Abstract
One seminal aspect in autoimmune diabetes is antigen presentation of beta cell antigens by the diabetes-propensity class II histocompatibility molecules. The binding properties of I-Ag7 molecules are reviewed here and an emphasis is placed on their selection of peptides with a highly specific sequence motif, in which one or more acidic amino acids are found at the carboxy end interacting at the P9 anchoring site of I-Ag7. The reasons for the central role of I-Ag7 in the autoimmune response are analyzed. The insulin B chain segment 9-23 is a hot spot for T cell selection and a striking example of a weak MHC binding peptide that triggers autoreactivity.
Introduction
The direct association of Major Histocompatibility Complex (MHC) molecules in the onset of various autoimmune disorders, including type 1 diabetes mellitus (T1DM), points a central role to their peptide binding features. Several attempts have been made to identify the natural peptides bound to MHC molecules, starting from the pioneering studies of Rammensee [1-3], and continuing with the introduction of mass spectrometry [4,5]. In our own experience, we have evaluated naturally processed peptides selected by various murine (I-Ak, I-Ad, I-Ek, I-Ep, I-Ag7, I-Ag7PD and Kd) and human (DQ8) MHC molecules. A conclusion from the identification of large numbers of natural peptides is that each MHC molecule selects for a unique repertoire of peptides in an allele-specific manner.
Biochemical, Structural and Functional Properties of diabetes-associated class II MHC molecules
A notable feature shared between the murine (I-Ag7) and human (DQ2 or DQ8) diabetogenic class II MHC molecules is the expression of a non-Asp amino acid at position 57 of the β chain [6]; most other class II MHC molecules contain a conserved Asp at β57, which forms an ion-pair with an opposing Arg at α76 that defines the rim and the structural features of the P9 pocket of the binding groove [7,8]. The absence of this salt bridge in the case of diabetogenic class II MHC molecules generated a P9 pocket that was wider, shallower and open towards the C-terminus [9-11]. However, the effect of these unique structural features on peptide selection remained unclear: earlier reports that analyzed binding interactions with synthetic peptides or peptide libraries suggested that diabetogenic MHC molecules bound peptides promiscuously with little or no specificity [12-15]. These conclusions were further bolstered by previous work from different laboratories, demonstrating that the diabetogenic class II MHC molecules, were unstable in SDS, i.e. they dissociated into the α- and β-subunits under non-denaturing conditions [13,16-21]. Prior work with other alleles, such as I-Ak or I-Ab, had established a clear correlation between SDS stability and peptide quality: high affinity peptides formed SDS-stable heterodimers, while low affinity peptides did not [16,22,23]. Since I-Ag7 and DQ8 were noticeably SDS-unstable it was proposed that they must bind promiscuously and to low affinity peptides [16]. However, mass-spectrometry based identification of naturally processed peptides selected by I-Ag7 and DQ8 generated very different results: both the murine and human alleles selected for similar peptides with a very well defined binding motif characterized by multiple C-termini acidic amino acids [17,24-26].
Binding studies demonstrated that the acidic amino acids interacted with the P9 pocket and contributed significantly towards binding affinity [24,25]. And moreover, the specificity of the P9 pocket, composed of the non-Asp β57 residue, was crucial in determining selection of peptides [24,26]. Table 1 depicts an example of a peptide family from the E2B integral membrane protein highly selected by APCs expressing either I-Ag7 or DQ8. Note that every member contains a stretch of 3 acidic amino acids (“EED”) towards the C-terminus. Binding assays to soluble I-Ag7 or DQ8 identified the same 9-mer binding core (shown in bold and underlined in Table 1) that contained an acidic P9 anchor [24,25]. Hence, SDS-instability is an intrinsic feature of diabetogenic class II MHC molecules, which is not reflective of the quality or specificity of peptides that are bound by these molecules. It is important to note that I-Ag7 binds to many of its ligands in the low micro-molar range, which is relatively weaker when compared to other murine alleles such as I-Ak that bind to its peptides in the nano-molar range.
Table 1.
