca2+-mediated conformational changes are essential for the function of p22 in membrane traffic (Barroso et al., 1996).
Here, we show that p22 distributes along microtubule tracks in different cell lines during interphase and mitosis. p22’s N-myristoylation is required for the association of p22 with microtubules, suggesting that the role of p22 in membrane traffic and its association with microtubules are functionally related. Also, we have shown that p22’s Ca2+-binding and Ca2+-mediated conformational changes are not required for its association with microtubules. Moreover, we show that p22 associates with microtubules indirectly via a cytosolic 70- to 30-kDa microtubule-binding factor distinct from classic microtubule-associated proteins (MAPs) and motor proteins. In summary, we have characterized the mechanism of interaction of p22 with microtubules that is essential to furthering our understanding of the cellular function(s) of p22.
MATERIALS AND METHODS
Generation of Antibodies against p22 Peptides
Antibodies were raised in rabbits against a synthetic peptide, pep2, (CNEKSKDVNGPEPLNSRSN; residues 96–113 in p22’s amino acid sequence) (see Figure 1A) coupled to keyhole limpet hemocyanin (Pierce, Rockford, IL). Immune serum was affinity-purified (APpep2) using the pep2 peptide immobilized on Sulfolink coupling gel as described previously (Barroso et al., 1996). Immunoblots were performed as described below, and APpep2 antibodies were used at a dilution of 1:400.
Cell Culture and Immunofluorescence
Normal rat kidney (NRK) cells were cultured in MEM with 10% fetal bovine serum and antibiotics (100 μg/ml penicillin and streptomycin). For immunofluorescence, cells were grown on glass coverslips, washed three times briefly in PBS, and fixed for 15–20 min with 4% paraformaldehyde in cytoskeleton buffer (10 mM MES, pH 6.1, 138 mM KCl, 3 mM MgCl2, 2 mM EGTA, 0.32 M sucrose) (Cramer and Mitchison, 1995). The cells were then washed three times briefly in PBS and permeabilized three times for 5 min in PBS/0.1% Triton X-100 (PBS-TX). After they were blocked with 0.5% fish gelatin in PBS-TX for 10 min, the fixed cells were incubated with APpep2 polyclonal antibodies (1:50 dilution) and anti-tubulin monoclonal antibodies (1:200 dilution) for 30 min at room temperature. Then, the cells were washed three times briefly with PBS-TX and incubated with secondary antibodies (donkey anti-mouse lissamine rhodamine or donkey anti-rabbit FITC; Jackson ImmunoResearch, West Grove, PA; 1:100 dilution) for 30 min. Rhodamine–phalloidin (Molecular Probes, Eugene, OR) was used at a dilution of 1:100 for 20 min. The cells were washed briefly in PBS-TX and mounted on glass slides using 50% glycerol in PBS. To depolymerize microtubules, NRK cells were incubated with nocodazole (final concentration of 2.5 μg/ml) for 1 h at 37°C in serum-free MEM containing 20 mM HEPES, pH 7.5, before being processed for immunofluorescence as described above.
Preparation of N-myristoylated Recombinant p22
Wild-type p22 was cloned into the bacterial expression vector PET3a (Novagen, Madison, WI) using the NdeI and BamHI sites, and the resulting vector was called p22PET3a. p22PET3a and N-myristoyl transferase (pBB131; kindly provided by Dr. J. I. Gordon and Monsanto Corporation, Skokie, IL) were cotransformed into BL21(DE3) competent cells. One transformant colony was grown in 150 ml of Luria-Bertani medium in the presence of ampicillin (100 μg/ml) and kanamycin (100 μg/ml) and centrifuged when the OD600 reached 0.6–0.8. The pellet was stored at 4°C overnight. The pellet was divided into 2700 ml of Luria-Bertani medium and grown in the presence of ampicillin and kanamycin until the OD600 reached 0.6. The cultures were induced with a final concentration of 0.4 mM IPTG, and one-twentieth the volume of myristic acid (3% polyoxyethylene 20 cetyl ether, 10 mM myristic acid) was added to each culture. The cultures were grown for another 3–4 h, centrifuged, and washed with PBS. Cell lysates were prepared by sonication, protamine sulfate treatment, and ammonium sulfate precipitation as previously described (Barroso et al., 1996). The resulting pellet was resuspended in TDE (20 mM Tris, pH 8, 5 mM EDTA, 0.5 mM DTT) and dialyzed against TDE. The sample was applied to a DEAE-Sepharose column that was equilibrated with 50 mM KCl in TDE. The column was eluted with a 50–500 mM KCl in TDE gradient. The p22-containing fractions were pooled, dialyzed against TDE, and concentrated to 200 μl. The protein was run over a Superdex 75 gel filtration column (Pharmacia, Piscataway, NJ), and the two fractions containing purified N-myristoylated p22 (p22-myr) were pooled. We currently can express significant amounts (∼250 μg/l of bacteria) of p22-myr, of which ∼98% is N-myristoylated as shown by mass spectroscopy analysis (our unpublished results; work performed in collaboration with Dr. Hunt’s laboratory, Chemistry, University of Virginia). N-myristoylated p22-E134A, an EF-3 mutant where the conserved 12th amino acid (Asp; position 134) of the Ca2+-binding loop was replaced by an Ala (Barroso et al., 1996), was expressed and purified in a similar manner.
Preparation of Nonmyristoylated p22
Nonmyristoylated p22 (p22-rec) was prepared as described above, except that the cells were not transformed with N-myristoyl transferase, and myristic acid was not added upon IPTG-mediated induction of the cultures.
Tubulin Purification and Preparation of Rat Liver Cytosol
Tubulin was purified from bovine brain as previously described in Williams and Lee (1982). Rat liver cytosol was prepared by homogenizing rat livers in PEM (0.1 M PIPES, pH 6.6, 1 mM EGTA, 1 mM MgSO4) at 30% wt/vol and centrifuging at 30,000 × g for 20 min at 2°C. The resulting supernatant was centrifuged again at 180,000 × g for 90 min at 2°C. The rat liver cytosol supernatant was aliquoted, frozen in liquid nitrogen, and stored at −80°C. Before use, the cytosol was centrifuged at 174,000 × g for 30 min at 4°C, and any insoluble material that pelleted was discarded.
Microtubule Cosedimentation Assay
Bovine brain tubulin (final concentration of 0.2–0.4 mg/ml) was incubated for 30 min at 37°C with 20 μM taxol (Sigma T-7402, Sigma, St. Louis, MO), 5 ng/μl aprotinin (Sigma A-1153), 1 ng/μl leupeptin (Sigma L-2884), 1 ng/μl pepstatin (Sigma P-4265), 0.2 μg/μl PMSF (Sigma P-7626), and PEM buffer in the presence or absence of different concentrations of rat liver cytosol (Rickard and Kreis, 1991; Masson and Kreis, 1993; Sontag et al., 1995). Certain reagents, such as 5 mM ATP, 5 mM GTP, 100–500 mM NaCl, or an ATP-depleting system of 10 U/ml hexokinase and 10 mM glucose, were added to the cosedimentation assay as described in the figure legends. The 200-μl reactions were layered over 1 ml of a 1-M sucrose cushion containing the same concentration of protease inhibitors and taxol as described above. The reactions were centrifuged in a swinging bucket TLS55 rotor (Beckman, Fullerton, CA) at 30,000 × g for 30 min at 37°C (Rickard and Kreis, 1991; Masson and Kreis, 1993; Sontag et al., 1995). Supernatants (total volume 200 μl) were added to 100 μl of 2× SDS-PAGE sample buffer, whereas the microtubule pellets were resuspended in 300 μl of 1× SDS-PAGE sample buffer. Then, equal amounts of supernatants and microtubule pellets (15 μl) were analyzed by SDS-PAGE and/or immunoblotting.
Gel Filtration Chromatography
Superose 12 gel filtration chromatography was performed in a Beckman HPLC system as per Pharmacia (Piscataway, NJ) instructions. The column was equilibrated in PBS, and 9 mg of rat liver cytosol were centrifuged at 14000 rpm for 5 min and applied to the column at 0.4 ml/min. Fractions (0.5 ml) were collected and fractions 19–22, 23–25, and 26–28 were pooled and concentrated. Single and pooled fractions were analyzed by SDS-PAGE and immunoblotting. Single and pooled fractions were also assayed for their ability to support the binding of p22-myr to microtubules in vitro.
SDS-PAGE and Immunoblotting
Samples were processed for SDS-PAGE and immunoblotting as previously described (Barroso et al., 1996). Immunoblots were processed by chemiluminescence (ECL, Amersham, Buckinghamshire, UK). Nonsaturated film exposures of ECL-treated blots were scanned and quantitated using the integrated density function in NIH Image. APpep1 (Barroso et al., 1996) and APpep2 antibodies against p22 were used at a 1:400 dilution. Monoclonal antibodies against dynein intermediate chain (74.1), used at a 1:2500 dilution, were kindly provided by Dr. K. K. Pfister (University of Virginia Medical School). Monoclonal antibodies against tubulin, used at a 1:12,000 dilution, and against Tau (Tau1 antibodies), used at a 1:2 dilution, were kindly provided by Dr. A. Frankfurter (University of Virginia). Polyclonal antibodies against frequenin (1:750 dilution) were kindly provided by Dr. A. Jeromin (Mt. Sinai Hospital, Toronto, Canada). Polyclonal antibodies against p115 (1:15,000 dilution) were kindly provided by Dr. E. Sztul (University of Alabama, Birmingham, AL). Monoclonal antibodies against p58 (Sigma) were used at a dilution of 1:7500. Polyclonal antibodies against actin (Sigma) were used at a dilution of 1:400. α55 polyclonal antibodies against CLIP-170 (1:500 dilution) were kindly provided by Dr. T. Kreis (University of Geneva, Geneva, Switzerland).
RESULTS
Generation and Characterization of New Antibodies against Specific p22 Peptide Sequences
Although affinity-purified antibodies against pep1 (APpep1 antibodies) specifically recognize p22 in Western blots (Barroso et al., 1996), they show significant nonspecific staining in immunofluorescence using methanol or paraformaldehyde fixations. Thus, we generated polyclonal antibodies against another p22 peptide, pep2, which is unique to the rat and human p22 sequences (Figure 1A). These antibodies were affinity-purified on a pep2 column (APpep2 antibodies) and are characterized in Figure 1B. APpep2 antibodies recognize all three forms of bacterially expressed p22, p22-myr, p22-rec, and the N-myristoylated mutant p22-E134A, by immunoblotting (Figure 1B, lanes 3–5). APpep2 and APpep1 antibodies recognize a protein of the same apparent molecular weight in rat liver cytosol (Figure 1B, lanes 1–2).
p22 Displays a Microtubular Pattern in NRK Cells
To understand the cellular function(s) of p22, it is essential to determine its intracellular distribution. Interestingly, NRK cells display a typical microtubule pattern when fixed with paraformaldehyde (Figure 1, C and D, pep2 and p22, respectively) or methanol (our unpublished results) and stained with APpep2 antibodies. In Figure 1C (pep2-competition), APpep2 antibodies preincubated with pep2 peptides show a markedly reduced staining in NRK cells, indicating that APpep2 antibodies recognize p22. APpep2 antibodies may also recognize other unidentified members of the p22 subfamily. When pep2 peptides are preincubated with anti-tubulin antibodies, the tubulin staining is not affected (our unpublished results), indicating that pep2 peptides do not cause random aggregation of antibodies. Addition of pep1 peptides to APpep2 antibodies does not reduce the microtubule-like pattern of p22 (our unpublished results). These results show that pep2 binds to APpep2 antibodies, which leads to a marked reduction in the p22 staining in NRK cells.
