Abstract
Development of methods to engineer gamma-retroviral vectors capable of transducing target cells in a cell-specific manner could impact the future of the clinical application of gene therapy as well as the understanding of the biology of transfer gene vectors. Two molecular events are critical for controlling the entry of gamma-retroviral vectors to target cells: binding to cell-surface receptors and the subsequent fusion of viral vector membrane and cellular membrane. In this report, we evaluated a method to incorporate a membrane-bound antibody and a fusogenic molecule to provide binding and fusion functions respectively, into gamma-retroviral vectors for targeted gene delivery. An anti-CD20 antibody and a fusogenic protein derived from Sindbis virus glycoprotein could be efficiently co-displayed on the surface of viral vectors. Vectors bearing anti-CD20 antibody conferred their binding specificity to cells expressing CD20. Enhanced in vitro transduction towards CD20-expressing cells was observed for gamma-retroviral vectors displaying both an antibody and a fusogen. We found that the biological activity of the fusogen played an important role on the efficiency of such a targeting strategy and were able to engineer several mutant forms of the fusogen exhibiting elevated fusion function to improve the overall efficiency of targeted transduction. We devised an animal model to show that subcutaneous injection of such engineered vectors to the areas xenografted with target cells could achieve targeted gene delivery in vivo. Taken together, we demonstrated as proof-of-principle a flexible and modular two-molecule strategy for engineering targeting gamma-retroviral vectors.
Introduction
Recombinant gamma-retroviral vectors have emerged as an efficient gene vector for the stable modification of target cells to deliver a therapeutic effect (Somia and Verma 2000). This vector system has been demonstrated clinically as one of the most efficient and powerful gene delivery systems capable of transducing a broad range of cell types (Cavazzana-Calvo et al. 2000; Morgan et al. 2006). The targeted cells that allowed for transduction by these vectors were largely determined by the envelope proteins incorporated in the outer lipid layer of the viral particles. The ability to pseudotype gamma-retroviral vectors with glycoproteins derived from various viruses has been shown to be an effective means to alter the cell tropism of these vectors for genetic modifications (Lavillette et al. 2001; Sandrin et al. 2003; Waehler et al. 2007). Although envelope glycoproteins derived from vesicular stomatitis virus G (VSVG), murine leukemia virus, and gibbon ape leukemia virus, are widely used to pseudotype gamma-retroviral vectors, they lack cell-type specificity, which limits the current gene transfer process to be an in vitro procedure of transducing pre-purified cells.
Development of methods capable of engineering gamma-retroviral vectors to be cell type-specific could substantially change the current practice of gene therapy and greatly expand the scope of gene therapy for disease treatment (Lavillette et al. 2001; Sandrin et al. 2003; Waehler et al. 2007). It can address some of the most desirable features for gamma-retrovirus-mediated gene transfer, such as the precise introduction of the therapeutic nucleic acid into expected cells, alleviating the concern of an off-targeting effect (Waehler et al. 2007). Many attempts have been made towards generating gamma-retroviral vectors that target particular cells by manipulating the molecules on the vector surface (Lavillette et al. 2001; Sandrin et al. 2003; Waehler et al. 2007). A common strategy is to genetically modify envelope glycoproteins to incorporate targeting ligands into gamma-retroviral vectors. It was found that several glycoproteins had structures that were able to tolerate the insertion of binding motifs such as peptide ligands, single chain antibodies, growth factors, etc (Waehler et al. 2007). These engineered glycoproteins can retarget vectors to cells expressing their corresponding target moieties. Another popular approach is to introduce a “molecular bridge” to direct vectors to specific cells (Waehler et al. 2007). The molecular bridge has dual specificities: one end can recognize viral glycoproteins, and the other end can bind to the molecular determinant on the target cell. Such a molecule can direct the attachment of viral vectors to target cells for transduction. To date, ligand-receptor, avidin-biotin, and chemical conjugations have been exploited for the creation of such molecular bridges to retarget gamma-retroviral vectors (Boerger et al. 1999; Snitkovsky and Young 1998). Recently, monoclonal antibodies have been introduced as a new kind of molecular bridge to allow retroviral vectors to preferentially transduce cells expressing cognate surface antigens both in vitro (Morizono et al. 2001) and in vivo (Morizono et al. 2005). In such studies, an E2 protein of the Sindbis virus glycoprotein was modified to contain the Fc-binding domain of protein A. Thus, one end of the monoclonal antibody could bind to viral vectors and the antigen recognition end could direct vectors to antigen-expressing cells.
Functions of binding and fusion of some natural viruses such as paramyxovirus are attributed to two proteins: an attachment protein and a fusion protein (Lamb 1993). Thus, mimicking such viruses to separate the binding and fusion functions as two distinct envelope molecules on the surface of gamma-retroviral vectors represents another attractive strategy for targeting. Lin et al. incorporated a binding-defective but fusion-competent hemagglutinin (HA) protein as a fusion protein and a chimeric glycorprotein engineered to contain specificity for the Flt-3 receptor as a binding protein, into gamma-retroviral vectors (Lin et al. 2001). It was shown that such two proteins could complement each other to mediate preferential modification of cells expressing Flt-3 in vitro (Lin et al. 2001). We have demonstrated successful targeting lentiviral vectors by co-display of membrane-bound antibody as the binding protein and fusogenic molecule derived from Sindbis virus glycoprotein as the fusion protein (Yang et al. 2006). Efficient and specific transduction was accomplished by a two-stage process: endocytosis induced by antibody-antigen interaction and fusion triggered by the acidic pH within the endosomic compartment.
In this study, we extended this approach to the gamma-retroviral vector system and showed that a similar method can be applied as a general strategy to engineering targeting gamma-retroviral vectors. We further engineered several fusogens, based on the original fusogenic molecule used for the targeting lentiviral vectors, which exhibited enhanced fusion behavior to coordinate with antibody to mediate targeted transduction.
Results
Construction and production of recombinant gamma-retroviral vectors
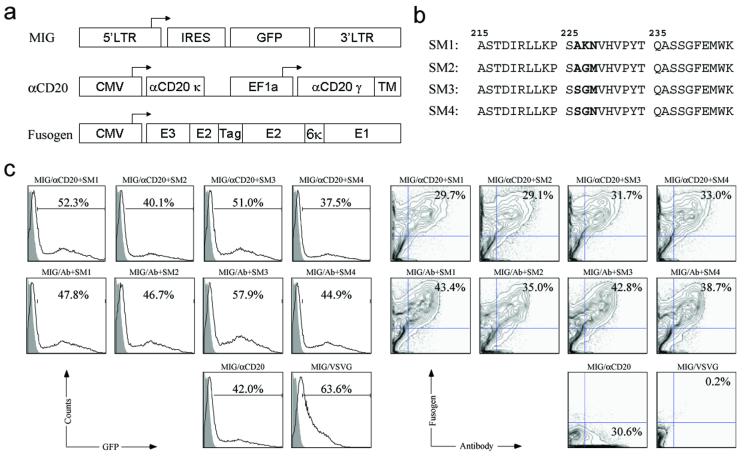
Anti-human CD20 (αCD20), a mouse/human chimeric antibody with a human membrane-bound IgG constant chain that is specific for human CD20, was constructed in our laboratory (Yang et al. 2006) and thus chosen as a model antibody for this study (designated pαCD20, Figure 1a). The human CD20 antigen is a pan-B cell marker and passive immunotherapy using anti-CD20 antibody has been shown to be effective in treating B-cell non-Hodgkin’s lymphoma (Hiddemann et al. 2005). A construct capable of expressing human Igα and Igβ proteins (designated pIgαβ) was used to assist the display of antibody to the surface of cells and subsequently, viral vectors. We used a MIG vector originating from a murine stem-cell virus as the transfer gamma-retroviral vector (Figure 1a) (Yang et al. 2002). MIG contains a DNA element for an internal ribosome entry site and a GFP reporter gene that is driven by the wild-type LTR promoter (Hawley et al. 1994).