An example of a dominant peptide family from the E2B integral membrane protein that is selected by APCs expressing I-Ag7 or DQ8. Note the presence of acidic amino acids towards the c-terminus of each family member. The 9-mer binding core is shown in bold and underlined. In other results, the Glu124 was identified as the P9-MHC anchor residue
 |
Recently, we utilized the vast datasets of natural peptides to develop a computational program that efficiently predicts epitopes selected by diabetogenic class II MHC molecules [27]. This approach had several unique aspects: (i) only the naturally selected peptides isolated from APCs were used in the development of this algorithm; (ii) the physical and structural characteristics of the binding pockets were used to refine the preferred amino acid anchors; and (iii) hindering residues, i.e. amino acids that are disfavored at a particular position, were used to screen potential binding peptides. All these factors, when applied in concert, enabled this algorithm to identify the preferred binding motif for I-Ag7, as shown in Figure 1. When tested against many natural peptides, the algorithm successfully assigned high scores to high affinity binders and lower scores to peptides that bound poorly [27]. Moreover, the algorithm successfully predicted I-Ag7 peptides from hen egg lysozyme that were recognized by T cells in NOD mice [27]. Hence, in this case, predictive algorithms that have evolved from analysis of naturally processed peptides from class II MHC molecules can be powerful tools for future analysis of unknown epitopes from target islet beta cell antigens. On the other hand, programs that have been developed for class I MHC epitope prediction [28] have been successful because of the fact that class I MHC molecules select for a very homogenous repertoire of peptides, i.e. they are all between 8-10 amino acids in length and exhibit a very well-defined binding motif. In contrast, class II MHC molecules select for peptides with varying length and many are selected as families (Table 1), which makes it difficult to identify the 9-mer binding core. This is further complicated by the possibility that some peptides can bind in multiple registers (as discussed later for the dominant insulin epitope in T1DM) and that class II bound peptides are edited by H-2 DM.
Figure 1.

I-Ag7 binding motif as determined by the alignment results from the expectation-maximization alignment algorithm of Chang et al [27] and shown by WebLogo. Preferred amino acids from positions P1 to P9 are indicated by their one letter code.
What are the consequences of the peptide binding properties of class II MHC molecules on T cell selection and peripheral activation? Earlier studies by Kanagawa et al and Ridgway et al noted that the expression of I-Ag7 alone, independent of the genetic background, was sufficient for escape of autoreactive T cells - i.e. these were CD4+ T cells that reacted with syngeneic APCs alone with no need for any exogenous antigens [29-31]. Note in Table 2 from the experiments of Kanagawa et al [29], the frequencies of autoreactive T cells was significantly higher in I-Ag7-expressing strains and independent of the NOD background genes.
Table 2.
Frequency of Autoreactive T cellsa
| Mouse Strain | Background | Class II MHC | Frequency of Autoreactive T cells |
|---|---|---|---|
| NOD | NOD | I-Ag7 | ∼1:1174 |
| NOD.H2b | NOD | I-Ab | <1: 10,000 |
| B6 | B6 | I-Ab | <1:10,000 |
| B6.G7 | B6 | I-Ag7 | ∼1:1216 |
Adapted from the published study of Kanagawa et al [29]
More recently, Stratmann et al generated I-Ag7 tetramers using a synthetic ‘mime’ peptide known to stimulate diabetogenic CD4+ T cells, including the well-characterized BDC T cell clone. Here again, tetramer-positive CD4+ T cells were identified in the periphery in both NOD and B6.G7 (congenic B6 mice expressing H-2g7) mouse strains [32]. However, whether escape of these T cells is a result of inadequate amounts of thymic self-peptides due to expression levels or low-affinity binders, or both, remains to be determined. In another study, Ferlin et al used transgenic NOD mice that expressed the Leishmania Antigen receptor for C Kinase (LACK) as a self protein to study T cell tolerance [33]. In this report it was noted that the LACK peptides that bound to I-Ag7 with low-affinity induced T cell tolerance [33]. However, in this case a high level of expression of this low-affinity ligand may also be a factor in tolerance induction. The relationship between peptide binding and specific beta cell antigens has best been studied against peptides from the insulin molecules, as described next.