To confirm that the staining pattern of p22 overlaps with that of the microtubule cytoskeleton, we performed double-label immunofluorescence of p22 and tubulin in NRK cells using APpep2 polyclonal antibodies and anti-tubulin monoclonal antibodies. A strong colocalization between the staining patterns of p22 (p22) and microtubules (tubulin) is detected in NRK cells (Figure 1D). p22 staining concentrates at the microtubule-organizing center (MTOC) and extends toward the periphery along microtubules. When visualized at a higher magnification, p22 shows a punctate distribution when compared with the continuous tubulin staining. A small fraction of p22-containing structures was found, not in association with microtubules, underneath the plasma membrane or in the cytosol (our unpublished results).
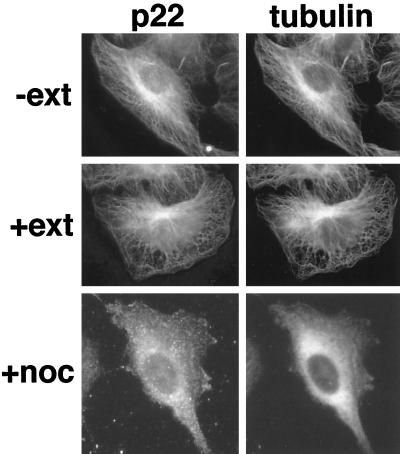
To obtain further evidence that p22 interacts with microtubules, NRK cells were selectively extracted with Triton X-100, a nonionic detergent, before fixation and immunofluorescence. Triton extraction removes ∼80% of the total cellular protein (Brown et al., 1976), but in the presence of PEM buffer, a microtubule-stabilizing buffer, most microtubules remain intact (Osborn and Weber, 1977). In Figure 2, Triton-extracted NRK cells (+ext) show an unchanged microtubule and p22 staining patterns relative to intact cells (−ext). We then tested whether treatment with microtubule-depolymerizing drugs like nocodazole would affect the distribution pattern of p22. As shown in Figure 2 (+noc/tubulin), tubulin staining shows a diffuse pattern throughout the cell, indicating that microtubules were depolymerized by the nocodazole treatment. Accordingly, a diffuse staining for p22 (+noc/p22) is seen in nocodazole-treated NRK cells. The Triton X-100 resistance and nocodazole sensitivity displayed by the distribution pattern of p22 clearly indicate that p22 associates specifically with microtubules.
Figure 2.
p22 colocalizes with microtubules in NRK cells. Immunofluorescence and image collection were performed as in Figure 1C using APpep2 (p22) and anti-tubulin (tubulin) antibodies in NRK cells. NRK cells were selectively extracted with (+ext) or without (−ext) Triton X-100 before fixation and immunofluorescence analysis. Triton X-100–extracted NRK cells show a normal microtubule staining pattern as well as an unchanged p22 pattern relative to intact cells. Nocodazole-treated NRK cells (+noc) show a diffuse staining for p22 and tubulin, although the staining pattern of p22 appears more punctate.
p22 Is Associated with Microtubules during Mitosis in Several Cell Lines
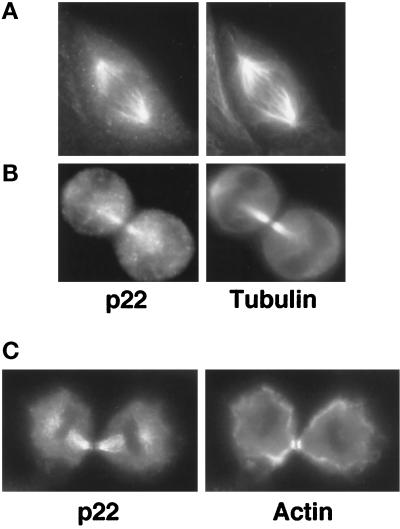
p22 is found in association with the mitotic spindle microtubules at different stages of mitosis in NRK, Madin–Darby canine kidney (MDCK), and CHO (Chinese hamster ovary) cells (Figure 3). In Figure 3A, we show an example of such an association during anaphase in MDCK cells. In Figure 3B, a strong colocalization between p22 and microtubule staining patterns is detected in CHO cells undergoing telophase. In Figure 3C, we show that p22 does not localize with the actin-rich contractile ring that encircles the equator of dividing NRK cells during cytokinesis. Because p22 is found associated with interphase, mitotic spindle, and intercellular bridge microtubules in several cell lines, we conclude that p22 remains associated with microtubules during the cell cycle.
Figure 3.
p22 associates with microtubules during mitosis. MDCK (A), CHO (B), and NRK cells (C) were plated at low confluency and processed for immunofluorescence as in Figure 1C using APpep2 antibodies (p22) and either anti-tubulin antibodies (Tubulin) or rhodamine–phalloidin to label actin filaments (Actin). An MDCK cell is shown undergoing anaphase (A), whereas CHO (B) and NRK (C) cells are shown undergoing telophase.
p22 Associates with Polymerized Microtubules In Vitro
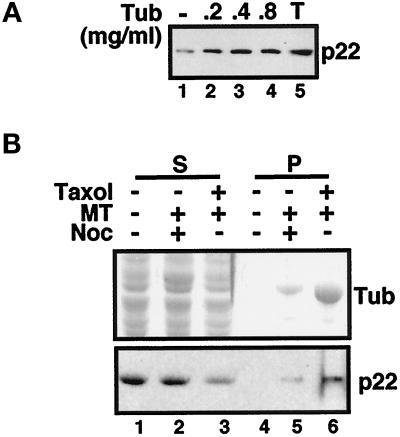
Our results obtained by immunofluorescence indicate that p22 colocalizes with microtubules in vivo. To address this question at a biochemical level and to confirm these immunofluorescence results independently of the use of APpep2 antibodies, we tested whether cytosolic p22 could associate with taxol-polymerized microtubules in cosedimentation assays. Rat liver cytosol (500 μg) was incubated in the presence of PEM buffer, protease inhibitors, and taxol at 37°C for 30 min and then centrifuged through a sucrose cushion to pellet cytosolic polymerized microtubules and their associated proteins. In Figure 4A, we show that ∼30% of rat liver cytosolic p22 can pellet with endogenous taxol-polymerized microtubules (lane 1). To test whether we could achieve a more efficient cosedimentation of cytosolic p22, we added exogenous taxol-polymerized brain microtubules to rat liver cytosol in the cosedimentation assays. When 0.2 mg/ml brain taxol-polymerized microtubules are added to the cosedimentation assay, twice as much p22 associates with the microtubule pellet (∼60–65%) (lane 2). Addition of 0.4 or 0.8 mg/ml brain microtubules does not result in a significant increase in the cosedimentation of p22 with microtubules (lanes 3–4). Total rat liver cytosol added to the in vitro cosedimentation assay is shown in lane 5. Recently, Bashour and Bloom (1998) showed that p58, a rat liver protein, associated with brain microtubules specifically but not with rat liver microtubules. It appears that p58 may bind brain microtubules via polyglutamates that are added posttranslationally to tubulin in brain but not to tubulin in liver (Bashour and Bloom, 1998; Hennig et al., 1998). Thus, p58 is not expected to bind microtubules in the only tissue in which it is abundant, namely liver. Because p22 is widely expressed in various tissues, including brain (Barroso et al., 1996), its association with brain microtubules should be relevant for its functional activity.
Figure 4.
p22 associates with taxol-polymerized microtubules in vitro. (A) Microtubule-binding proteins were purified from rat liver cytosol by incubation with taxol to polymerize endogenous microtubules (lane 1) or with 0.2 mg/ml (lane 2), 0.4 mg/ml (lane 3), or 0.8 mg/ml (lane 4) taxol-polymerized brain microtubules. The 200-μl reaction mixture was layered over 1 ml of a 1 M sucrose cushion and centrifuged in a TLS55 rotor for 30 min at 37°C at 21,000 rpm. The total amount of p22 present in the cytosol is shown in lane 5. Equal amounts of pellets (lanes 1–4) were collected and analyzed by immunoblotting with anti-p22 (p22). (B) Microtubule-binding proteins were purified from rat liver cytosol by incubation with nocodazole-treated (lane 5) or taxol-treated (lane 6) brain microtubules. Taxol-treated microtubules were not added to rat liver cytosol to test for nonspecific pelleting of p22 (lane 4). The 200-μl reaction mixture was centrifuged through a sucrose cushion as described for A. Equal amounts of supernatants (lanes 1–3) and pellets (lanes 4–6) were collected and analyzed by SDS-PAGE and Coomassie blue staining (Tub) or by immunoblotting with anti-p22 (p22). Nonsaturated film exposures of ECL-treated blots were scanned and quantitated using the NIH Image program.
To test whether the cosedimentation of p22 is dependent on the presence of polymerized microtubules, we incubated rat liver cytosol in PEM buffer and protease inhibitors in the absence (Figure 4B, lanes 1 and 4) or presence of nocodazole-treated (lanes 2 and 5) or taxol-treated brain microtubules (lanes 3 and 6) for 30 min at 37°C. In the absence of polymerized microtubules, most of p22 remains in the supernatant (lane 1). Only reduced amounts of cytosolic p22 pellet in the presence of nocodazole-treated brain microtubules (lane 5), whereas a significant amount of cytosolic p22 associates with microtubule pellets in the presence of taxol-polymerized microtubules (lane 6). In summary, these results demonstrate that the sedimentation of p22 is microtubule dependent.