Figure 1. Co-transfection of virus-producing cells to make the recombinant targeting gamma-retroviral vectors.
(a) Schematic diagrams of constructs encoding transfer gamma-retroviral vector MIG, membrane-bound human/mouse chimeric antibody against CD20 (αCD20), and fusogenic molecule derived from Sindbis virus glycoprotein. LTR: long terminal repeat; IRES: internal ribosome entry site; GFP: green fluorescence protein; CMV: human cytomegalovirus immediate-early gene promoter; EF1a: human elongation factor 1 α promoter; αCD20κ: immunoglobulin κ light chain of anti-CD20 antibody; αCD20γ: immunoglobulin γ heavy chain of anti-CD20 antibody; TM: transmembrane domain of immunoglobulin γ heavy chain; E1, E2: E1, E2 proteins of Sindbis virus glycoprotein (E1 for induction of fusion and E2 for receptor binding); E3: signal peptide of Sindbis virus glycoprotein; Tag: 10-residue epitope HA tag sequence (MYPYDVPDYA); Several alterations (Morizono et al. 2005; Yang et al. 2006), including deletion of amino acids 61-64 of wild-type E3 and mutations of 157KE158 into 157AA158 of wild-type E2, were introduced to disrupt the binding to heparin sulfate glycosaminoglycan, resulting in a binding-deficient but fusion-active fusogenic protein SM1. (b) Amino acid sequences of the E1 226 region of various engineered fusogens. The sequence starts at amino acid 215 and ends with amino acid 244 of wild-type E1. Mutations involved in the generation of new forms of fusogen (SM2, SM3, and SM4) are shown in bold. (c & d) FACS analysis of co-transfected vector-producing cells. 293T cells were transiently transfected with plasmids for transfer gamma-retroviral vector MIG, membrane-bound human/mouse chimeric anti-CD20 antibody (αCD20), fusogenic protein (SM1-4), and other packaging construct (pIgαβ, gag-pol). Co-transfection using a non-relevant antibody lacking the binding specificity to CD20 (Ab), as well as co-transfection using αCD20 alone (without fusogen) or using a plasmid encoding vesicular stomatitis virus glycoprotein (VSVG), was performed as controls. Two days post-transfection, GFP expression from the plasmid MIG was analyzed by FACS and gating is based on the total live cell population (c). The antibody and fusogen expressions were measured by co-staining using anti-human IgG antibody and anti-HA tag antibody and analyzed by FACS. αCD20 and various fusogenic molecule expressions were gated on the GFP-positive cell populations (d).
Previous study has demonstrated that the modified forms of Sindbis virus glycoprotein (SVG) can be effectively incorporated onto the surface of gamma-retrovirus and lentivirus (Morizono et al. 2001; Morizono et al. 2005; Yang et al. 2006). It is known that fusion of SVG is pH-sensitive and independent of receptor binding (Lu et al. 1999; Smit et al. 1999). We adopted the series of alterations reported previously into the E2 domain of SVG (Figure 1a) and showed that this version of SVG (designated SM1) is binding-defective but fusion-active and can be used as a fusogen to mediate targeted transduction of an engineered lentiviral vector bearing a surface antibody (Yang et al. 2006). Further study in my laboratory showed that the performance of the fusogen plays a critical role in enabling efficient transduction by the engineered viral vectors (Joo and Wang 2008). We thus sought other forms of SVG that might be able to induce different or even improved fusogenic activity. Studies conducted by Kielian and co-workers showed that Sindbis virus is dependent on the cholesterol in the target membrane for entry and exit from the cell (Lu et al. 1999). Further characterization of SVG revealed that that requirement of cholesterol for viral infection could be modulated by three amino acids within the E1 226 region of the glycoprotein. The study identified 3 mutants that could increase cholesterol-independence of Sindbis virus in infecting cells (Lu et al. 1999). We decided to evaluate the function and efficiency of these mutants as a fusogen coordinating with αCD20 to mediate targeted transduction of gamma-retroviral vectors. These new forms of fusogen were constructed and designated SM2, SM3, SM4, respectively (Figure 1b).
To generate gamma-retroviral vectors enveloped with both αCD20 and fusogenic molecular (designated MIG/αCD20+fusogen), 293T cells were transiently transfected by a standard calcium phosphate-mediated precipitation method with the transfer vector MIG, packaging plasmids encoding gag and pol, pαCD20, pIgαβ, and fusogen. MIG pseudotyped by the envelope protein derived from vesicular stomatitis virus (designated MIG/VSVG), was used as a non-targeting vector control; vectors pseudotyped by VSVG are known to have broad tropism and can modify many cell types. Another set of controls was provided by making gamma-retroviral vectors either co-displaying a non-relevant antibody (Ab) and a fusogen (MIG/Ab+fusogen), or displaying only αCD20 (MIG/αCD20). FACS analysis of the transfected, virus-producing 293T cells showed that 40 ∼ 50% of live cells had expression of GFP (Figure 1c, left). Among these GFP-positive cells, approximately 30% of the cells co-expressed a αCD20 and one form of fusogen (Figure 1c, right). A slightly higher percentage (35-40%) of co-expression was seen for co-transfection with a non-relevant antibody and a fusogen (Figure 1c, right). Transfection with VSVG gave higher GFP expression (Figure 1c, left) that could be due to higher transfection efficiency and/or super-infection of MIG/VSVG; no antibody and fusogen expression was detected. As expected, no fusogen was detected on cells transfected with only αCD20 (Figure 1c). A close examination of the expression pattern for various fusogens (SM1-4) revealed that the similar level of transfection and expression was obtained among these four fusogens, suggesting that they could be incorporated into the gamma-retroviral surface with similar efficiency.
Co-display of a fusogen and an antibody on the gamma-retroviral surface
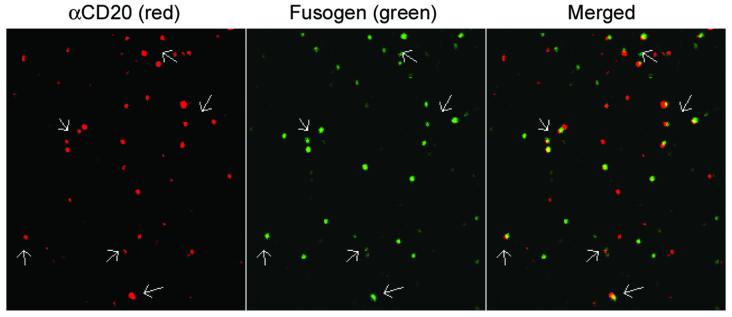
We used a previously reported method of confocal imaging of individual gamma-retroviral particles (Andrawiss et al. 2003) to directly visualize the co-incorporation of antibody and fusogen on the surface of engineered vector virion. Fresh supernatant of cells transfected with constructs for making the gamma-retroviral vector MIG/αCD20+SM3 was harvested and filtered through 450- and 220-nm filters. The resulting supernatant was spun down to a poly-lysine coated coverslip and fixed. As shown in Figure 2, display of αCD20 on the individual viral vector surface could be stained by anti-human IgG antibody and visualized in the red channel. Some of this red fluorescence was colocalized with the fusogenic protein SM3, detected with anti-HA antibody and shown in the green channel, indicating that a good fraction of vector particles contained both αCD20 and SM3.