While these studies have pointed to a MHC-centric role for I-Ag7 in thymic selection, other reports have identified a role for the NOD background genes in generating a compromised thymic selection milieu [34-36]. Kishimoto et al noted that NOD thymocytes were more resistant to deletion upon crosslinking of the TCR via monoclonal antibodies [34], while other studies with CD4+ TCR transgenic mice concluded that antigen encounter resulted in inefficient deletion of thymocytes on the NOD background when compared to non-NOD strains [35,36]. It is likely that both MHC and non-MHC background genes contribute towards selection of a self-reactive T cell repertoire.
Another feature of I-Ag7 that may contribute towards autoimmunity relates to its unique properties of peptide selection. We and others have noted that I-Ag7-bound peptides contain multiple C-termini acidic residues that contribute towards binding affinity [17,24]. More importantly, >80-90% of peptides isolated from I-Ag7 contain C-terminal stretches of two or three acidic residues [24,25] (Figure 1). A consequence of this highly selective repertoire may mean that the diversity of peptides selected by I-Ag7 in the thymus may be narrower when compared to other class II MHC molecules. If so, then this would directly impact T cell selection. Similarly, the unique features of peptide selection by I-Ag7 may allow for this molecule to efficiently select for islet β-cell-derived peptides that may be targets of autoreactive T cells. Indeed, many of the known epitopes from autoantigens in T1DM (including insulin) exhibit the preferred I-Ag7 binding motif. The role for the non-Asp β57 (β57Ser) in selection of these peptides is foremost: APCs expressing a modified I-Ag7 wherein the β57 was changed to the conserved Asp (termed I-Ag7PD) selected for entirely different sets of peptides that did not exhibit the presence of c-terminal acidic amino acids [24,26]. These results support the observations of Kanagawa et al who followed the thymic selection and peripheral activation of the diabetogenic CD4+ BDC T cell in mice expressing either I-Ag7 or I-Ag7PD. Results from this study demonstrated that while BDC T cells were selected by both I-Ag7- and I-Ag7PD-expressing thymi, only I-Ag7 APCs in the periphery were able to activate this pathogenic T cell [37].
Weak MHC binding peptides and autoreactivity: the case of the dominant insulin T cell epitope
Insulin, and particularly the 9-23 peptide of the insulin beta chain, is a primary target of T cell reactivity in both the human disease and the NOD mouse [38,39] (in this issue, contribution by Eisenbarth). Initial binding studies of the B:(9-23) peptide to soluble I-Ag7 in detergent free conditions revealed poor binding and fast dissociation rates [10,13,14,40]. The IC50 values for the B:(9-23) peptide were measured in the 5-10 μM range at endosomal pH and the half-life of the complexes was less than two hours. Recently, a detailed analysis of the binding of this epitope to I-Ag7 was made which confirmed these general findings and revealed several interesting new features [41]. First, the B:(9-23) peptide bound weakly to I-Ag7 in two contiguous registers, using two neighboring binding core segments, as shown in Table 3. Note that one register utilized the preferred glutamic acid as the P9 anchor, while the other register utilizes a glycine, which can also be accommodated at this position. Changing either of these to a lysine, which should result in a loss of binding, had no effect on binding. However, a noticeable loss of binding was evident when both the glycine at 20 and glutamic acid at 21 were mutated to lysines (Table 3). To confirm the presence of two registers, we demonstrated that the minimal 9-mer binding core of each segment did indeed bind to I-Ag7 (Table 3).
Table 3.
Binding of Insulin B Chain Peptidesb
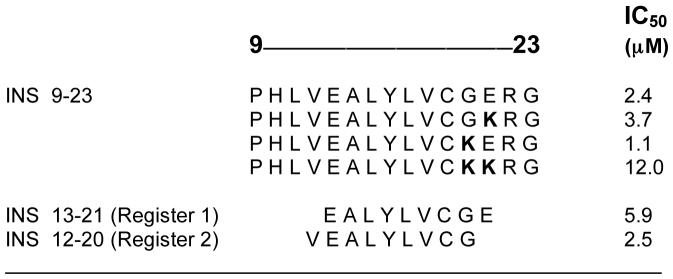 |
Binding analysis of synthetic peptides to soluble I-Ag7. Binding studies were performed at pH 5.5 [41]. Replacement of the natural residues with lysines allowed for the identification of the P9-MHC contact amino acid.