Quantitation of the Association of p22 with Microtubules
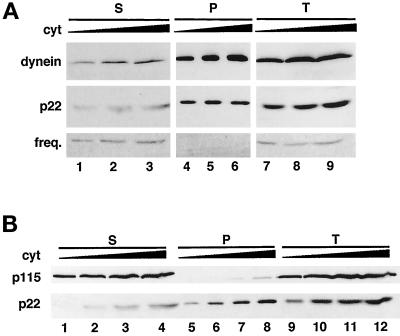
To confirm the results shown in Figure 4B and to further characterize the microtubule-binding ability of cytosolic p22, we subjected different amounts of rat liver cytosolic proteins (250, 500, 750, and 1000 μg) to the cosedimentation assay in the presence of taxol-polymerized brain microtubules (Figure 5). In Figure 5A, we show that ∼60% of cytosolic p22 (p22, lanes 4–6) and ∼70% of the microtubule motor cytoplasmic dynein (Paschal et al., 1987; Schroer et al., 1989) (dynein, lanes 4–6) can associate with taxol-polymerized microtubules, considering the total amount of p22 and cytoplasmic dynein present in cytosol (p22 and dynein, lanes 7–9) as 100%. A cytosolic protein such as TAP/p115 (Barroso et al., 1995) (Figure 5B, p115, lanes 5–8) and an irrelevant soluble protein such as Protein A show negligible binding (our unpublished results). In summary, our cosedimentation results demonstrate that <60% of p22 in rat liver cytosolic extracts is able to associate with microtubules in vitro. For comparison, 50–75% of the phosphatase 2A cytosolic pool associates with microtubule pellets in cosedimentation assays (Sontag et al., 1995).
Figure 5.
Binding to microtubules is not general to all EF-hand Ca2+ binding proteins. (A) Microtubule-binding proteins were purified from different amounts of rat liver cytosol: 500 μg (lanes 1, 4, and 7), 750 μg (lanes 2, 5, and 8), and 1000 μg (lanes 3, 6, and 9) by incubation with taxol-polymerized brain microtubules as described in Figure 4. (B) Microtubule-binding proteins were purified from different amounts of rat liver cytosol: 250 μg (lanes 1, 5, and 9), 500 μg (lanes 2, 6, and 10), 750 μg (lanes 3, 7, and 11), and 1000 μg (lanes 4, 8, and 12) as described above. The total amount of p22 present in the different amounts of rat liver cytosol is shown in lanes 7–9 (A) and 9–12 (B). Equal amounts of supernatants (lanes 1–3 in A and 1–4 in B) and pellets (lanes 4–6 in A and 5–8 in B) were collected and analyzed by immunoblotting with anti-p22 (p22), anti-dynein antibodies (dynein), anti-frequenin antibodies (freq.), and anti-p115 antibodies (p115). Nonsaturated film exposures of ECL-treated blots were scanned and quantitated using the NIH Image program.
We also assayed the microtubule cosedimentation assay for the presence of frequenin, an N-myristoylated member of the EF-hand superfamily of Ca2+-binding proteins, which was suggested to facilitate neurotransmitter release in neuromuscular junctions (Pongs et al., 1993; Olafsson et al., 1995; McFerran et al., 1998). As shown in Figure 5A (freq., lanes 4–6), frequenin does not associate with microtubules, indicating that the association of p22 with microtubules is not general to all N-myristoylated EF-hand Ca2+-binding proteins.
It is also important to notice that the in vitro microtubule cosedimentation assay is performed in the presence of PEM buffer, which contains 1 mM EGTA, a Ca2+ chelating agent. Also the addition of 5 mM CaCl2 to the microtubule cosedimentation assay does not affect the binding of p22 to microtubules (our unpublished results). Because p22 is found associated with the microtubule pellets in the presence of EGTA as well as of Ca2+, we do not expect Ca2+-binding and Ca2+-mediated conformational changes to be required for the binding of p22 to microtubules (see below).
The Association of p22 with Microtubules Requires a Cytosolic Factor
p22 does not contain characteristic microtubule-binding sites, for example, CAP-Gly domains that are present in CLIP-170 (Pierre et al., 1992) and p150dynactin (Gill et al., 1991) or tandem repeats that are present in Tau, MAP2, and MAP4 proteins (Lewis et al., 1989; Aizawa et al., 1990). In general, microtubule-binding domains are defined by positively charged regions. Several other microtubule-binding basic domains that do not show significant homology with CAP-Gly domains or Tau repeats have been identified in structural MAPs, for example, E-MAP-115 (Masson and Kreis, 1993) and MAP1B (Noble et al., 1989). Because p22 does not possess any characteristic basic domain, the p22–microtubule interaction is not expected to be charge dependent. Polyaspartic acid is an anionic compound that blocks the charge-dependent interaction between MAP1 and MAP2 and tubulin (Nakamura et al., 1989; Fujii et al., 1990). Polyaspartic acid (10–40 μg/ml) does not inhibit the cosedimentation of p22 with taxol-stabilized microtubules (our unpublished results), suggesting that the p22–microtubule interaction is not charge dependent and that p22 is not binding nonspecifically to acidic molecules like tubulin and/or actin.
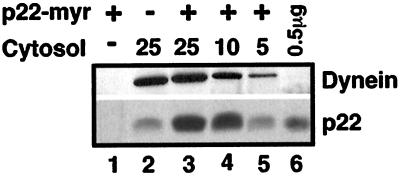
Because p22 does not possess any characteristic microtubule-binding domain, we expect p22 to associate with microtubules indirectly via linker protein(s); however, p22 may still associate directly with microtubules through a yet unidentified microtubule-binding site. Thus, to determine whether p22 binds directly or indirectly to microtubules, we have assayed whether purified bacterially expressed p22-myr binds to taxol-polymerized microtubules (prepared from purified brain tubulin free of associated proteins, as described in Williams and Lee, 1982) in the presence or absence of cytosol. In Figure 6 (p22, lane 1), we showed that only negligible amounts of p22-myr associate with the microtubule pellet in the absence of cytosol, suggesting that p22 binds microtubules through a microtubule-binding factor. As shown in Figure 6 (Dynein, lanes 2–3), the same amount of cytoplasmic dynein associates with microtubule pellets in the absence or presence of p22-myr. We then tested whether p22-myr could bind microtubules in the presence of cytosol. When 25 μl of cytosol (23 mg/ml) were added to 2 μg of p22-myr, the amount of microtubule-associated p22 increased by threefold (p22, lane 3) in comparison with the amount of cytosolic p22 that associates with the microtubule pellet in the absence of p22-myr (p22, lane 2). Thus, the amount of p22–microtubule-binding factor should be in a threefold excess, at least, relative to that of cytosolic p22 and should not be a rate-limiting step for the association of p22 to microtubules in an in vitro assay.
Figure 6.
p22 binds indirectly to microtubules via a cytosolic microtubule-binding factor. p22-myr (2 μg) (lanes 1 and 3–5) and/or rat liver cytosol (25, 10, or 5 μl) (lanes 2–5) at a concentration of 23 mg/ml were incubated with purified brain tubulin (0.2 mg/ml) in the presence of PEM buffer and taxol, and processed as described in Figure 4. Equal amounts of microtubule pellets (lanes 1–5) and 0.5 μg of p22-myr were subjected to immunoblotting using anti-dynein (dynein) and anti-p22 (p22). Dynein was used as an internal control for the various dilutions of cytosol. Nonsaturated film exposures of ECL-treated blots were scanned and quantitated using the NIH Image program.
When 10 or 5 μl of cytosol were added to the microtubule-binding assay in the presence of 2 μg of p22-myr, we observed a reduction of 1.4- and 3.2-fold, respectively (Dynein, lanes 4 and 5), in the binding of dynein to microtubules in comparison to the amount of dynein that associates with the microtubule pellet in the presence of 25 μl of cytosol and 2 μg of p22-myr (Dynein, lane 3). A similar reduction of 1.2- and 3.4-fold in the amount of p22 binding to microtubules was detected in the presence of 2 μg of p22-myr and 10 or 5 μl of cytosol, respectively (p22, lanes 4 and 5), relative to the amount of p22 that associates with the microtubule pellet in the presence of 25 μl of cytosol and 2 μg of p22-myr (p22, lane 3). These results show that dilution of cytosol in the microtubule-binding assay leads to a similar level of reduction in the amount of microtubule-associated dynein and p22, strongly suggesting that the p22–microtubule-binding factor is a cytosolic protein. In agreement, p22 associates with microtubules in the presence of Triton X-100, a nonionic detergent, and thus in the absence of organized membranes both in vivo (Figure 2, +ext) and in vitro (our unpublished results).
p22–Microtubule Association Is Sensitive to 500 mM NaCl but It Is Insensitive to Nucleotides
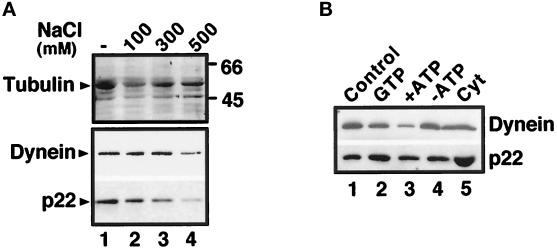
To investigate the nature of the cytosolic p22–microtubule-binding factor, we took advantage of various reagents that have been shown to modulate the association of microtubule-binding proteins with microtubules. As shown in Figure 7A, both dynein and p22 dissociate from microtubules in the presence of 500 mM NaCl, as has been shown for several MAPs and motor proteins (Vallee, 1986), whereas tubulin is found associated with the microtubule pellets independent of the salt concentration. Increasing the salt concentration linearly from 0 to 500 mM results in increased dissociation of p22 from microtubules (Figure 7A, p22).
Figure 7.
p22–microtubule association is sensitive to 500 mM NaCl but insensitive to nucleotides. (A) Equal amounts of microtubule pellets from microtubule-binding assays treated with increasing amounts of NaCl (100–500 mM NaCl) (lane 2–4) or not (lane 1) were analyzed by SDS-PAGE and Coomassie blue staining (tubulin) and immunoblotted using anti-p22 (p22) and anti-dynein (dynein). (B) Equal amounts of microtubule pellets from microtubule-binding assays treated with 5 mM GTP (lane 2), 5 mM ATP (lane 3), an ATP-depleting system (lane 4), or not (lane 1) were immunoblotted as described in A. Lane 5 shows the total amount of p22 present in the cytosol added to the cosedimentation assay. Nonsaturated film exposures of ECL-treated blots were scanned and quantitated using the NIH Image program.
As shown in Figure 7B (p22, lanes 1–4), p22 cosediments with microtubules under all the conditions tested, suggesting that its binding to microtubules is nucleotide insensitive. In the presence of 5 mM ATP, the majority of cytoplasmic dynein dissociates from microtubules, as has been shown previously (Paschal et al., 1987, 1991; Schroer et al., 1989). These results suggest that the binding of p22 to microtubules is not mediated by microtubule motor proteins, for example cytoplasmic dynein and kinesin, because these proteins are expected to be released from microtubules in the presence of high concentrations of ATP or GTP, respectively (Vale et al., 1985; Kuznetsov and Gelfand, 1986; Paschal et al., 1987; Schroer et al., 1989; Gelfand and Bershadsky, 1991; Paschal et al., 1991).