Figure 2. Confocal imaging analysis for the co-display of an antibody and a fusogenic molecule on the surface of individual gamma-retroviral particles.
Supernatants of 293T cells transfected with various plasmids (MIG, pαCD20, Igαβ, SM3, and gag-pol) was sequentially filtered through 450- and 220-nm pore-size filters. The resulting supernatants were adhered to poly-L-lysine-coated coverslips and imaged using a laser scanning confocal microscope (Zeiss LSM 510). Coverslips were rinsed and immunostained using Alexa 594 anti-human IgG against αCD20 (red) and anti-HA tag antibody against fusogen SM3 (green), respectively. Images for red and green fluorescence were acquired separately. Overlapping red and green signals appears as yellow in a merged image. Scale bar represents 2 μm. The similar virtualization of gamma-retroviral particles was previously reported by Andrawiss et al. (Andrawiss et al. 2003).
Study of viral vector-cell binding
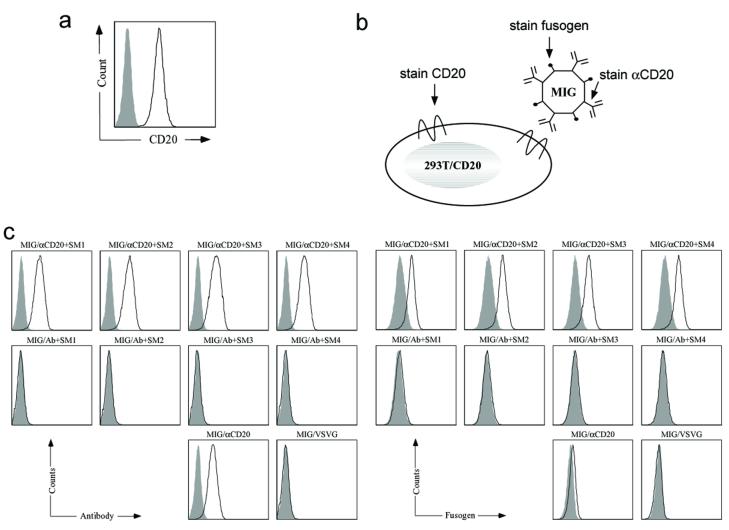
A virus-cell binding assay was conducted to further demonstrate the co-display of antibody and fusogen in the same virion and the specific targeting of αCD20 antibody to CD20 antigen. We used 293T/CD20, a 293T cell line stably expressing the CD20 protein antigen, as a target cell line and 293T cells lacking the expression of CD20 as a negative control (Figure 3a). Cells were incubated with gamma-retroviral supernatants at 4°C for 30 minutes and then co-stained for αCD20 and fusogen by anti-human IgG antibody and anti-HA tag antibody, respectively (Figure 3b). FACS analysis showed that there was clear binding between 293T/CD20 cells and gamma-retroviral vectors bearing αCD20 and fusogen (Figure 3c). In contrast, no binding was observed for either 293T/CD20 cells incubated with gamma-retroviral vectors bearing a non-relevant antibody (Ab) and a fusogen, or 293T cells incubated with MIG/αCD20+fusogen (Figure 3c), indicating that the detected virus-cell binding stems from the specific interaction between the viral surface αCD20 and the cell surface CD20 antigen. Observed binding between the gamma-retroviral vector bearing only αCD20 (MIG/αCD20) and 293T/CD20 cells implied that the viral vector binding to cells is only attributed to the recognition between antibody and cognate antigen and is fusogen-independent, which was further supported by the similar binding efficiencies detected among the various gamma-retroviral vectors bearing different fusogens on their surfaces (Figure 3c).
Figure 3. Antigen-binding specificity of gamma-retroviral vector particles containing an antibody and a fusogenic protein.
(a) FACS analysis of the targeting 293T/CD20 cell line by surface staining using anti-human CD20 antibody. Solid line: CD20 expression on 293T/CD20 cell line; Shaded area: CD20 expression on 293T cell line (as a control). (b) Schematic representation of the binding of gamma-retroviral vectors bearing αCD20 and fusogen to targeting 293T/CD20 cells. Arrows indicate the parts that must be co-stained to detect the three different components on either cell surface (CD20) or cell surface-bounded viral vector (αCD20 and fusogen). (c) FACS analysis of the 293T/CD20 cells incubated with various gamma-retroviral vectors (the text label on the top of each sub-figure indicates the antibody and fusogen displayed on viral vector surface). After incubation at 4°C for 30 minutes and extensive washing using cold PBS, anti-human IgG antibody against αCD20 and anti-HA tag antibody against fusogen were used to detect viral vectors bound to the 293T/CD20 cells. (c, left) FACS analysis of staining for the presence of fusogen. (c, right) FACS analysis of staining for the presence of αCD20. Solid line: analysis on 293T/CD20 cells incubated with vectors; Shaded area, analysis on 293T cells incubated with vectors (as a control).
Targeted transduction by gamma-retroviral vectors in vitro
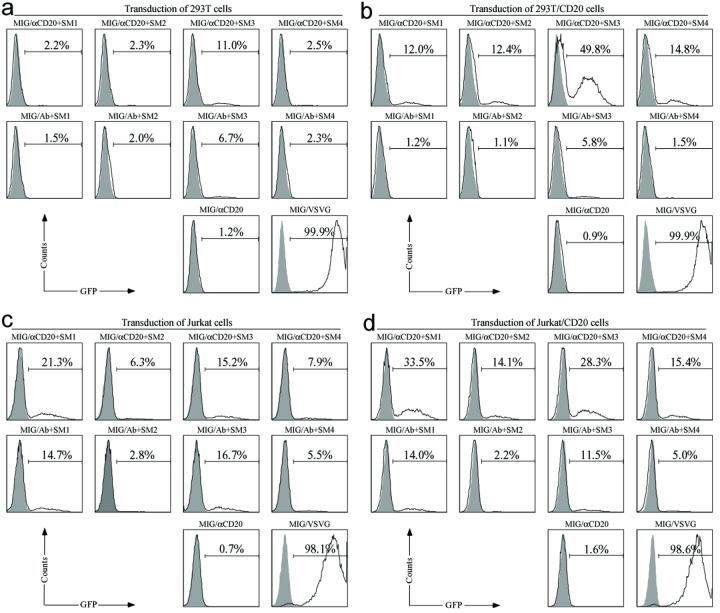
We next wanted to study the in vitro transduction efficiency and specificity of gamma-retroviral vectors bearing both αCD20 and fusogen molecules to specific cells. CD20-expressing cells, 293T/CD20 and Jurkat/CD20 (a human Jurkat cell line constantly expressing CD20), were used as targets and correspondingly, 293T and Jurkat cells served as non-CD20-expressing cell controls. MIG/VSVG was included as a non-targeting vector control. We also used gamma-retroviral vectors bearing a non-relevant antibody and fusogen as a viral vector control to demonstrate the requirement of the specificity of αCD20 for targeting CD20-expressing cells. Cells were exposed to various gamma-retroviral vectors bearing an antibody and a fusogen. After four days post-transduction, FACS analysis of GFP expression was employed to measure the efficiency of targeted transduction (Figure 4).
Figure 4. Gamma-retroviral vector transduction of targeted cells in vitro.