The presence of two binding registers had a biological correlate: naturally occurring insulin-reactive T cells isolated from pancreatic nodes and infiltrated islets of NOD mice recognized either one or the other register, as shown by the truncation analysis of the insulin epitope to identify the minimal T cell epitope (Figure 2). Moreover, insulin T cells were sensitive to amino acid changes in either the flanks or the core segments of the peptide-affected binding and T cell responses [41].
Figure 2.

Examples of two natural T cell clones from NOD mice that recognize different registers of the insulin peptide. The optimal register encompassed the entire 9-23 segment.
Taken together, these data suggest that the B:(9-23) peptide and the I-Ag7 molecule form a loose and flexible complex. Furthermore, these results established a direct relationship between a weak peptide-MHC and the development of an autoimmune T cell repertoire. The great variability in sensitivity to amino acid changes of the anti-(9-23) T cells described in the report may be a reflection of the many conformations that the peptide-MHC exhibits. Whether this great heterogeneity in TCR recognition of single peptide-MHC complexes is a universal feature of autoimmune specificities for weakly interacting peptides will have to be further examined when other epitopes are fully characterized. Nevertheless, insulin appears to be an autoantigen in which the binding of the immunodominant epitope, B:(9-23), to the class II MHC molecule is weak, and yet it correlates with a high representation of autoreactive T cells and a strong influence on the development of disease.
If weak peptide-MHC complexes allow for the escape of autoreactive T cells from the thymus, the obvious question remains, how are these T cells activated in the periphery? One explanation may simply be related to the anatomic distribution and relative concentrations of the given autoantigen. In the case of beta cell specific autoantigens, such as insulin, the large quantity of these secreted proteins in a local milieu may be sufficient to overcome the inherent weakness of the peptide-MHC interaction. Intra-islet dendritic cells must encounter very large quantities of insulin, both secreted and contained in granules of beta cells, such that the number of processed and presented peptides is of a sufficient threshold to activate insulin reactive T cells. This idea is supported by evidence that early diabetogenic T cell priming occurs in the draining peri-pancreatic lymph node, where naïve T cells encounter APCs charged with abundant beta-cell antigens [42,43]. A second component that most likely plays a role in resolving the apparent paradox between the failure of negative selection and peripheral activation is that of costimulation. Presumably, autoimmune T cells recognizing weak peptide-MHC complexes that escape the thymus receive additional signals in the periphery that push them over a threshold for activation. However this idea alone, in the case of the autoantigen insulin which circulates throughout the body, cannot explain the localization of T cell priming to the peri pancreatic lymph nodes, but costimulation, in concert with the presence of a quantitative excess of antigen seems a plausible mechanism for the loss of tolerance to weakly interacting self peptides. In this manner, protein and peptide secreting endocrine glands, such as the pancreatic islets seem to represent prime targets for weak peptide-MHC targeted T cell mediated autoimmunity. Moreover, the T cell activation thresholds for such weak ligands may be enhanced by the repertoire of self-peptides present on an APC. Recent reports with both CD4+ and CD8+ T cells have suggested that normal self peptides contribute towards the formation of an immunological synapse and T cell activation [44,45]. The extent and specificity of these self peptide-MHC complexes in facilitating activation of antigen-specific T cells needs to be examined in greater detail. As a side note, earlier work in the experimental autoimmune encephalomyelitis model had also suggested a relationship between low affinity peptides and T cell autoreactivity [46,47]. In this inducible disease model, the autoantigenic peptide, Ac1-11 of myelin basic protein, bound poorly to the class II MHC molecule, I-Au, and autoreactive T cells were shown to escape negative selection in the thymus [46,47].