To test whether CLIP-170, a protein involved in linking endocytic vesicles to microtubules (Pierre et al., 1992), is required for the binding of p22 to microtubules, we assayed the effect of the serine phosphatase inhibitor okadaic acid on the p22-microtubule association, because binding of CLIP-170 is inhibited in the presence of okadaic acid (Rickard and Kreis, 1991). Okadaic acid had no effect on the binding of p22 to microtubules (our unpublished results), suggesting that CLIP-170 is not involved in the binding of p22 to microtubules.
N-myristoylation Is Required for p22 to Associate with Microtubules
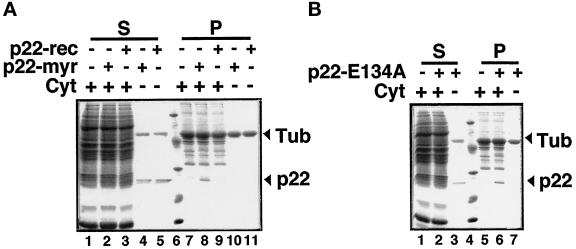
To assay the importance of N-myristoylation and Ca2+-binding in the p22–microtubule association, we used our cosedimentation assay to follow the binding of p22-myr, p22-rec, and N-myristoylated p22-E134A to microtubules using SDS-PAGE and Coomassie Blue staining. When 500 μg of cytosol were added to the cosedimentation assay in Figure 5, A (lanes 1, 4, and 7) and B (lanes 2, 6, and 10), we used immunoblotting to detect the amount of p22 associated with the microtubule pellet, as shown in Figure 5, A (lane 4) and B (lane 6). In Figure 8, when a similar amount of cytosol (500 μg) was added to the taxol-polymerized microtubules in the cosedimentation assay in the absence of added recombinant p22, no detectable amounts of proteins within the 31- to 21-kDa range were detected associated with the microtubule pellet using Coomassie blue staining of SDS-PAGE gels (Figure 8, A [lane 7] and B [lane 5]). Coomassie blue staining is less sensitive than immunoblotting, which explains the inability to detect a protein of a molecular weight similar to p22 in Figure 8, A (lane 7) and B (lane 5). Then, we incubated 12 μg of purified p22-myr (final concentration of 62 μg/ml in the cosedimentation assay) with taxol-polymerized microtubules in the presence or absence of cytosol and subjected this reaction mixture to the microtubule cosedimentation assay described above. As expected, in the absence of cytosol, the majority of p22-myr (arrow) is found in the supernatant (Figure 8A, lane 4), with no significant direct binding to the microtubule pellet (Figure 8A, lane 10). Detectable amounts of p22-myr (arrow) can only be seen in association with microtubule pellets in the presence of cytosol (Figure 8A, lane 8). Three sets of p22-myr–microtubule-binding experiments were analyzed by SDS-PAGE and Coomassie blue staining. These Coomassie blue-stained gels were dried and scanned, and the amount of p22-myr was quantitated using the NIH Image program. Considering the amount of p22-myr present in the supernatant in the absence of cytosol as 100%, we determined that ∼50% of p22-myr is found associated with microtubule pellets in the presence of cytosol. Moreover, we subjected these three sets of p22-myr–microtubule-binding experiments to immunoblotting using anti-p22 antibodies, and we have determined that ∼45% of total added p22-myr cosediments with microtubules in the presence of cytosol (our unpublished results). We have used higher dilutions of anti-p22 antibodies to quantitate the p22-myr added to the microtubule cosedimentation assays. Thus, 50–60% of p22-myr as well as of cytosolic p22 can associate with microtubules in vitro. Sedimentation of p22-myr does not occur under conditions that do not favor the sedimentation of microtubules, for example, under nocodazole and/or ice treatment. These results are in agreement with those shown in Figure 6, indicating that p22-myr cannot associate with microtubules directly and that the p22–microtubule interaction requires a cytosolic factor.
Figure 8.
N-myristoylation is required for the association of p22 with microtubules. (A) p22-myr (12 μg), p22-rec (12 μg), and/or rat liver cytosol (500 μg) were incubated with purified tubulin (0.4 mg/ml) in the presence of PEM buffer and taxol, and processed as described in Figure 4. Equal amounts of supernatants (lanes 1–5) and microtubule pellets (lanes 7–11) were subjected to SDS-PAGE and Coomassie blue staining. Arrows indicates the position of p22 and tubulin. In lane 6, the molecular weights in kDa are as follows: 97, 45, 31, 21, 14. (B) N-myristoy-lated p22-E134A (12 μg) and/or rat liver cytosol (500 μg) were incubated with purified tubulin (0.4 mg/ml) in the presence of PEM buffer and taxol, and processed as described in Figure 4. Equal amounts of supernatants (lanes 1–3) and microtubule pellets (lanes 5–7) were subjected to SDS-PAGE and Coomassie blue staining. In lane 4, the molecular weights in kDa are as follow: 97, 45, 31, 21, 14. Arrows indicates the position of p22 and tubulin.
To assay the functional significance of N-myristoylation in the association of p22 with microtubules, we added purified bacterially expressed p22-rec (nonmyristoylated p22) to the microtubule cosedimentation assay in the presence of cytosol (Figure 8A). p22 expressed in bacteria is nonmyristoylated, because bacteria do not perform this cotranslational modification. In Figure 8A, we show that the association of p22-rec with the microtubule pellet is dramatically reduced (lane 9), whereas p22-myr is found associated with the microtubule pellet (lane 8). As expected, in the absence of cytosol, most of p22-myr and p22-rec is found in the supernatant (lanes 4–5), with no significant direct binding to microtubules (Figure 8A, lanes 10 and 11). These results suggest that N-myristoylation is essential for p22 to associate with microtubules. As shown in Figure 5, frequenin, another N-myristoylated EF-hand Ca2+-binding protein, does not associate with microtubules, indicating that the requirement for N-myristoylation is specific to p22’s association with microtubules.
Because the cosedimentation assay is performed in the presence of 1 mM EGTA (from the PEM buffer), Ca2+ does not appear to be required for p22 to associate with microtubules. To test directly whether Ca2+-binding and Ca2+-mediated conformational changes are required for p22 to associate with microtubules, we added bacterially expressed N-myristoylated p22-E134A, which is unable to undergo Ca2+-mediated conformational changes and shows reduced Ca2+-binding ability (Barroso et al., 1996) to the microtubule cosedimentation assay (Figure 8B). In the presence of cytosol, N-myristoylated p22-E134A can associate with the microtubule pellet (Figure 8B, lane 6). In the absence of cytosol, the majority of N-myristoylated p22-E134A is found in the supernatant (Figure 8B, lane 3), with no significant direct binding to microtubules (Figure 8B, lane 7). Our results suggest that Ca2+-binding as well as Ca2+-mediated conformational changes are not required for the association of p22 with microtubules.
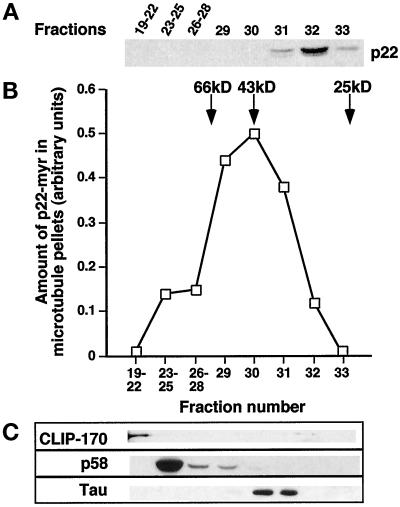
p22–Microtubule-Binding Factor Has a Molecular Weight in the Range of 70–30 kDa and Is Distinct from Classic MAPs
Rat liver cytosol was fractionated on a Superose 12 gel filtration column (fractionation range: 1–300 kDa). Cytosolic p22 elutes between the 43 and 25 kDa markers (Figure 9A), which is consistent with p22’s retarded electrophoretic gel migration of 27 kDa in relation to its predicted molecular mass of 22 kDa (Barroso et al., 1996). The same phenomenon is observed for bacterially expressed p22 as well as for other EF-hand proteins, such as recoverin. Then, single or pooled fractions were added to p22-myr and microtubules in the cosedimentation assay and tested for their ability to support the binding of p22-myr to microtubules, as described for Figure 8. As shown in Figure 9B, the p22–microtubule binding activity elutes in fractions 29–31. Although it is still unclear whether the p22–microtubule binding activity represents a single protein, fractionation of rat liver cytosol on Superose 12 as well as on a Superdex-75 gel filtration column (fractionation range: 3–70 kDa; our unpublished results) yields a single peak of p22–microtubule binding activity that correspond to a globular protein of ∼70–30 kDa. Thus, p22 and its microtubule-binding factor elute in different fractions upon Superose 12 chromatography (31–33 and 29–31, respectively), indicating that they do not associate to form a heterodimer in cytosol. Western blot analyses of microtubule pellets show that CLIP-170 (Pierre et al., 1992), p58 (Bashour and Bloom, 1998; Hennig et al., 1998), and Tau (isoform recognized in rat liver extracts by Tau-1 antibodies) (Kosik et al., 1988, 1989; Michalik et al., 1995; Gu et al., 1996) associate with microtubules in fractions 19–22, 23–29, and 30–31, respectively (Figure 9C). These results exclude high molecular weight MAPs, such as MAP2 and MAP4, as well as CLIP-170, p58, and Tau as candidates for the p22–microtubule binding factor, because their peak of microtubule-binding activity does not comigrate with the p22–microtubule-binding activity.
Figure 9.
The cytosolic p22–microtubule-binding factor behaves as 70- to 30-kDa globular protein upon fractionation on Superose 12. (A) Cytosol was fractionated on Superose 12 (fractionation range: 1–300 kDa) and pooled, or single fractions were analyzed by SDS-PAGE and immunoblotting with anti-p22. p22 elutes in fractions 31–33. (B) Pooled or single fractions were tested for their ability to support the binding of p22-myr to microtubules in the cosedimentation assay. Fractionation of the cytosol yielded a peak of p22–microtubule-binding activity around 70–30 kDa (fractions 29–31). Coomassie blue-stained SDS-PAGEs were scanned, and the amount of p22-myr found associated with the microtubule pellet was quantitated using the NIH Image program. (C) Immunoblotting of microtubule pellets with anti-CLIP-170 (α55), anti-p58, and anti-Tau (Tau 1) shows that the microtubule-binding profiles of these proteins do not overlap with the p22–microtubule-binding activity.