Fresh unconcentrated recombinant gamma-retroviral vectors (2 ml) either bearing αCD20 and the indicated fusogen (MIG/αCD20+fusogen) or bearing Ab (control antibody, no specificity to CD20) and the indicated fusogen (MIG/Ab+fusogen), were added to CD20-negative 293T (a), or CD20-negative Jurkat (c), or CD20-positive 293T (b), or CD20-positive Jurkat (d) cells. Vectors bearing αCD20 only (MIG/aCD20) or bearing glycoprotein derived from vesicular stomatitis virus (MIG/VSVG) were included as controls. Four days post-transduction, the resulting GFP expression was taken as an indication of transduction efficiency and was analyzed by FACS. Solid line: analysis on transduced cells; Shaded area, analysis on non-transduced cells (as a control).
Gamma-retroviral vectors MIG/αCD20+fusogen, which contained both an antibody (αCD20) and a fusogenic molecule, were able to transduce 293T/CD20 and Jurkat/CD20 cells (Figures 4b and 4d). This observed transduction was dependent on CD20 because only background transduction was obtained when the same vectors were used to transduce the control 293T and Jurkat cells lacking the expression of CD20 (Figures 4a and 4c). As a non-targeting control, MIG/VSVG vector was used to transduce various cell lines and transduction was found to be independent of CD20 expression (Figures 4). We also observed greater cell toxicity for MIG/VSVG-exposed cells as compared to cells transduced with the engineered targeting vectors (MIG/αCD20+fusogen) (data not shown). As another control, gamma-retroviral vectors MIG/Ab+fusogen, which displayed both a non-relevant antibody and a fusogenic molecule, exhibited the similar low background transduction for both CD20-expressing cell lines (293T/CD20 and Jurkat/CD20) (Figures 4b and 4d) and control cells (293T and Jurkat) (Figures 4a and 4c); the background transduction might have stemmed from the residual binding capacity of the E2 protein of the engineered fusogens. This finding further confirmed the role of αCD20 on mediating the specific targeting to CD20 antigen on the cell surface. Although the gamma-retroviral vector MIG/αCD20, which only displayed αCD20 on the vector surface, could clearly bind to 293T/CD20 (Figure 3), no detectable transduction was obtained for this vector because of the lack of fusogen. This result supports our hypothesis that two molecules on the surface of the gamma-retroviral vector-antibody, determining the specific targeting, and fusogen, triggering the fusion activity-are both necessary for targeted transduction. Moreover, gamma-retroviral vectors bearing the same antibody but different fusogens on their surfaces showed distinctly different transduction efficiencies of both target cells and control cells. Among the various engineered gamma-retroviral vectors, MIG/αC20+SM3 gave the highest transduction and could transduce approximately 50% of 293T/CD20 cells and 30% of Jurkat/CD20 cells using unconcentrated fresh viral supernatant. The specific transduction titers of different vectors against 293T/CD20 cells were measured and given in Table 1; the titers were based on GFP expression and determined in dilution ranges that exhibited a linear response between the number of transduction events and the volume of vector added. Due to the higher titer, we decided to use the vector MIG/αCD20+SM3 for the in vivo experiment to target 293T/CD20 cells.
Study of binding and fusion activity of engineered gamma-retroviral vectors
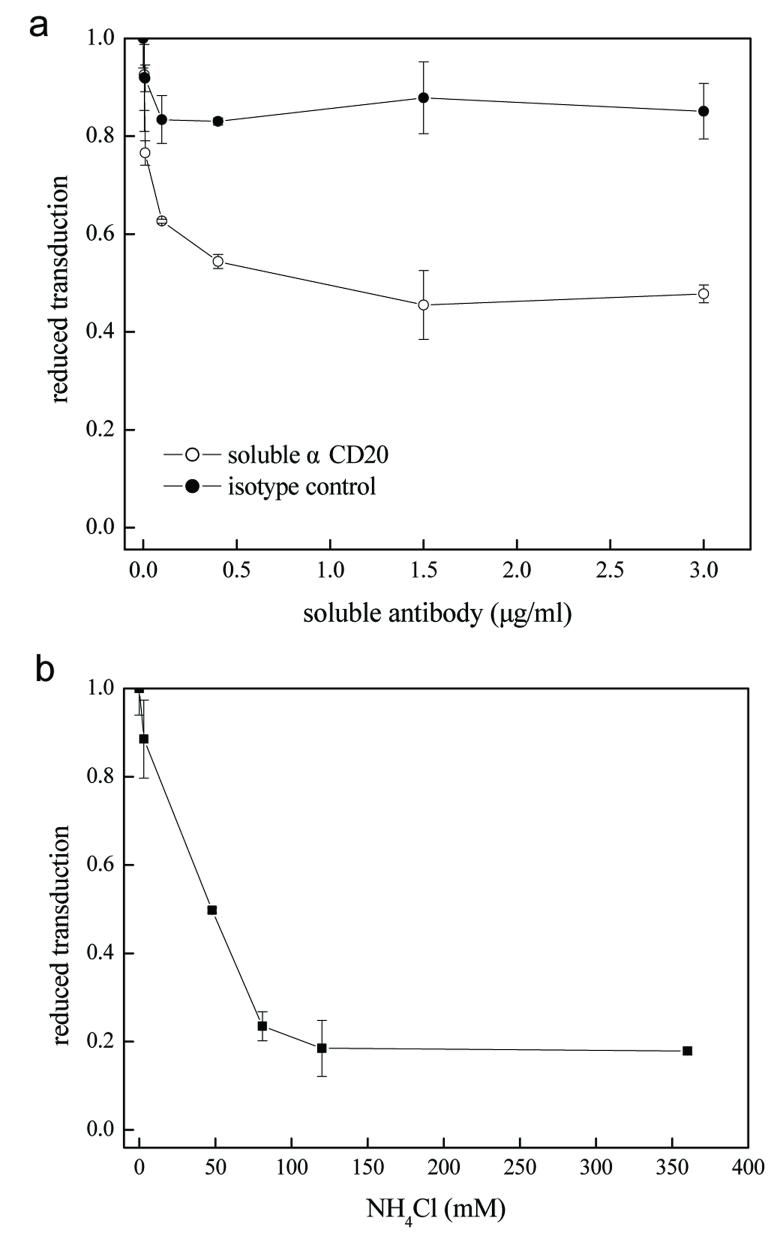
Understanding of viral vector entry could potentially offer more opportunities for the development of efficient viral delivery systems (Dimitrov 2004). Our intention in studying the binding and fusion activities of engineered gamma-retroviral vectors is to further provide the evidence to support our postulation that the transduction specificity was a consequence of the interaction between αCD20 and surface antigen CD20 and that low pH was required for fusogenic molecules to trigger membrane fusion. An competitive blocking assay using a soluble anti-CD20 antibody was used to assess the necessity of the specific interaction between αCD20 and CD20 to targeted transduction. 293T/CD20 cells were exposed with the gamma-retroviral supernatant MIG/αCD20+SM3 in the presence of various concentrations of the soluble αCD20 antibody. As we expected, levels of transduction decreased significantly (Figure 5a), presumably due to the competitive inhibition of the soluble αCD20 in blocking the binding of viral vectors bearing surface αCD20 to CD20-expressing cells.
Figure 5. Study of the effects of antibody competition and endosome neutralization on the transduction efficiency in vitro.