Concluding remarks
An understanding of the biochemical basis of peptides selected by diabetogenic class II MHC molecules is key to gaining insights into the identity and nature of islet β cell antigens that are targeted in diabetes. Analysis of natural peptides has provided useful information with regards to the sequence specificity that can now be applied to predictive algorithms. However, further work needs to clarify the role of the highly specific binding motif of I-Ag7 peptides and thymic selection of the T cell repertoire. On the other hand, a role for weak binding ligands such as the insulin peptides in autoimmune diabetes, is also emerging. Whether such peptides form a significant fraction of the repertoire of islet β cell epitopes that are targeted by diabetogenic T cells needs to be examined in finer detail.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Falk K, Rotzschke O, Rammensee HG. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990;348:248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- 2.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 3.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Pool sequencing of natural HLA-DR, DQ, and DP ligands reveals detailed peptide motifs, constraints of processing, and general rules. Immunogenetics. 1994;39:230–242. doi: 10.1007/BF00188785. [DOI] [PubMed] [Google Scholar]
- 4.Chicz RM, Lane WS, Robinson RA, Trucco M, Strominger JL, Gorga JC. Self-peptides bound to the type I diabetes associated class II MHC molecules HLA-DQ1 and HLA-DQ8. Int Immunol. 1994;6:1639–1649. doi: 10.1093/intimm/6.11.1639. [DOI] [PubMed] [Google Scholar]
- 5.Hunt DF, Michel H, Dickinson TA, Shabanowitz J, Cox AL, Sakaguchi K, Appella E, Grey HM, Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992;256:1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- 6.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 7.Fremont DH, Monnaie D, Nelson CA, Hendrickson WA, Unanue ER. Crystal structure of I-Ak in complex with a dominant epitope of lysozyme. Immunity. 1998;8:305–317. doi: 10.1016/s1074-7613(00)80536-1. [DOI] [PubMed] [Google Scholar]
- 8.Scott CA, Peterson PA, Teyton L, Wilson IA. Crystal structures of two I-Ad-peptide complexes reveal that high affinity can be achieved without large anchor residues. Immunity. 1998;8:319–329. doi: 10.1016/s1074-7613(00)80537-3. published erratum appears in Immunity 1998 Apr;8(4):531. [DOI] [PubMed] [Google Scholar]
- 9.Corper AL, Stratmann T, Apostolopoulos V, Scott CA, Garcia KC, Kang AS, Wilson IA, Teyton L. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science. 2000;288:505–511. doi: 10.1126/science.288.5465.505. [DOI] [PubMed] [Google Scholar]
- 10.Latek RR, Suri A, Petzold SJ, Nelson CA, Kanagawa O, Unanue ER, Fremont DH. Structural basis of peptide binding and presentation by the type I diabetes-associated MHC class II molecule of NOD mice. Immunity. 2000;12:699–710. doi: 10.1016/s1074-7613(00)80220-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee KH, Wucherpfennig KW, Wiley DC. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immunol. 2001;2:501–507. doi: 10.1038/88694. [DOI] [PubMed] [Google Scholar]
- 12.Gregori S, Bono E, Gallazzi F, Hammer J, Harrison LC, Adorini L. The motif for peptide binding to the insulin-dependent diabetes mellitus-associated class II MHC molecule I-Ag7 validated by phage display library. Int Immunol. 2000;12:493–503. doi: 10.1093/intimm/12.4.493. [DOI] [PubMed] [Google Scholar]
- 13.Hausmann DH, Yu B, Hausmann S, Wucherpfennig KW. pH-dependent peptide binding properties of the type I diabetes-associated I-Ag7 molecule: rapid release of CLIP at an endosomal pH. J Exp Med. 1999;189:1723–1734. doi: 10.1084/jem.189.11.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stratmann T, Apostolopoulos V, Mallet-Designe V, Corper AL, Scott CA, Wilson IA, Kang AS, Teyton L. The I-Ag7 MHC class II molecule linked to murine diabetes is a promiscuous peptide binder. J Immunol. 2000;165:3214–3225. doi: 10.4049/jimmunol.165.6.3214. [DOI] [PubMed] [Google Scholar]