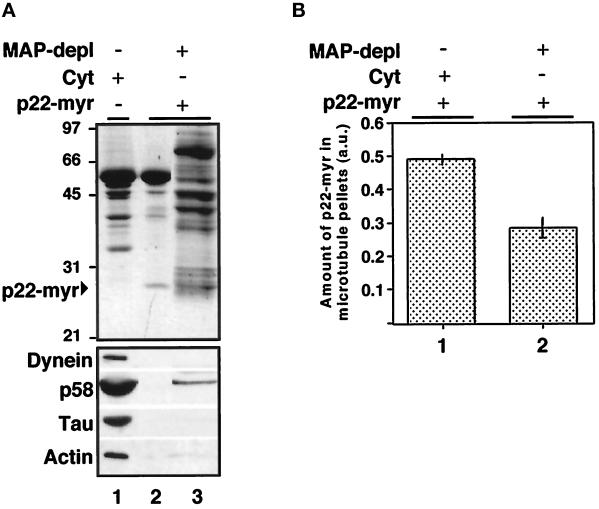
To test whether the p22–microtubule-binding activity behaves as a classic MAP, we used microtubule affinity depletion to remove these proteins from rat liver cytosol (Vallee, 1986; Blocker et al., 1996). A microtubule cosedimentation assay was performed, as described for Figure 5, and proteins bound to microtubules were removed from rat liver cytosol by pelleting (Figure 10A, lane 1). To test whether the supernatant (MAP-depleted cytosol) could support the binding of p22-myr to microtubules, we subjected the MAP-depleted cytosol to a subsequent cosedimentation assay in the presence of extra microtubules and p22-myr (Figure 10A, lane 2). Western blot analyses of the microtubule pellets and supernatants confirmed the specific depletion of microtubule-binding proteins, such as Tau, p58, and dynein. As shown in Figure 10A (bottom panel), the majority of dynein, p58, and Tau was found associated with the first microtubule pellet (lane 1), with no significant binding to the second microtubule pellet (lane 2). Nevertheless, the MAP-depleted cytosol was able to support the binding of p22-myr to microtubules (Figure 10A, top panel, lane 2), indicating that the cytosolic p22–microtubule-binding factor cannot be completely removed by microtubule affinity depletion, as has been shown for MAPs and motors. Because actin has been found associated with microtubules (Sider et al., 1999), we used immunoblotting to assay whether actin was found associated with the first and/or second microtubule pellets. As shown in Figure 10A (actin), actin was found associated with the first microtubule pellet (lane 1) with no significant binding to the second pellet (lane 2). We also tested whether actin is involved in the p22–microtubule association by incubating actin and p22-myr in the presence of taxol-polymerized microtubules and subjecting this reaction mixture to the microtubule cosedimentation assay. The great majority of p22-myr was found in the supernatant, indicating that actin is not involved in the association of p22 with microtubules (our unpublished results). Thus actin, as well as p58, dynein, and Tau, are not good candidates for the p22–microtubule-binding factor. When p22-myr was added to MAP-depleted cytosol, the amount of p22-myr that was found associated with the microtubule pellet was reduced by 1.7-fold (Figure 10B, lane 2), in comparison with the amount of p22-myr that associates with microtubules in the presence of total cytosol (Figure 10B, lane 1). Thus, ∼50–40% of the p22–microtubule-binding factor is removed from cytosol by microtubule pelleting. These results indicate that MAPs, which bind to microtubules with high affinity and thus are completely removed by microtubule pelleting, are not involved in the association of p22 with microtubules and that the cytosolic p22–microtubule-binding factor has an intrinsically low affinity for microtubules.
Figure 10.
MAPs and motor proteins that are removed from cytosol by microtubule affinity depletion are not necessary for the binding of p22 to microtubules. (A) A microtubule cosedimentation assay was performed in the presence of cytosol and microtubules as described above to remove MAPs and motor proteins from cytosol by microtubule pelleting (lane 1). The supernatant (MAP-depleted cytosol: MAP-depl −) was then tested for its ability to support the binding of p22-myr to extra microtubules in a second microtubule cosedimentation assay (lane 2). Equal amounts of microtubule pellets from the first and second cosedimentation assay (lanes 1 and 2) and supernatant from the second cosedimentation assay (lane 3) were subjected to SDS-PAGE and Coomassie blue staining (top panel) as well as to immunoblotting with anti-dynein, anti-p58, anti-Tau (Tau 1), and anti-actin (bottom panel). (B) Equal amounts of microtubule pellets from cosedimentation assays performed in the presence of microtubules, p22-myr and cytosol (lane 1), or MAP-depleted cytosol (lane 2) were subjected to SDS-PAGE and Coomassie blue staining. The Coomassie blue-stained SDS-PAGE was scanned, and the amount of p22-myr found associated with the microtubule pellet was quantitated using the NIH Image program. The bars represent the mean ± SD from three experiments.
DISCUSSION
Here we show that p22, a conserved and widely expressed N-myristoylated EF-hand Ca2+-binding protein, associates indirectly with microtubules via a cytosolic factor. Originally, we showed that p22 is required for membrane traffic in a cell-free assay that reconstitutes the targeting and fusion of membrane vesicles with the plasma membrane (Barroso et al., 1996). The association of p22 with microtubules requires the N-myristoylation of p22 but does not involve p22’s Ca2+-binding activity, suggesting that the p22–microtubule association and the role of p22 in membrane traffic are functionally related, because N-myristoylation is required for both events. Significantly, p22 is one of the first identified nonmotor proteins that is likely to regulate membrane traffic through its association with the microtubule cytoskeleton. Using immunofluorescence and microtubule cosedimentation assays, we found that p22 associates with microtubules in vivo as well as in vitro. p22 associates indirectly with microtubules via a cytosolic microtubule binding factor that binds N-myristoylated p22, but not nonmyristoylated p22, does not require Ca2+, behaves as a 70- to 30-kDa globular protein, and is distinct from classic MAPs and motor proteins. Thus, we propose that p22 associates with microtubules via a novel N-myristoylation–dependent mechanism, which does not involve classic MAPs and motor proteins.
We have shown that p22 distributes along microtubule tracks in different cell types. During interphase, p22 associates with the MTOC and extends toward the cell periphery along microtubule tracks. The staining of p22 is more punctate than that of tubulin, suggesting that p22 may associate with other proteins or membranes intermittently spaced along microtubules. p22’s localization is specific to microtubules in that p22 remains associated with microtubules after detergent extraction and its microtubule pattern collapses upon treatment with nocodazole to depolymerize microtubules. Our results do not exclude the possibility that a small fraction of p22 might be able to associate with cellular organelles and/or behave as soluble cytoplasmic protein. During mitosis, p22 is found associated with the mitotic spindle and intercellular bridge microtubules. p22 might have two distinct functions in interphase and mitosis as was shown for cytoplasmic dynein, which transports organelles along microtubules in interphase, whereas in metaphase it has been implicated in mitotic spindle formation and orientation as well as in chromosome segregation (Niclas et al., 1996).
N-myristoylation involves the cotranslational addition of a fatty acyl moiety to the extreme amino terminus of the protein and has been shown to be required for protein–protein (Chow et al., 1987; Song et al., 1996) and protein–membrane interactions (Swierczynski and Blackshear, 1995; Franco et al., 1996), Ca2+-binding (Ames et al., 1995), and protein stability and folding (Yonemoto et al., 1993; Kennedy et al., 1996). The finding that p22, but not frequenin, another N-myristoylated EF-hand Ca2+-binding protein, associates with microtubules in an N-myristoylation–dependent manner, suggests that in addition to the N-myristoyl group, other p22 domains are required for the specific association of p22 with microtubules. For example, the N-myristoyl group of p22 might cooperate with other p22 domain(s) for the formation of a novel binding site specific for the p22–microtubule binding factor. Also, N-myristoylation might be required for the correct folding of p22 to allow its binding to the microtubule binding factor. Thus, the p22–microtubule binding factor should be able to recognize the N-myristoyl group of its binding targets as well as other p22 domain(s). Similarly, Ki-Ras, but not Ha-Ras, associates with microtubules in a prenylation-dependent manner, indicating that prenylation and other determinants are involved in the specific binding of Ki-Ras to microtubules (Thissen et al., 1997).
Previously, we have shown that N-myristoylation and Ca2+-mediated conformational changes are required for the function of p22 in membrane traffic (Barroso et al., 1996). Here, we report that the association of p22 with microtubules requires the N-myristoylation of p22 but does not involve p22’s Ca2+-binding or Ca2+-mediated conformational changes. These results suggest that the p22–microtubule association and the role of p22 in membrane traffic should be functionally related, because the N-myristoylation of p22 is required for both events. Therefore, p22 may regulate membrane traffic through its association with the microtubule cytoskeleton.
A myristoyl “switch” requires a protein to undergo a signal-mediated conformational change to expose its myristoyl moiety, which can then interact with other proteins or membranes (Zozulya and Stryer, 1992). EF-hand proteins, such as recoverin, have been shown to use Ca2+–myristoyl “switches” to transduce Ca2+ signals (Zozulya and Stryer, 1992; Tanaka et al., 1995). Because N-myristoylated p22-E134A, a p22 EF-3 mutant that is unable to undergo Ca2+-mediated conformational changes, associates with microtubules, a Ca2+–myristoyl switch is not involved in the p22–microtubule association. Because we have shown previously that Ca2+-mediated conformational changes are required for the function of p22 in membrane traffic (Barroso et al., 1996), we propose that p22’s Ca2+-mediated conformational changes are required for the regulation of downstream effectors involved specifically in the targeting/docking/fusion membrane traffic steps (Barroso et al., 1996).
Both p22 and frequenin belong to the EF-hand superfamily and have been suggested to be involved in membrane trafficking (Pongs et al., 1993; Olafsson et al., 1995; Barroso et al., 1996; McFerran et al., 1998), but they localize to different subcellular compartments. An interesting emerging concept is that EF-hand Ca2+-binding proteins may localize to different subcellular compartments according to their functionality. Thus, EF-hand proteins, such as p22, frequenin, and calmodulin, may transduce Ca2+ signals to regulate different downstream effectors that are involved in the regulation of the organization of cytoskeleton, membrane traffic, and other cellular processes.
p22 requires the presence of a cytosolic factor to associate with microtubules, as has been indicated for a growing number of EF-hand Ca2+-binding proteins that have been shown to associate with the cellular cytoskeleton. For example, the Ca2+–calmodulin system regulates the organization and dynamics of microtubules by its ability to interact directly with MAPs, such as tau and MAP-2 (Maccioni and Cambiazo, 1995; Henriquez et al., 1996). Other members of the EF-hand superfamily, such as neurocalcin (Iino et al., 1995a,b), centrin (Salisbury, 1995; Paoletti et al., 1996), NCS-1 (Martone et al., 1999), and S-100 proteins (Richter-Landsberg and Heinrich, 1995), can be found associated with the microtubule cytoskeleton, whereas VILIP, an EF-hand protein of the recoverin subfamily, has been found associated with the actin cytoskeleton (Lenz et al., 1996). Recently, a dynein light chain from the outer arm of Chlamydomonas flagella was identified as an EF-hand Ca2+-binding protein (King and Patel-King, 1995). Also, a calmodulin-binding protein was identified as a new member of the kinesin superfamily that appears to be present only in plants (Narasimhulu and Reddy, 1998). S100B, a member of the EF-hand superfamily, has been shown to bind and regulate the activity of CapZ (Ivanenkov et al., 1995; Kilby et al., 1997), an actin capping protein that is part of the dynactin complex, a protein complex that associates with microtubules through one of its protein components, p150glued (Holleran et al., 1998).