(a) 293T/CD20 targeting cells were pre-incubated with various concentrations of soluble anti-CD20 antibody with the same clonal origin of the membrane-bound αCD20 used in vector targeting for 30 min. Subsequently, the fresh unconcentrated supernatant of gamma-retroviral vector MIG/αCD20+SM3 (1 ml) was added to allow transduction to occur. After replacement with fresh medium, cells were placed in the incubator for three days before FACS analysis of GFP expression. (b) Various concentrations of NH4Cl (dissolved in PBS, pH=7.4) were added into viral supernatant (MIG/αCD20+SM3) for eight hours, after which the medium was replaced with fresh medium and cells were further cultured for three days before FACS analysis of GFP-positive cells. All data is presented as the percentage of reduced transduction as compared to the result of transduction without treatment of either antibody (a) or NH4Cl (b).
In another set of experiments, we used a similar protocol described above to test the effect of ammonium chloride (NH4Cl), which could neutralize the endosomal compartment, on the transduction efficiency of the gamma-retroviral vectors. We observed a dramatic decrease in transduction efficiency in response to an increase of in concentration of NH4Cl (Figure 5b). This result shows that the viral membrane fusion is low pH-dependent, which is consistent with the mechanisms of our engineered fusogens. Thus, this confirms that the fusion function of the engineered recombinant gamma-retroviral vector is mediated by the incorporated fusogen molecules.
Targeted transduction of gamma-retroviral vectors in vivo
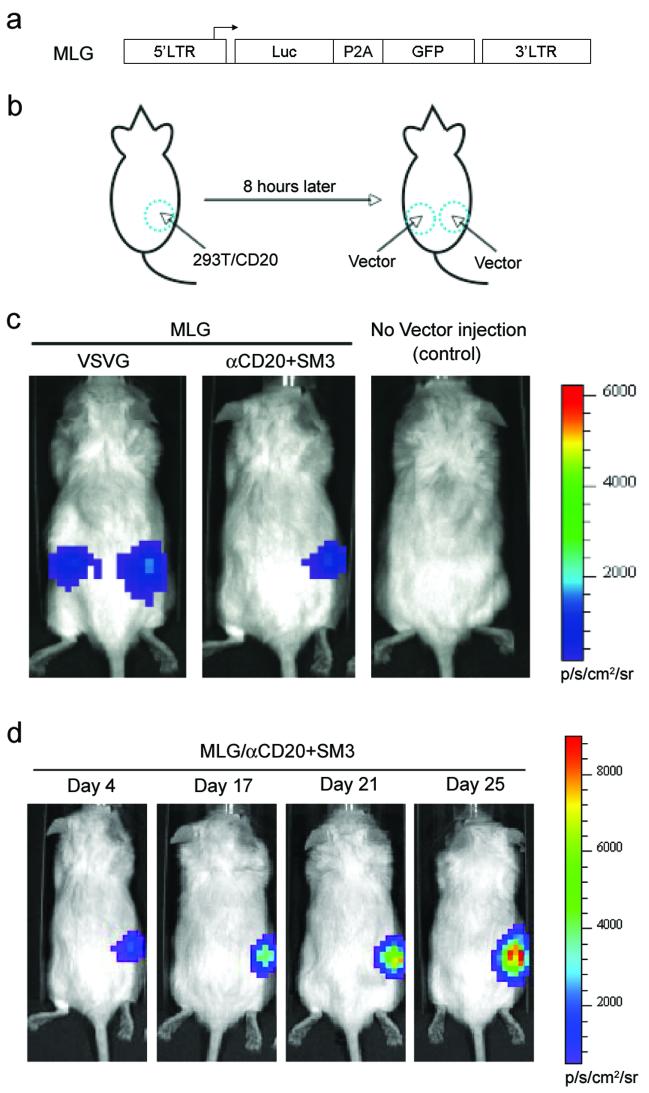
We set up a model system to investigate the ability of recombinant gamma-retroviral vectors to deliver genes to specific cells in vivo. RAG2-/-γc-/- immunocompromised mice xenografted with target cells (293T/CD20) were employed as the animal model system for the in vivo experiment. Target cells (293T/CD20, 10×106) were injected subcutaneously on the right flank of the mouse, which served as a target for the gamma-retroviral vector (Figure 6b). No xenograft was performed on the left side of the same mouse, which could be used as a control site that lacked targeting cells (Figure 6b). A bioluminescence imaging (BLI) assay was employed to monitor the efficiency of in vivo transduction of 293T/CD20 cells using the targeting vector. For this purpose, we constructed a new transfer gamma-retroviral vector, MLG, by inserting both the firefly luciferase gene and the GFP gene into the mouse stem cell virus (MSCV) backbone (Figure 6a). The two genes were linked by a 2A sequence derived from the Porcine Teschovirus (P2A) that ensures the co-expression of these two genes under the control of the MSCV LTR promoter (Szymczak et al. 2004). This dual-reporter vector enabled us to detect the MLG transduced cells in vivo using BLI, and in vitro using flow cytometry. Eight hours post-injection of 293T/CD20 cells, the concentrated gamma-retroviral vector MLG bearing αCD20 and SM3 (MLG/αCD20+SM3) was subcutaneously injected (10×106 Transduction Units (TU), 2 Multiplicity of Infection (MOI)) to the 293T/CD20-injected area on the right flank of the mouse, and also to the left flank, in which no target cells were xenografted. VSVG-enveloped vector (MLG/VSVG, MOI=5) was injected in the same manner as the target vector (MLG/αCD20+SM3) and was used as a non-targeting vector control. We monitored the luciferase expression by injection of the substrate D-Luciferin intraperitonially and imaged using an IVIS200 imaging system. The data for day 4 post-injection shown in Figure 6c is representative of three independent experiments. We detected a bioluminescence signal in the area xenografted with 293T/CD20 cells and injected it with the targeting vector MLG/αCD20+SM3, demonstrating the success of the delivery of luciferase gene to target cells. This vector exhibited certain targeting specificity in vivo as there was no detectable signal in the left flank, suggesting that fewer mouse cells were transduced to express the reporter gene. When the same amount of the vector MLG/αCD20+SM3 was injected to a mouse xenografted with 293T cells, no detectable bioluminescence was obtained (data not shown). As expected, the luciferase signal was observed on the both sides of mouse flanks injected with MLG/VSVG, confirming the broad specificity of VSVG-enveloped gamma-retroviral vector. Following the growth of the 293T/CD20 cells, we could detect the gradual increase of luciferase signals on right side of the mouse (Figure 6d). FACS analysis of xenografted cells harvested from the scarified mouse confirmed the expression of GFP in the 293T/CD20 cells (data not shown).
Figure 6. Targeted transduction of 293T/CD20 cells xenografted on immunodeficient mice by engineered gamma-retroviral vectors.
(a) Schematic diagram of transfer gamma-retroviral vector (MLG) encoding firefly luciferase and GFP. LTR: long terminal repeat; Luc: firefly luciferase gene; P2A: a self-cleavage peptide sequence derived from Porcine Teschovirus. (b) Schematic representation of the procedure used to evaluate the targeting ability of engineered gamma-retroviral vectors to modify targeted cells in vivo. 293T/CD20 cells (5×106) were injected subcutaneously on the right flank side of an immunodeficient RAG2-/-γc-/- mouse. After eight hours, concentrated recombinant viral vectors (10×106 for MLG/aCD20+SM3, 25×106 for MLG/VSVG as a non-target vector control) were injected subcutaneously on both right (the area grafted with 293T/CD20 cells) and left sides. Several days later, the mouse was subjected to analysis using bioluminescence imaging. (c & d) Mice were injected individually with 1.5 mg of D-luciferin into the interperitonial cavity and imaged using the Xenogen IVIS 2000 system. (c) Representative images of mice with the injection of the targeting vector MLG/αCD20+SM3, with an injection of non-targeting vector MLG/VSVG, or without injection. Images were acquired four days post-vector injection. (d) Images acquired from a mouse injected with targeting MLG/αCD20+SM3 vector at the indicated times of post-vector injection.