- 15.Bhatnagar A, Milburn PJ, Lobigs M, Blanden RV, Gautam AM. Nonobese diabetic mice display elevated levels of class II-associated invariant chain peptide associated with I-Ag7 on the cell surface. J Immunol. 2001;166:4490–4497. doi: 10.4049/jimmunol.166.7.4490. [DOI] [PubMed] [Google Scholar]
- 16.Carrasco-Marin E, Shimizu J, Kanagawa O, Unanue ER. The class II MHC I-Ag7 molecules from non-obese diabetic mice are poor peptide binders. J Immunol. 1996;156:450–458. [PubMed] [Google Scholar]
- 17.Munz C, Hofmann M, Yoshida K, Moustakas AK, Kikutani H, Stevanovic S, Papadopoulos GK, Rammensee HG. Peptide analysis, stability studies, and structural modeling explain contradictory peptide motifs and unique properties of the NOD mouse MHC class II molecule H2-A(g7) Eur J Immunol. 2002;32:2105–2116. doi: 10.1002/1521-4141(200208)32:8<2105::AID-IMMU2105>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Peterson M, Sant AJ. The inability of the nonobese diabetic class II molecule to form stable peptide complexes does not reflect a failure to interact productively with DM. J Immunol. 1998;161:2961–2967. [PubMed] [Google Scholar]
- 19.Reizis B, Altmann DM, Cohen IR. Biochemical characterization of the human diabetes-associated HLA-DQ8 allelic product: similarity to the major histocompatibility complex class II I-A(g)7 protein of non-obese diabetic mice. Eur J Immunol. 1997;27:2478–2483. doi: 10.1002/eji.1830271003. [DOI] [PubMed] [Google Scholar]
- 20.Reizis B, Eisenstein M, Bockova J, Konen-Waisman S, Mor F, Elias D, Cohen IR. Molecular characterization of the diabetes-associated mouse MHC class II protein, I-Ag7. Int Immunol. 1997;9:43–51. doi: 10.1093/intimm/9.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Ettinger RA, Liu AW, Nepom GT, Kwok WW. Beta 57-Asp plays an essential role in the unique SDS stability of HLA-DQA1*0102/DQB1*0602 alpha beta protein dimer, the class II MHC allele associated with protection from insulin-dependent diabetes mellitus. J Immunol. 2000;165:3232–3238. doi: 10.4049/jimmunol.165.6.3232. [DOI] [PubMed] [Google Scholar]
- 22.Nelson CA, Petzold SJ, Unanue ER. Identification of two distinct properties of class II major histocompatibility complex-associated peptides. Proc Natl Acad Sci U S A. 1993;90:1227–1231. doi: 10.1073/pnas.90.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson CA, Viner N, Young S, Petzold S, Benoist C, Mathis D, Unanue ER. Amino acid residues on the I-Ak alpha-chain required for the binding and stability of two antigenic peptides. J Immunol. 1996;156:176–182. [PubMed] [Google Scholar]
- 24.Suri A, Vidavsky I, van der Drift K, Kanagawa O, Gross ML, Unanue ER. In APCs, the autologous peptides selected by the diabetogenic I-Ag7 molecule are unique and determined by the amino acid changes in the P9 pocket. J Immunol. 2002;168:1235–1243. doi: 10.4049/jimmunol.168.3.1235. [DOI] [PubMed] [Google Scholar]
- 25.Suri A, Walters JJ, Gross ML, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity. J Clin Invest. 2005;115:2268–2276. doi: 10.1172/JCI25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.•Suri A, Walters JJ, Kanagawa O, Gross ML, Unanue ER. Specificity of peptide selection by antigen-presenting cells homozygous or heterozygous for expression of class II MHC molecules: The lack of competition. Proc Natl Acad Sci U S A. 2003;100:5330–5335. doi: 10.1073/pnas.0330859100.These three papers examine large numbers of naturally selected peptides bound to either I-Ag7 or DQ8. They establish the key role of non-Asp β57 in peptide selection
- 27.Chang KY, Suri A, Unanue ER. Predicting peptides bound to I-Ag7 class II histocompatibility molecules using a novel expectation-maximization alignment algorithm. Proteomics. 2007;7:367–377. doi: 10.1002/pmic.200600584. [DOI] [PubMed] [Google Scholar]