Several lines of evidence suggest that the p22–microtubule binding factor is distinct from classic motor proteins and MAPs. First, the insensitivity of the p22–microtubule interaction to nucleotides such as ATP or GTP suggests that p22 does not associate with microtubules via motor proteins, like cytoplasmic dynein or kinesin, because these proteins are expected to be released from microtubules in the presence of high concentrations of ATP or GTP (Vale et al., 1985; Paschal et al., 1987, 1991; Schroer et al., 1989). Second, the consistent association of ∼60% of p22 with microtubules in cosedimentation assays suggests a low affinity for this interaction. Third, the p22–microtubule-binding factor does not behave as a classic MAP because it shows a low affinity for microtubules and cannot be completely removed from cytosol by microtubule affinity depletion, as has been shown for MAPs and motor proteins (Vallee, 1986; Blocker et al., 1996). Although we have shown that okadaic acid, a phosphatase inhibitor, does not affect the binding of p22 to microtubules, we have not completely ruled out the possible involvement of a cytosolic protein modifying agent, such as a kinase or phosphatase, in the association of p22 with microtubules.
Two other interesting features of the p22–microtubule-binding factor indicate that p22 associates with microtubules via a novel mechanism, which does not involve MAPs and motor proteins. First, the p22–microtubule-binding factor behaves as 70- to 30-kDa globular protein upon gel filtration chromatography. Our results indicate that high molecular weight proteins or multimeric complexes (100 kDa or greater) that are able to associate with microtubules as well as other microtubule-binding proteins such as p58 and tau are not required for the p22–microtubule association. Second, the p22–microtubule-binding factor is the first protein to be identified that targets cytosolic proteins such as p22 to the microtubule cytoskeleton in an N-myristoylation–dependent manner.
Recently, it has been proposed that different MAPs could be involved in targeting kinases and phosphatases to microtubules (Liao et al., 1998). MAP2 targets the regulatory subunit of type II cAMP-dependent protein kinase and MAP kinase to microtubules (Obar et al., 1989; Rubino et al., 1989), whereas MAP4 binds cyclin B, which may direct Cdc2 kinase to microtubules (Ookata et al., 1995). Protein phosphatase 1 and phosphatase 2A appear to associate with microtubules via the formation of a complex with tau (Sontag et al., 1995, 1996; Liao et al., 1998). Although many of these proteins have been shown to bind microtubules through classic MAPs or motor proteins, p22 appears to use a distinct N-myristoylation–dependent mechanism to associate with microtubules. We propose that p22 associates with microtubules through a microtubule interacting protein in the range of 70–30 kDa, distinct from classic MAPs and motors. Candidates for the p22–microtubule-binding factor are 67-kDa bovine pancreas microtubule-interacting protein (Michalik et al., 1993), MIP-90 (Gonzalez et al., 1998), mapmodulin (Ulitzur et al., 1997), PAT-1 (Zheng et al., 1998), or other unidentified microtubule-interacting proteins (Schoenfeld and Obart, 1994; Maccioni and Cambiazo, 1995; Mandelkow and Mandelkow, 1995). Although CLIMP-63, an integral membrane protein that can bind to microtubules as well as membranes (Klopfenstein et al., 1998), was shown to possess a low affinity for microtubules, we do not expect it to be involved in the p22–microtubule association, because p22 binds to microtubules in a detergent-resistant membrane-independent manner both in vivo and in vitro. Our results could also be explained by the association of p22 with microtubules via signal-transducing proteins that have been shown to associate directly or even indirectly with microtubules. For example, trimeric GTP-binding proteins (Roychowdhury and Rasenick, 1997), small GTP-binding proteins (Thissen et al., 1997), and guanine nucleotide exchange factors (Glaven et al., 1999), as well as G protein-coupled receptor kinases (Carman et al., 1998; Pitcher et al., 1998), have been shown to associate with microtubules. We are currently working on the identification of the p22–microtubule-binding factor, which will allow us to understand further the cellular roles of p22.
Considering our results, we suggest that the association of p22 with microtubules is mediated by a microtubule-interacting protein that shows low affinity for microtubules. p22’s interaction with its cytosolic microtubule-binding factor could result in the formation of a new microtubule-binding site or could increase the specificity of p22 toward microtubules. Gundersen and Cook (1999) proposed that the microtubule surface may behave as a scaffold to induce the association of two or more factors that otherwise would not interact. In agreement, p22 and its microtubule-binding factor elute in different fractions upon gel filtration chromatography (31–33 and 29–31, respectively), indicating that they do not associate to form a heterodimer in cytosol. It is possible that the microtubule surface is responsible for bringing together these two low-affinity components, which otherwise would not interact with each other. Considering that p22 associates with microtubules via a novel N-myristoylation–dependent mechanism and that a growing number of signal transduction proteins have been found to associate with microtubules, we suggest that other N-myristoylated signal transduction proteins may associate with microtubules via a similar mechanism.
Recently, Barber and coworkers have shown that p22 binds to NHE-1, a 100-kDa ubiquitous transmembrane Na/H+ exchanger involved in pH regulation (Lin and Barber, 1996). Thus, p22, as calmodulin and other members of the EF-hand family, might have different functions in the regulation of membrane traffic, microtubule organization, and ion exchangers. Several proteins involved in membrane traffic have been shown to have other functions as well. For example, syntaxin 1A, a key molecule involved in diverse vesicle docking/fusion events, was shown to physically interact with CFTR, cystic fibrosis transmembrane conductance regulator, chloride channels and regulate CFTR-mediated currents both in Xenopus oocytes and in epithelial cells that normally express these proteins (Naren et al., 1997).
Although several EF-hand Ca2+-binding proteins, such as p22 (Barroso et al., 1996), calcineurin B (Grasso et al., 1990; Apodaca et al., 1994; Hunziker, 1994; Colombo et al., 1997), frequenin (Pongs et al., 1993; Olafsson et al., 1995; McFerran et al., 1998), and calmodulin (Kerboeuf et al., 1993; Kübler et al., 1994; Chamberlain et al., 1995; de Figueiredo and Brown, 1995; Wang and Kelly, 1995; Kibble and Burgoyne, 1996), have been shown to be required for membrane trafficking events, the specific mechanism used by them to regulate membrane traffic remains largely unknown. EF-hand Ca2+-binding microtubule-interacting proteins, such as p22 and calmodulin, may act directly in membrane trafficking by modulating an essential component of the vesicular transport machinery. Alternatively, they may modulate membrane traffic indirectly by affecting the organization of the cellular cytoskeleton, because microtubule dynamics appear to play a role in the regulation of several membrane trafficking events such as phagosome movement (Blocker et al., 1998) and endoplasmic reticulum organization (Waterman-Storer and Salmon, 1998).
Based on its structural homology to such signal transducers as calcineurin and calmodulin, our current model for the function of p22 is that p22 acts by transducing Ca2+ signals to downstream effectors. Considering that our previous studies implicate a possible role of p22 in donor vesicle–acceptor membrane interactions leading to membrane fusion (Barroso et al., 1996) and in thinking about the potential functions of p22, we propose that the role of the p22–microtubule association in membrane traffic might reflect a function in facilitating membrane movement along microtubules or in regulating the organization and dynamics of microtubules that are also involved, albeit indirectly, in membrane trafficking.
ACKNOWLEDGMENTS
We thank Christine Brown for the cloning of p22-E134A into the PET vector and Kristin Sharman for her help with the deconvolution. We also thank Dr. A. Periasamy (W. M. Keck Center for Cellular Imaging, University of Virginia, Charlottesville, VA) for his help with the image collection and processing as well as Wilson McIvor for his help with the HPLC. We also express our thanks to Drs. K. Howell, K. Pfister, David Castle, and Elizabeth Sztul for critically reading this manuscript. In addition, we are grateful to Drs. K. Pfister, A. Frankfurter, and G. Bloom for helpful advice and discussion. Initially, this work was aided by grant IRG-149L from the American Cancer Society. Since 1998, this work has been supported by grant R01-GM57519 from National Institutes of Health.