Discussion
The purpose of this study is to explore the possibility of incorporating membrane-bound antibody and fusogenic protein to the surface of gamma-retroviral vectors for targeted gene delivery. We separated binding and fusion functions into two distinct molecules on the vector surface with the hope that binding could induce endocytosis of viral vectors to endosome, after which fusogen could respond to the endosomal pH and trigger virus-membrane fusion to empty the vector core to the cytosol. This mimics the naturally occurring paramyxoviruses (Lamb 1993), whose binding and fusion activities are controlled by the attachment protein (HN) and fusion protein (F), respectively. Such a concept has been previously explored for targeting gamma-retroviral vectors. Paula and co-workers reported the early success of cell-targeting in vitro by engineering a gamma-retroviral vector co-displaying a binding-defective but fusion-competent hemagglutinin (HA) protein and a chimeric murine leukemia virus envelope protein capable of binding to the Flt-3 receptor (Lin et al. 2001). We demonstrated previously that the antibody could be used as the binding protein to coordinate with a fusogenic protein for producing targeting lentiviral vector in vitro and in vivo (Yang et al. 2006). We also learned from that study that the property of the fusogen could play a critical role on the transduction efficiency of engineered lentiviral vector (Yang et al. 2006).
In this study, a model antibody (αCD20) and several forms of the fusogen derived from Sindbis virus glycoprotein were incorporated into the gamma-retroviral vector. This was done by co-transfecting producing 293T cells with plasmids encoding αCD20, Igαβ, fusogen, gag-pol, and transfer gamma-retroviral backbone. FACS analysis showed that fusogen and αCD20 could be co-expressed efficiently in 293T cells. Co-incorporation of both proteins on the surface of virions was confirmed by confocal imaging of viral particles; similar imaging analysis was previously used to study viral particle assembly of murine leukemia virus (Andrawiss et al. 2003) and to demonstrate the encapsulation of GFP-Vpr protein into HIV-1-based lentiviral particles (McDonald et al. 2002). Gamma-retroviral vectors bearing αCD20 and fusogen was shown to be able to specifically bind to 293T/CD20 cells and transduce those cells 6.8 ∼ 44.0-fold more efficiently than the parent 293T cells (Table 1). The enhanced transduction needed the specific interaction between antibody and cognate antigen, as only background transduction was obtained when a non-relevant antibody was used and the addition of soluble form of targeting antibody could significantly reduce the transduction efficiency. Fusogen was absolutely required for transduction because vector lacking fusogen resulted in no detectable transduction. We further set up an assay in a xenografted mouse model to show that concentrated gamma-retroviral vector MLG/αCD20+SM3 subcutaneously injected near the site of grafted 293T/CD20 cells could preferentially deliver firefly luciferase to target cells (right side), while the non-targeting MLG/VSVG vector equally transduced both grafted 293T/CD20 tumor cells (right side) and murine tissue cells (left side). This in vivo experiment confirmed that our targeting gamma-retroviral vector could partially alleviate the general off-target effect produced by vector systems with broad specificity such as the VSVG-pseudotyped vector. Since approximately 95% of B-cell lymphoma constantly expresses the CD20 antigen (Davis et al. 1999), we are currently designing experiments to further evaluate this CD20-targeting vector system for its ability to deliver a suicide gene (Blumenthal et al. 2007) to eradicate human lymphoma cells in a xenografted lymphoma animal model.
The experimental results for targeted transduction in vitro showed that the various forms of engineered fusogen coordinated differently with antibody to mediate infection by gamma-retroviral vector. These mutant forms of fusogen were adapted from the previous study conducted by Lu et al., in which mutation in the E1 226 region of Sindbis virus glycoprotein could alter the cholesterol dependence of Sindbis viral infection (Lu et al. 1999). The study conducted by Lu et al. demonstrated that several sequences in the 226 region of the E1 domain play a critical role in virus fusion. The original form of fusogen (SM1) in this study was constructed previously in my laboratory (Yang et al. 2006), which contained alterations reported by Morizono et al. (Morizono et al. 2005) to disrupt the binding capability of the glycoprotein to heparin sulfate structures on the surface of many cell types. It was found that the resulting protein SM1 was a binding-defective but fusion-competent molecule that could be used for engineering viral vectors for targeted delivery (Morizono et al. 2005; Yang et al. 2006). We incorporated mutations in the E1 226 region of SM1 that made infection by Sindbis virus less dependent of cholesterol and this resulted in three new mutants (SM2, SM3 and SM4). Flow cytometry analysis of virus-producing cells transiently transfected with these mutants (SM1-4) showed a similar level of expression, suggesting that they could be incorporated onto the gamma-retroviral surface with a similar efficiency. Results from the in vitro transduction experiment showed that all three new fusogens (SM2-4) outperformed SM1 in terms of their capability to mediate specific infection. Although the highest titer against 293/CD20 was obtained for MIG/αCD20+SM3, this vector had the highest background transduction against 293T cells. Both MIG/αCD20+SM2 and MIG/αCD20+SM4 could transduce 293T/CD20 cells approximately 40-fold more efficiently than the control 293T cells.
Although much more study is needed to further characterize these mutants in order to understand the different targeting behaviors observed, we speculate, based on the recently published crystal structure of the fusion protein of Semiliki Forest virus (Gibbons et al. 2003; Gibbons et al. 2004), an alphavirus that is closely related to Sindbis virus, that the mutations in the fusion loop region could facilitate the conformational rearrangement of the E1 protein induced by low pH within the endosome to form a trimer; this is the active configuration required to trigger fusion. An experiment that can be used to test this hypothesis is to measure the required pH for the different mutants to induce fusion in vitro (Smit et al. 1999). The mutations could also stabilize the necessary configuration of the fusion loop of the E1 trimer to favor fusion. The experiments in the laboratory for targeting other cell types (such as CD34-expressing cells) using corresponding antibodies (such as anti-CD34 antibody) showed that although SM2-4 always performed better than SM1 as a fusogen in our two-molecule targeting system, the exact mutant that gave the highest transduction titer varied upon which cell types were targeted and which antibody was used as the binding molecule (Zeigler and Wang 2008). Nevertheless, our study clearly shows that the fusogenic protein is amenable for engineering to improve the efficiency of gene delivery.
Our targeting model also requires specific binding between antibody and its target antigen and that this binding should induce endocytosis of gamma-retroviral vectors. Addition of the soluble antibody was found to be able to inhibit transduction, confirming the binding requirement for the observed targeting. Confocal imaging studies showed that the engineered vector bearing αCD20 can be internalized into the endocytic vesicles of 293T/CD20 cells upon binding to cognate surface antigen CD20 (Joo and Wang 2008). When ammonium chloride was added to neutralize the acidic endosomal compartments, targeted transduction was significantly reduced, which was expected, due to the pH-dependent nature of the fusogenic protein incorporated in the recombinant gamma-retroviral vector. These studies demonstrate that binding and fusion are two molecular events that we consider essential for our targeting strategy to be successful.