- 28.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 29.Kanagawa O, Martin SM, Vaupel BA, Carrasco-Marin E, Unanue ER. Autoreactivity of T cells from nonobese diabetic mice: an I-Ag7-dependent reaction. Proc Natl Acad Sci U S A. 1998;95:1721–1724. doi: 10.1073/pnas.95.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ridgway WM, Fasso M, Lanctot A, Garvey C, Fathman CG. Breaking self-tolerance in nonobese diabetic mice. J Exp Med. 1996;183:1657–1662. doi: 10.1084/jem.183.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridgway WM, Ito H, Fasso M, Yu C, Fathman CG. Analysis of the role of variation of major histocompatibility complex class II expression on nonobese diabetic (NOD) peripheral T cell response. J Exp Med. 1998;188:2267–2275. doi: 10.1084/jem.188.12.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.•Stratmann T, Martin-Orozco N, Mallet-Designe V, Poirot L, McGavern D, Losyev G, Dobbs CM, Oldstone MB, Yoshida K, Kikutani H, et al. Susceptible MHC alleles, not background genes, select an autoimmune T cell reactivity. J Clin Invest. 2003;112:902–914. doi: 10.1172/JCI18337.An important study that indicated a role for I-Ag7 in selection of autoreactive T cells
- 33.Ferlin WG, Mougneau E, Hugues S, Appel H, Jang MH, Cazareth J, Beaudoin L, Schricke C, Lehuen A, Wucherpfennig KW, et al. Self-peptides that bind with low affinity to the diabetes-associated I-A(g7) molecule readily induce T cell tolerance in non-obese diabetic mice. Eur J Immunol. 2004;34:2656–2663. doi: 10.1002/eji.200425413. [DOI] [PubMed] [Google Scholar]
- 34.Kishimoto H, Sprent J. A defect in central tolerance in NOD mice.[see comment] Nature Immunology. 2001;2:1025–1031. doi: 10.1038/ni726. [DOI] [PubMed] [Google Scholar]
- 35.Zucchelli S, Holler P, Yamagata T, Roy M, Benoist C, Mathis D. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity. 2005;22:385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Lesage S, Hartley SB, Akkaraju S, Wilson J, Townsend M, Goodnow CC. Failure to censor forbidden clones of CD4 T cells in autoimmune diabetes. J Exp Med. 2002;196:1175–1188. doi: 10.1084/jem.20020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanagawa O, Vaupel BA, Xu G, Unanue ER, Katz JD. Thymic positive selection and peripheral activation of islet antigen- specific T cells: separation of two diabetogenic steps by an I-A(g7) class II MHC beta-chain mutant. J Immunol. 1998;161:4489–4492. [PubMed] [Google Scholar]
- 38.Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur J Immunol. 1994;24:1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 39.•Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430.Important papers identifying insulin-reactive T cells
- 40.Yu B, Gauthier L, Hausmann DH, Wucherpfennig KW. Binding of conserved islet peptides by human and murine MHC class II molecules associated with susceptibility to type I diabetes. Eur J Immunol. 2000;30:2497–2506. doi: 10.1002/1521-4141(200009)30:9<2497::AID-IMMU2497>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 41.•Levisetti MG, Suri A, Petzold SJ, Unanue ER. The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J Immunol. 2007;178:6051–6057. doi: 10.4049/jimmunol.178.10.6051.The hot-spot insulin reactive peptides bind poorly to I-Ag7
- 42.Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J Exp Med. 2002;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 45.Yachi PP, Ampudia J, Gascoigne NR, Zal T. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat Immunol. 2005;6:785–792. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fairchild PJ, Wildgoose R, Atherton E, Webb S, Wraith DC. An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Int Immunol. 1993;5:1151–1158. doi: 10.1093/intimm/5.9.1151. [DOI] [PubMed] [Google Scholar]
- 47.•Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–415. doi: 10.1016/1074-7613(95)90170-1.The findings that weak MHC interacting peptides can induce autoreactivity