Abbreviations used:
- MAP
microtubule-associated protein
- p22-myr
N-myristoylated bacterially expressed p22
- p22-rec
nonmyristoylated bacterially expressed p22
REFERENCES
- Aizawa H, Emori Y, Murofushi H, Kawasaki H, Sakai H, Suzuki K. Molecular cloning of a ubiquitously distributed microtubule-associated protein with Mr 190,000. J Biol Chem. 1990;265:13849–13855. [PubMed] [Google Scholar]
- Ames JB, Porumb T, Tanaka T, Ikura M, Stryer L. Amino-terminal myristoylation induces cooperative Ca2+ binding to recoverin. J Biol Chem. 1995;270:4526–4533. doi: 10.1074/jbc.270.9.4526. [DOI] [PubMed] [Google Scholar]
- Apodaca G, Enrich C, Mostov KE. The calmodulin antagonist, W-13, alters transcytosis, recycling, and the morphology of the endocytic pathway in Madin-Darby canine kidney cells. J Biol Chem. 1994;269:19005–19013. [PubMed] [Google Scholar]
- Barroso M, Bernd K, DeWitt N, Chang A, Mills K, Sztul E. A novel Ca2+-binding protein, p22, is required for constitutive membrane traffic. J Biol Chem. 1996;271:10183–10187. doi: 10.1074/jbc.271.17.10183. [DOI] [PubMed] [Google Scholar]
- Barroso M, Nelson DS, Sztul E. Transcytosis-associated protein (TAP)/p115 is a general fusion factor required for binding of vesicles to acceptor membranes. Proc Natl Acad Sci USA. 1995;92:527–531. doi: 10.1073/pnas.92.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashour A-M, Bloom GS. 58K, a microtubule-binding Golgi protein, is a formiminotransferase cyclodeaminase. J Biol Chem. 1998;273:19612–19617. doi: 10.1074/jbc.273.31.19612. [DOI] [PubMed] [Google Scholar]
- Blocker A, Griffiths G, Olivo J-C, Hyman AA, Severin FF. A role for microtubule dynamics in phagosome movement. J Cell Sci. 1998;111:303–312. doi: 10.1242/jcs.111.3.303. [DOI] [PubMed] [Google Scholar]
- Blocker A, Severin FF, Habermann A, Hyman AA, Griffiths G, Burkhardt JK. Microtubule-associated protein-dependent binding of phagosomes to microtubules. J Biol Chem. 1996;271:3803–3811. doi: 10.1074/jbc.271.7.3803. [DOI] [PubMed] [Google Scholar]
- Bloom GS, Goldstein LSB. Cruising along microtubule highways: how membranes move through the secretory pathway. J Cell Biol. 1998;140:1277–1280. doi: 10.1083/jcb.140.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SW, Levinson W, Spudich JA. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5:119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Carman CV, Som T, Kim CM, Benovic JL. Binding and phosphorylation of tubulin by G protein-coupled receptor kinases. J Biol Chem. 1998;273:20308–20316. doi: 10.1074/jbc.273.32.20308. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, Roth D, Morgan A, Burgoyne RD. Distinct effects of alpha-SNAP, 14–3-3 proteins, and calmodulin on priming and triggering of regulated exocytosis. J Cell Biol. 1995;130:1063–1070. doi: 10.1083/jcb.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow M, Newman JF, Filman D, Hogle JM, Rowlands DJ, Brown F. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature. 1987;327:482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- Cole NB, Lippincott-Schwartz J. Organization of organelles and membrane traffic by microtubules. Curr Opin Cell Biol. 1995;7:55–64. doi: 10.1016/0955-0674(95)80045-x. [DOI] [PubMed] [Google Scholar]
- Colombo MI, Beron W, Stahl PD. Calmodulin regulates endosome fusion. J Biol Chem. 1997;272:7707–7712. doi: 10.1074/jbc.272.12.7707. [DOI] [PubMed] [Google Scholar]
- Cramer LP, Mitchison TJ. Myosin is involved in postmitotic cell spreading. J Cell Biol. 1995;131:179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Figueiredo P, Brown WJ. A role for calmodulin in organelle membrane tubulation. Mol Biol Cell. 1995;6:871–887. doi: 10.1091/mbc.6.7.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M, Chardin P, Chabre M, Paris S. Myristoylation-facilitated binding of the G protein ARF1GDP to membrane phospholipids is required for its activation by a soluble nucleotide exchange factor. J Biol Chem. 1996;271:1573–1578. doi: 10.1074/jbc.271.3.1573. [DOI] [PubMed] [Google Scholar]
- Fujii T, Nakamura A, Ogoma Y, Kondo Y, Arai T. Selective purification of microtubule-associated proteins 1 and 2 from rat brain using poly(L-aspartic acid) Anal Biochem. 1990;184:268–273. doi: 10.1016/0003-2697(90)90679-4. [DOI] [PubMed] [Google Scholar]
- Gelfand VI, Bershadsky AD. Microtubule dynamics: mechanism, regulation, and function. Annu Rev Cell Biol. 1991;7:93–116. doi: 10.1146/annurev.cb.07.110191.000521. [DOI] [PubMed] [Google Scholar]
- Gill SR, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaven JA, Whitehead I, Bagrodia S, Kay R, Cerione RA. The Dbl-related protein, Lfc, localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J Biol Chem. 1999;274:2279–2285. doi: 10.1074/jbc.274.4.2279. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Cambiazo V, Maccioni RB. The interaction of Mip-90 with microtubules and actin filaments in human fibroblasts. Exp Cell Res. 1998;239:243–253. doi: 10.1006/excr.1997.3875. [DOI] [PubMed] [Google Scholar]
- Goodson HV, Valetti C, Kreis TE. Motors and membrane traffic. Curr Opin Cell Biol. 1997;9:18–28. doi: 10.1016/s0955-0674(97)80147-0. [DOI] [PubMed] [Google Scholar]
- Grasso JA, Bruno M, Yates AA, Wei LT, Epstein PM. Calmodulin dependence of transferrin receptor recycling in rat reticulocytes. Biochem J. 1990;266:261–272. doi: 10.1042/bj2660261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Oyama F, Ihara Y. Tau is widely expressed in rat tissues. J Neurochem. 1996;67:1235–1244. doi: 10.1046/j.1471-4159.1996.67031235.x. [DOI] [PubMed] [Google Scholar]
- Gundersen GG, Cook T. Microtubules and signal transduction. Curr Opin Cell Biol. 1999;11:81–94. doi: 10.1016/s0955-0674(99)80010-6. [DOI] [PubMed] [Google Scholar]
- Hennig D, Scales SJ, Moreau A, Murley LL, DeMey J, Kreis TE. A formiminotransferase cyclodeaminase isoform is localized to the Golgi complex and can mediate interaction of trans-Golgi network-derived vesicles with microtubules. J Biol Chem. 1998;273:19602–19611. doi: 10.1074/jbc.273.31.19602. [DOI] [PubMed] [Google Scholar]
- Henriquez JP, Cambiazo V, Maccioni RB. Tubulin domains for the interaction of microtubule associated protein DMAP-85 from Drosophila melanogaster. Mol Cell Biochem. 1996;158:149–159. doi: 10.1007/BF00225841. [DOI] [PubMed] [Google Scholar]
- Holleran EA, Karki S, Holzbaur ELF. The role of the dynactin complex in intracellular motility. Int Rev Cytol. 1998;182:69–110. doi: 10.1016/s0074-7696(08)62168-3. [DOI] [PubMed] [Google Scholar]
- Hunziker W. The calmodulin antagonist W-7 affects transcytosis, lysosomal transport, and recycling but not endocytosis. J Biol Chem. 1994;269:29003–29009. [PubMed] [Google Scholar]
- Iino S, Kobayashi S, Okazaki K, Hidaka H. Immunohistochemical localization of neurocalcin in the rat inner ear. Brain Res. 1995a;680:128–134. doi: 10.1016/0006-8993(95)00253-m. [DOI] [PubMed] [Google Scholar]
- Iino S, Kobayashi S, Okazaki K, Hidaka H. Neurocalcin-immunoreactive receptor cells in the rat olfactory epithelium and vomeronasal organ. Neurosci Lett. 1995b;191:91–94. doi: 10.1016/0304-3940(95)11568-2. [DOI] [PubMed] [Google Scholar]
- Ikura M. Ca2+-binding and conformational response in EF-hand proteins. Trends Biochem Sci. 1996;21:14–17. [PubMed] [Google Scholar]
- Ivanenkov VV, Jamieson GA, Gruenstein E, Dimlich RV. Characterization of S-100b binding epitopes. Identification of a novel target, the actin capping protein, CapZ. J Biol Chem. 1995;270:14651–14658. doi: 10.1074/jbc.270.24.14651. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Kretsinger RH. Ca2+-binding proteins 1: EF-hands. Protein Profile. 1995;1:343–517. [PubMed] [Google Scholar]
- Kennedy MT, Brockman H, Rusnak F. Contributions of myristoylation to calcineurin structure/function. J Biol Chem. 1996;271:26517–26521. doi: 10.1074/jbc.271.43.26517. [DOI] [PubMed] [Google Scholar]
- Kerboeuf D, Le Berre A, Dedieu J-C, Cohen J. Calmodulin is essential for assembling links necessary for exocytotic membrane fusion in Paramecium. EMBO J. 1993;12:3385–3390. doi: 10.1002/j.1460-2075.1993.tb06012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibble AV, Burgoyne RD. Calmodulin increases the initial rate of exocytosis in adrenal chromaffin cells. Pflügers Arch Eur J Physiol. 1996;431:464–466. doi: 10.1007/BF02207288. [DOI] [PubMed] [Google Scholar]
- Kilby PM, Van-Eldik LJ, Roberts GC. Identification of the binding site on S100B protein for the actin capping protein CapZ. Protein Sci. 1997;6:2494–2503. doi: 10.1002/pro.5560061202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Patel-King RS. Identification of a Ca2+-binding light chain within Chlamydomonas outer arm dynein. J Cell Sci. 1995;108:3757–3764. doi: 10.1242/jcs.108.12.3757. [DOI] [PubMed] [Google Scholar]
- Klee CB, Draetta GF, Hubbard JM. Calcineurin. Adv Enzymol. 1988;61:149–200. doi: 10.1002/9780470123072.ch4. [DOI] [PubMed] [Google Scholar]
- Klopfenstein DRC, Kappeler F, Hauri H-P. A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 1998;17:6168–6177. doi: 10.1093/emboj/17.21.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Crandall JE, Mufson EJ, Neve RL. Tau in situ hybridization in normal and Alzheimer brain: localization in the somatodendritic compartment. Ann Neurol. 1989;26:352–361. doi: 10.1002/ana.410260308. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Orecchio LD, Binder L, Trojanowski JQ, Lee VM, Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988;1:817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Kübler E, Schimmöller F, Riezman H. Ca2+-independent calmodulin requirement for endocytosis in yeast. EMBO J. 1994;13:5539–5546. doi: 10.1002/j.1460-2075.1994.tb06891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov SA, Gelfand VI. Bovine brain kinesin is a microtubule-activated ATPase. Proc Natl Acad Sci USA. 1986;83:8530–8534. doi: 10.1073/pnas.83.22.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz SE, Braunewell KH, Weise C, Nedlina-Chittka A, Gundelfinger ED. The neuronal EF-hand Ca(2+)-binding protein VILIP: interaction with cell membrane and actin-based cytoskeleton. Biochem Biophys Res Commun. 1996;225:1078–1083. doi: 10.1006/bbrc.1996.1298. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Ivanov IE, Lee GH, Cowan NJ. Organization of microtubules in dendrites and axons is determined by a short hydrophobic zipper in microtubule-associated proteins MAP2 and tau. Nature. 1989;342:498–505. doi: 10.1038/342498a0. [DOI] [PubMed] [Google Scholar]
- Liao H, Li Y, Brautigan DL, Gundersen GG. Protein phosphatase 1 is targeted to microtubules by the microtubule-associated protein Tau. J Biol Chem. 1998;273:21901–21908. doi: 10.1074/jbc.273.34.21901. [DOI] [PubMed] [Google Scholar]
- Lin X, Barber DL. A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc Natl Acad Sci USA. 1996;93:12631–12636. doi: 10.1073/pnas.93.22.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni RB, Cambiazo V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol Rev. 1995;75:835–864. doi: 10.1152/physrev.1995.75.4.835. [DOI] [PubMed] [Google Scholar]