The observed background transduction and the modest increase of transduction (6.8∼44 folds) to target cells suggest that further characterization and optimization is needed in order to convert this system into a generally applicable method for engineering targeting gamma-retroviral vectors. For example, we need to know the average number of antibody and fusogenic protein incorporated on the surface of a virion and the optimal number that can render targeting with the highest efficiency. The background transduction likely stems from the residual binding of fusogen, thus we need to further engineer our fusogen to be more blind to the cell surface but more responsive for fusion. The choice of antibody and antigen could also be important and further understanding of the system to allow us to select the right pair for targeting could be extremely useful for the design of new targeting systems. An in vivo assay for the systemic delivery of the engineered vector bearing antibody and fusogen is needed to further characterize its targeting specificity and efficiency. However, we have demonstrated in this study a modular and flexible two-molecule system to engineer gamma-retroviral vectors that can be potentially used as a general method for targeted gene delivery. It should be noted that our targeting method is unable to address the intrinsic genotoxicity or insertional mutagenesis associated with gamma-retroviral vectors (Fehse and Roeder 2008). This remains to be a big challenge in order to safely use this vector system for gene therapy applications, although some promising results have emerged from the gene targeting field using the zinc finger nuclease method (Lombardo et al. 2007), which could potentially address this concern.
Materials and Methods
Cell lines
293T cells (human kidney epithelial cells transformed with the large T antigen of simian virus 40) and Jurkat cells were obtained from American Type Culture Collection (ATCC). 293T/CD20 and Jurkat/CD20 cells, both expressing the human CD20 antigen, were made by stable transduction of parent 293T and Jurkat cells respectively, with a lentiviral vector encoding the cDNA of human CD20. Transduced cells were subjected to sorting by flow cytometry to provide uniform populations of cells expressing CD20.
Construct Preparation
The plasmid pαCD20 for expression of human/mouse chimeric antibody (membrane bound form) against human CD20 was previously constructed in my laboratory (Yang et al. 2006). The construct to express antibody associated protein (pIgαβ) was described previously (Yang et al. 2006) and used in this study. MIG plasmid was reported previously (Yang et al. 2002) and kindly provided by the laboratory of David Baltimore (California Institute of Technology). To construct MLG to deliver transgenes of both GFP and firefly luciferase, cDNAs of GFP and luciferase were PCR-amplified. The DNA encoding a 2A sequence derived from Porcine Teschovirus (Szymczak and Vignali 2005; Szymczak et al. 2004), furin cleavage sequence (Fang et al. 2005), and flexible SGSG linker (totally termed as P2A linker) was ordered from an oligo synthesis company (Operon Biotechnology). P2A was firstly PCR-assembled with GFP to yield P2A-GFP. The resulting DNA was further assembled with the cDNA of luciferase to yield Luc-P2A-GFP. Luc-P2A-GFP was then cloned into a plasmid encoding the gamma-retroviral vector backbone to generate MLG.
The plasmid encoding the original fusogenic protein was previously constructed in my laboratory ((Yang et al. 2006), termed SINmu) and designated SM1. It contains an insertion of an HA tag sequence between amino acids 71 and 74 of the E2 protein of SVG for detection purposes and several other alterations adapted from Chen and colleagues (Morizono et al. 2005) for disrupting the binding of E2 to heparin sulfate structures. PCR mutagenesis was employed to introduce mutations at the E1 226 region to replace amino acids AKN in SM1 with AGM, or SGM, or SGN to generate new mutant forms of fusogen designated SM2, SM3 and SM4 respectively. The integrity of the DNA sequence for these mutants was confirmed by DNA sequencing.
Viral vector production
293T cells were seeded in a 6-cm culture dish in DMEM medium supplemented with Fetal Bovine Serum (Sigma, 10%) and L-glutamine (10 ml/L) and penicillin and streptomycin (100 units/ml). After 18∼20 hr, 293T cells with the confluence of ∼90% were transfected with appropriate plasmids per plate of 5 μg transfer gamma-retroviral vector (MIG or MLG), 2.5 μg each of pαCD20, pIgαβ, fusogen and other necessary packaging plasmids (gag-pol), using a standard calcium phosphate precipitation technique. Cells were replaced with pre-warmed fresh medium 4 hr after transfection and the viral supernatants were harvested between 48 ∼ 72 hr after additional incubation. After being filtered through a 0.45-μm pore size filter (Nalegene), the viral supernatant was ready for binding and transduction experiments. Viral supernatants could be further concentrated by using ultracentrifugation (Optima L-90K Ultracentrifuge, Beckman Coulter) for 90 min at 25,000 × g and 4°C and resuspended in an appropriate volume of cold PBS to obtain the high titer viral vector stock for in vivo study.
Direct visualization of viral particles by confocal imaging
For the detection of individual viral particles displaying the antibody (anti-CD20) and fusogenic protein (SM3), the fresh viral supernatant was overlaid upon poly-lysine-coated no.1 coverslips in a six-well culture dish and centrifuged at 3,700 × g at 4°C for 2 hr in a Sorval Legend RT centrifuge. The coverslips were rinsed with cold PBS twice and the adhered viruses were immunostained by Alexa 594 anti-human IgG (Molecular Probes) and anti-HA-biotin (Miltenyi Biotec Inc.) antibodies. They were then incubated with FITC-conjugated streptavidin (BD Phamingen). The coverslips were mounted in vectashield (Vector Laboratories) as an anti-fade mounting medium. The image was obtained with a Zeiss LSM-510 laser scanning confocal microscope (Carl Zeiss, Thornwood) using a plan-apochromat 63×/1.4 oil immersion objective. The image was processed using the LSM 510 software version 3.2 SP2.
Virus-cell binding assay
Approximately 0.5×106 cells (293T or 293T/CD20) were incubated with 2 ml of various gamma-retroviral supernatants at 4°C for 30 minutes. After extensive washing using cold PBS, anti-human IgG antibody (BD Bioscience) and anti-HA tag antibody (Miltenyi Biotec Inc.) were used to detect the presence of anti-human CD20 antibody and fusogenic protein on the viral vectors bound to target cells, respectively. After staining, FACSort (BD Bioscience) was use to perform flow cytometry analysis of virus-cell complexes to assess the binding.
Gamma-retroviral vector-mediated transduction in vitro
Cells (293T/CD20, 293T, Jurkat/CD20, and Jurkat) were seeded on a 24-well tissue culture plate with the density of 0.2×106 per well the day before transduction. The viral supernatants (1 ml per well) were added to individual wells, followed by spin-transduction at 2,500 rpm, 30°C for 90 min using a RT legend centrifuge (Sorval). Fresh medium was then applied and the place was placed back in incubator for another 3∼5 days at 37°C and 5% CO2. Transduction was determined by flow cytometry analysis of GFP-positive cells. For determination of transduction titer, serial dilutions of the viral supernatants were added into each well. The titer was determined by measuring GFP-positive cells in dilution ranges that resulted in a linear relationship between the number of transduction events and the volume of vector added.
Antibody competition and endosome neutralization
293T/CD20 cells (0.2×106) were pre-incubated with soluble anti-CD20 antibody (BD Bioscience) with the same clonal origin of the membrane-bound antibody used for targeting, followed by the usual transduction assay described above. The soluble isotype antibody was used as a control. For endosome neutralization, ammonium chloride (NH4Cl, dissolved in PBS, pH=7.4) was added into viral supernatants during transduction. Upon completion of spin-transduction, fresh medium was applied and cells were incubated for another 3 ∼ 5 days before flow cytometry analysis. .
Gamma-retroviral transduction of target cells in vivo
293T/CD20 cells (5×106) were xenografted into RAG2-/-γc-/- female mice (Taconic) of 6-8 weeks old by subcutaneous injection into their right flanks. After 8 hr, the concentrated gamma-retroviral vector bearing αCD20 and SM3 (MLG/ αCD20+SM3, 10×106 TU, MOI=2) was administered by subcutaneous injection on both sides of each mouse. As a non-targeting vector, VSVG-enveloped MLG vector (25×106 TU, MOI=5) was similarly injected to both sides of each mouse xenografted with 293T/CD20 on the right flank side. Several days later, the expression of the reporter firefly luciferase gene delivered by recombinant gamma-retroviral vectors was imaged. Mice were anesthetized in the presence of isoflurane and 1.5 mg of D-Luciferin (Xenogen) dissolved in 100 μL PBS was individually injected into the interperitonial cavity of the mice. Five minutes post-injection of the firefly luciferase substrate, the mice were imaged using a bioluminescence imaging system (Xenogen IVIS 200). Images were analyzed using Living Image 2.50.1 software. All injections were done using a 27G1/2 needle and 1 ml tuberculin slip tip syringe (BD Biosciences).
Acknowledgements
We thank April Tai for critical reading of the manuscript, and USC Norris Center Cell and Tissue Imaging Core. This work was supported by a National Institute of Health grant AI068978.
References
- Andrawiss M, Takeuchi Y, Hewlett L, Collins M. Murine leukemia virus particle assembly quantitated by fluorescence microscopy: role of Gag-Gag interactions and membrane association. J. Virol. 2003;77(21):11651–60. doi: 10.1128/JVI.77.21.11651-11660.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal M, Skelton D, Pepper KA, Jahn T, Methangkool E, Kohn DB. Effective suicide gene therapy for leukemia in a model of insertional oncogenesis in mice. Mol. Ther. 2007;15(1):183–92. doi: 10.1038/sj.mt.6300015. [DOI] [PubMed] [Google Scholar]
- Boerger AL, Snitkovsky S, Young JA. Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types. Proc. Natl. Acad. Sci. USA. 1999;96(17):9867–72. doi: 10.1073/pnas.96.17.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Hacein-Bey S, Basile CD, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288(5466):669–672. doi: 10.1126/science.288.5466.669. others. [DOI] [PubMed] [Google Scholar]
- Davis TA, Czerwinski DK, Levy R. Therapy of B-cell lymphoma with anti-CD20 antibodies can result in the loss of CD20 antigen expression. Clin Cancer Res. 1999;5(3):611–5. [PubMed] [Google Scholar]
- Dimitrov DS. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004;2(2):109–22. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Qian J-J, Yi S, Harding TC, Tu GH, VanRoey M, Jooss K. Stable antibody expression at therapeutic levels using the 2A peptide. Nat. Biotech. 2005;23(5):584–590. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- Fehse B, Roeder I. Insertional mutagenesis and clonal dominance: biological and statistical considerations. Gene Ther. 2008;15(2):143–53. doi: 10.1038/sj.gt.3303052. [DOI] [PubMed] [Google Scholar]
- Gibbons DL, Erk I, Reilly B, Navaza J, Kielian M, Rey FA, Lepault J. Visualization of the target-membrane-inserted fusion protein of Semliki Forest virus by combined electron microscopy and crystallography. Cell. 2003;114(5):573–83. doi: 10.1016/s0092-8674(03)00683-4. [DOI] [PubMed] [Google Scholar]
- Gibbons DL, Vaney MC, Roussel A, Vigouroux A, Reilly B, Lepault J, Kielian M, Rey FA. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature. 2004;427(6972):320–5. doi: 10.1038/nature02239. [DOI] [PubMed] [Google Scholar]
- Hawley RG, Lieu FH, Fong AZ, Hawley TS. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994;1(2):136–8. [PubMed] [Google Scholar]
- Hiddemann W, Buske C, Dreyling M, Weigert O, Lenz G, Forstpointner R, Nickenig C, Unterhalt M. Treatment strategies in follicular lymphomas: current status and future perspectives. J. Clin. Oncol. 2005;23(26):6394–9. doi: 10.1200/JCO.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Joo K-I, Wang P. Visualization of Targeted Transduction by Engineered Lentiviral Vectors. 2008 doi: 10.1038/gt.2008.87. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RA. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197(1):1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- Lavillette D, Russell SJ, Cosset FL. Retargeting gene delivery using surface-engineered retroviral vector particles. Curr. Opin. Biotechnol. 2001;12(5):461–6. doi: 10.1016/s0958-1669(00)00246-9. [DOI] [PubMed] [Google Scholar]
- Lin AH, Kasahara N, Wu W, Stripecke R, Empig CL, Anderson WF, Cannon PM. Receptor-specific targeting mediated by the coexpression of a targeted murine leukemia virus envelope protein and a binding-defective influenza hemagglutinin protein. Hum. Gene Ther. 2001;12(4):323–332. doi: 10.1089/10430340150503957. [DOI] [PubMed] [Google Scholar]
- Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA, Ando D, Urnov FD, Galli C, Gregory PD. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol. 2007;25(11):1298–306. doi: 10.1038/nbt1353. others. [DOI] [PubMed] [Google Scholar]
- Lu YE, Cassese T, Kielian M. The cholesterol requirement for sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J. Virol. 1999;73(5):4272–8. doi: 10.1128/jvi.73.5.4272-4278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Vodicka MA, Lucero G, Svitkina TM, Borisy GG, Emerman M, Hope TJ. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 2002;159:441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–9. doi: 10.1126/science.1129003. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K, Bristol G, Xie YM, Kung SKP, Chen ISY. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 2001;75(17):8016–8020. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizono K, Xie YM, Ringpis GE, Johnson M, Nassanian H, Lee B, Wu L, Chen ISY. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11(3):346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- Sandrin V, Russell SJ, Cosset FL. Targeting retroviral and lentiviral vectors. Curr. Top. Microbiol. Immunol. 2003;281:137–78. doi: 10.1007/978-3-642-19012-4_4. [DOI] [PubMed] [Google Scholar]
- Smit JM, Bittman R, Wilschut J. Low-pH-dependent fusion of Sindbis virus with receptor-free cholesterol- and sphingolipid-containing liposomes. J. Virol. 1999;73(10):8476–84. doi: 10.1128/jvi.73.10.8476-8484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitkovsky S, Young JA. Cell-specific viral targeting mediated by a soluble retroviral receptor-ligand fusion protein. Proc. Natl. Acad. Sci. USA. 1998;95(12):7063–8. doi: 10.1073/pnas.95.12.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somia N, Verma IM. Gene therapy: trials and tribulations. Nat. Rev. Genet. 2000;1(2):91–9. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- Szymczak AL, Vignali DA. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin. Biol. Ther. 2005;5(5):627–38. doi: 10.1517/14712598.5.5.627. [DOI] [PubMed] [Google Scholar]
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat. Biotech. 2004;22(5):589–94. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 2007;8(8):573–87. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Bailey L, Baltimore D, Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc. Natl. Acad. Sci. USA. 2006;103(31):11479–84. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Qin XF, Baltimore D, Van Parijs L. Generation of functional antigen-specific T cells in defined genetic backgrounds by retrovirus-mediated expression of TCR cDNAs in hematopoietic precursor cells. Proc. Natl. Acad. Sci. USA. 2002;99(9):6204–9. doi: 10.1073/pnas.092154599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler L, Wang P. 2008 Manuscript in preparation. [Google Scholar]