- Mandelkow E, Mandelkow E-M. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995;7:72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Martone ME, Edelmann VM, Ellisman MH, Nef P. Cellular and subcellular distribution of the calcium-binding protein NCS-1 in the CNS of the rat. Cell Tissue Res. 1999;295:395–407. doi: 10.1007/s004410051246. [DOI] [PubMed] [Google Scholar]
- Masson D, Kreis TE. Identification and molecular characterization of E-MAP-115, a novel microtubule-associated protein predominantly expressed in epithelial cells. J Cell Biol. 1993;123:357–371. doi: 10.1083/jcb.123.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFerran BW, Graham ME, Burgoyne RD. Neuronal Ca2+ sensor 1, the mammalian homologue of frequenin is expressed in chromaffin and PC12 cells and regulates neurosecretion from dense-core granules. J Biol Chem. 1998;273:22768–22772. doi: 10.1074/jbc.273.35.22768. [DOI] [PubMed] [Google Scholar]
- Michalik L, Neuville P, Vanier MT, Launay JF. Biochemical and immunochemical identification of a microtubule-binding protein from bovine pancreas. Cell Motil Cytoskeleton. 1993;25:381–390. doi: 10.1002/cm.970250408. [DOI] [PubMed] [Google Scholar]
- Michalik L, Neuville P, Vanier MT, Launay JF. Pancreatic tau related maps: biochemical and immunofluorescence analysis in a tumoral cell line. Mol Cell Biochem. 1995;143:107–112. doi: 10.1007/BF01816943. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Arai T, Kondo Y, Fujii T. Inhibitory effects of poly(L-aspartic acid) on the assembly of brain microtubules and the interaction of microtubule-associated protein 2 with F-actin in vitro. J Biochem (Tokyo) 1989;106:93–97. doi: 10.1093/oxfordjournals.jbchem.a122827. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Kretsinger RH. Evolution of the EF-hand family of proteins. Annu Rev Biophys Biomol Struct. 1994;23:473–507. doi: 10.1146/annurev.bb.23.060194.002353. [DOI] [PubMed] [Google Scholar]
- Narasimhulu SB, Reddy AS. Characterization of microtubule binding domains in the Arabidopsis kinesin-like calmodulin binding protein. Plant Cell. 1998;10:957–965. doi: 10.1105/tpc.10.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naren AP, Nelson DJ, Xie W, Jovov B, Pevsner J, Bennett MK, Benos DJ, Quick MW, Kirk KL. Regulation of CFTR chloride channels by syntaxin and Munc18 isoforms. Nature. 1997;390:302–305. doi: 10.1038/36882. [DOI] [PubMed] [Google Scholar]
- Niclas J, Allan VJ, Vale RD. Cell cycle regulation of dynein association with membranes modulates microtubule-based organelle transport. J Cell Biol. 1996;133:585–593. doi: 10.1083/jcb.133.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Lewis SA, Cowan NJ. The microtubule binding domain of microtubule-associated protein MAP1B contains a repeated sequence motif unrelated to that of MAP2 and tau. J Cell Biol. 1989;109:3367–3376. doi: 10.1083/jcb.109.6.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar RA, Dingus J, Bayley H, Vallee RB. The RII subunit of cAMP-dependent protein kinase binds to a common amino-terminal domain in microtubule-associated proteins 2A, 2B, and 2C. Neuron. 1989;3:639–645. doi: 10.1016/0896-6273(89)90274-2. [DOI] [PubMed] [Google Scholar]
- Olafsson P, Wang T, Lu B. Molecular cloning and functional characterization of the Xenopus Ca2+-binding protein frequenin. Proc Natl Acad Sci USA. 1995;92:8001–8005. doi: 10.1073/pnas.92.17.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Bulinski JC, Murofushi H, Aizawa H, Itoh TJ, Hotani H, Okumura E, Tachibana K, Kishimoto T. MAP4 is the in vivo substrate for CDC2 kinase in HeLa cells: identification of an M-phase specific and a cell cycle-independent phosphorylation site in MAP4. J Cell Biol. 1995;128:849–862. [Google Scholar]
- Osborn M, Weber K. The display of microtubules in transformed cells. Cell. 1977;12:561–571. doi: 10.1016/0092-8674(77)90257-4. [DOI] [PubMed] [Google Scholar]
- Paoletti A, Moudjou M, Paintrand M, Salisbury JL, Bornens M. Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J Cell Sci. 1996;109:3089–3102. doi: 10.1242/jcs.109.13.3089. [DOI] [PubMed] [Google Scholar]
- Paschal BM, Shpetner HS, Vallee RB. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987;105:1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal BM, Shpetner HS, Vallee RB. Purification of brain cytoplasmic dynein and characterization of its in vitro properties. Methods Enzymol. 1991;196:181–191. doi: 10.1016/0076-6879(91)96018-m. [DOI] [PubMed] [Google Scholar]
- Pierre P, Scheel J, Rickard JE, Kreis TE. CLIP-170 links endocytic vesicles to microtubules. Cell. 1992;70:887–900. doi: 10.1016/0092-8674(92)90240-d. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Hall RA, Daaka Y, Zhang J, Ferguson SSG, Hester S, Miller S, Caron MG, Lefkowitz RJ, Barak LS. The G protein-coupled receptor kinase 2 is a microtubule-associated protein kinase that phosphorylates tubulin. J Biol Chem. 1998;273:12316–12324. doi: 10.1074/jbc.273.20.12316. [DOI] [PubMed] [Google Scholar]
- Polans A, Baehr W, Palczewski K. Turned on by Ca2+! The physiology and pathology of Ca2+-binding proteins in the retina. Trends Neurosci. 1996;19:547–554. doi: 10.1016/s0166-2236(96)10059-x. [DOI] [PubMed] [Google Scholar]
- Pongs O, et al. Frequenin: a novel Ca2+-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron. 1993;11:15–28. doi: 10.1016/0896-6273(93)90267-u. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C, Heinrich M. S-100 immunoreactivity in rat brain glial cultures is associated with both astrocytes and oligodendrocytes. J Neurosci Res. 1995;42:657–665. doi: 10.1002/jnr.490420508. [DOI] [PubMed] [Google Scholar]
- Rickard JE, Kreis TE. Binding of pp170 to microtubules is regulated by phosphorylation. Biol Chem. 1991;266:17597–17605. [PubMed] [Google Scholar]
- Roychowdhury S, Rasenick MM. G proteins b1γ2 subunits promote microtubule assembly. J Biol Chem. 1997;272:31576–31581. doi: 10.1074/jbc.272.50.31576. [DOI] [PubMed] [Google Scholar]
- Rubino HM, Dammerman M, Shafit-Zagardo B, Erlichman J. Localization and characterization of the binding site for the regulatory subunit of type II cAMP-dependent protein kinase on MAP2. Neuron. 1989;3:631–638. doi: 10.1016/0896-6273(89)90273-0. [DOI] [PubMed] [Google Scholar]
- Salisbury JL. Centrin, centrosomes, and mitotic spindle poles. Curr Opin Cell Biol. 1995;7:39–45. doi: 10.1016/0955-0674(95)80043-3. [DOI] [PubMed] [Google Scholar]
- Schafer BW, Heizmann CW. The S100 family of EF-hand Ca2+-binding proteins: functions and pathology. Trends Biochem Sci. 1996;21:134–140. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Obart RA. Diverse distribution and function of fibrous microtubule-associated proteins in the nervous system. Int Rev Cytol. 1994;151:67–137. doi: 10.1016/s0074-7696(08)62631-5. [DOI] [PubMed] [Google Scholar]
- Schroer TA, Steuer ER, Sheetz MP. Cytoplasmic dynein is a minus end-directed motor for membranous organelles. Cell. 1989;56:937–946. doi: 10.1016/0092-8674(89)90627-2. [DOI] [PubMed] [Google Scholar]
- Sider JR, Mandato CA, Weber KL, Zandy AJ, Beach D, Finst RJ, Skoble J, Bement WM. Direct observation of microtubule-f-actin interaction in cell free lysates. J Cell Sci. 1999;112:1947–1956. doi: 10.1242/jcs.112.12.1947. [DOI] [PubMed] [Google Scholar]
- Song J, Hirschman J, Gunn K, Dohlman HG. Regulation of membrane and subunit interactions by N-myristoylation of a G protein alpha subunit in yeast. J Biol Chem. 1996;271:20273–20283. doi: 10.1074/jbc.271.34.20273. [DOI] [PubMed] [Google Scholar]
- Sontag E, Numbhakdi-Craig V, Bloom GS, Mumby MC. A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J Cell Biol. 1995;128:1131–1144. doi: 10.1083/jcb.128.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Cell. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- Swierczynski SL, Blackshear PJ. Membrane association of the myristoylated alanine-rich C kinase substrate (MARCKS) protein. Mutational analysis provides evidence for complex interactions. J Biol Chem. 1995;270:13436–13445. doi: 10.1074/jbc.270.22.13436. [DOI] [PubMed] [Google Scholar]
- Sztul E. Transcytotic vesicle fusion with plasma membrane. Methods Enzymol. 1992;219:44–52. doi: 10.1016/0076-6879(92)19008-t. [DOI] [PubMed] [Google Scholar]
- Sztul E, Colombo M, Stahl P, Samanta R. Control of protein traffic between distinct plasma membrane domains: requirement for a novel 108,000 protein in the fusion of transcytotic vesicles with the apical plasma membrane. J Biol Chem. 1993;268:1876–1885. [PubMed] [Google Scholar]
- Sztul E, Kaplin A, Saucan L, Palade G. Protein traffic between distinct plasma membrane domains: isolation and characterization of vesicular carriers involved in transcytosis. Cell. 1991;64:81–89. doi: 10.1016/0092-8674(91)90210-p. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ames JB, Harvey TS, Stryer L, Ikura M. Sequestration of the membrane-targeting myristoyl group of recoverin in the Ca2+-free state. Nature. 1995;376:444–447. doi: 10.1038/376444a0. [DOI] [PubMed] [Google Scholar]
- Thissen JA, Gross JM, Subramanian K, Meyer T, Casey PJ. Prenylation-dependent association of Ki-Ras with microtubules. J Biol Chem. 1997;272:30362–30370. doi: 10.1074/jbc.272.48.30362. [DOI] [PubMed] [Google Scholar]
- Ulitzur N, Rancano C, Pfeffer SR. Biochemical characterization of mapmodulin, a protein that binds microtubule-associated proteins. J Biol Chem. 1997;272:30577–30582. doi: 10.1074/jbc.272.48.30577. [DOI] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB. Purification of brain microtubules and microtubule-associated protein 1 using taxol. Methods Enzymol. 1986;134:104–115. doi: 10.1016/0076-6879(86)34079-5. [DOI] [PubMed] [Google Scholar]
- Vallee RB, Sheetz MP. Targeting of motor proteins. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- Wang JH, Kelly PT. Postsynaptic injection of CA2+/CaM induces synaptic potentiation requiring CaMKII and PKC activity. Neuron. 1995;15:443–452. doi: 10.1016/0896-6273(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr Biol. 1998;14:798–806. doi: 10.1016/s0960-9822(98)70321-5. [DOI] [PubMed] [Google Scholar]
- Williams RC, Lee JC. Preparation of tubulin from brain. Methods Enzymol. 1982;85:376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- Yonemoto W, McGlone ML, Taylor SS. N-myristoylation of the catalytic subunit of cAMP-dependent protein kinase conveys structural stability. J Biol Chem. 1993;268:2348–2352. [PubMed] [Google Scholar]
- Zheng P, Eastman J, Pol SV, Pimplikar SW. PAT-1, a microtubule-interacting protein, recognizes the basolateral sorting signal of amyloid precursor protein. Proc Natl Acad Sci USA. 1998;95:14745–14750. doi: 10.1073/pnas.95.25.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozulya S, Stryer L. Ca2+-myristoyl protein switch. Proc Natl Acad Sci USA. 1992;89:11569–11573. doi: 10.1073/pnas.89.23.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]