Introduction
Gastric cancer is the second most common cause of cancer death worldwide [1]. Its estimated incidence in the United States for 2008 is 21,500 with a mortality of 10,880 [2]. Gastric cancer is comprised of two major types [1,3]: 1) intestinal, which is the more common variant and which has a strong association with environmental factors, inclusive of cigarette smoking, dietary factors (particularly salted foods), and Helicobacter pylori [1]; 2) and diffuse gastric cancer (DGC), which is less common than the intestinal type but is more likely to be attributed to host factor effects [3].
Hereditary diffuse gastric cancer (HDGC) was initially described in 1964 in three Maori families from New Zealand [4]. It is an autosomal dominantly inherited syndrome attributed to mutations of the E-cadherin gene (CDH1, epithelial cadherin, OMIM#19209), identified by Guilford et al.[5] in 1998 in members of these Maori families. Approximately 40% of well-defined HDGC families may be found to harbor this mutation [6]. Women carrying the mutation also have an increased lifetime risk of lobular carcinoma of the breast [7,8].
Knowledge of the CDH1 mutation carrier status of a patient provides a level of certainty of DGC expression limited only by its reduced penetrance, which is estimated to be in the range of 70% [6,9,10]; this, along with its variable age of DGC onset, then becomes a factor of extreme importance in patients’ decision-making process when being counseled about the decision for prophylactic total gastrectomy [9]. Women carrying the mutation also have an increased lifetime risk of lobular carcinoma of the breast [7,8].
There is a wide range of variability in age of onset within and between HDGC families, a factor which may impact heavily upon the age when prophylactic total gastrectomy should be given serious consideration. In our experience, the average age of onset of HDGC has been 38 years, but it may range from 16 to 82 years [6]. Such variation in age of onset of DGC poses a challenge to genetic counselors as well as clinical geneticists when discussing genetic testing in concert with the timing for prophylactic total gastrectomy. Complex medical, ethical, and psychological, as well as medicolegal ramifications (e.g., the level of the patient’s understanding) may impact heavily upon decisions for both testing and surgery [11].
The identification of unaffected CDH1 mutation carriers
The identification of families with HDGC is usually prompted by a case of DGC occurring in an individual less than 50 years of age. In 1999, the International Gastric Cancer Linkage Consortium proposed the following criteria for identifying families for CDH1 genetic testing: “(1) two or more documented cases of diffuse gastric cancer in first/second degree relatives, with at least one diagnosed before the age of 50, or (2) three or more cases of documented diffuse gastric cancer in first/second degree relatives, independently of age of onset” [12]. An estimated mutation detection rate of 25% was suggested for such families [12]. The HDGC research program from the British Columbia Cancer Agency (DH) has modified the criteria to make them easier to use in environments where getting pathologic confirmation on multiple families is difficult. All criteria with an expected mutation detection rate are shown in Table 1. The common criteria for identifying mutation positive families are two or more cases of gastric cancer, with at least one DGC diagnosed before the age of 50 [6,8,13,14]. We have found mutations in 46% (38/83) of families meeting these criteria (D.H., unpublished data, 2008). These mutations include nonsense, frame-shift, splicing and missense mutations, and we are currently searching for nontraditional mutations that could account for some of the mutation negative cases. Although these testing criteria are readily applicable to North America or northern Europe, they would lead to a much lower mutation detection rate if used in populations with a higher background incidence of gastric cancer. After the family history has been confirmed and pretest genetic counseling has been given, genetic testing can be undertaken. A blood sample from an affected member of a prospective kindred is also the best substrate for index testing. If that is not available, testing an obligate carrier is a reasonable alternative. Often, as this disease is rapidly lethal, no living affected individuals are available for testing. The options remaining include testing DNA extracted from archival paraffin blocks from an affected family member, or testing unaffected, first-degree relatives. The former option is problematic as the DNA quality is often not sufficient for easy screening of a whole gene. Such blocks can be used to confirm mutations identified in unaffected at-risk relatives.
Table 1.
Testing Criteria
| Modified Testing Criteria* |
|
| Potential Additional Criteria |
|
Percentage of expected positive results based on the experience of the BCCA HDGC program
After the ascertainment of the proband’s DNA sample, primary screening can be accomplished using two different approaches, namely, mutation scanning with a method such as denaturing high performance liquid chromatography (DHPLC) [13] or direct sequencing [15]. As DHLPC can be used only as an initial screening method, samples that exhibit aberrant DHPLC chromatograms must still undergo direct sequencing to identify the exact mutation. Once a family mutation has been identified, it is simple to set up a mutation-specific assay to assess the mutation status of family members [6].
Inadequacies of DGC Screening
The medical, pathology, and surgical literature shows an extraordinarily high rate of metastasis of DGC with frequent mortality when symptoms appear [16,17]. Knowledge of HDGC’s natural history has, consequently, led to a recommendation for the option of prophylactic total gastrectomy among CDH1 germline mutation carriers in order to reduce its cancer morbidity and mortality [18]. The major clinical problem involving HDGC patients who are CDH1 mutation carriers, and which mandates consideration of prophylactic total gastrectomy, is the lack of effective DGC screening in pursuit of an early, life-saving diagnosis. This problem is a consequence of the submucosal expression of the signet cell cancer pathology in DGC, which severely limits ability to detect DGC sufficiently early in its clinical-pathology course to provide lifesaving benefit to the high-risk CDH1 mutation carrier. Given this screening deficit, surgical extirpation of the entire stomach before DGC metastasis takes place appears at this time to be the only way to achieve potential curative benefit to the patient.
Lewis et al. [17] were among the first investigators to recommend prophylactic total gastrectomy for patients with HDGC and the CDH1 mutation. This recommendation was based upon the finding of occult DGC in the prophylactic gastrectomy samples of six asymptomatic members of two families: two males and four females, with ages ranging from 22 to 40 [19]. The prophylactic surgical procedure involved total gastrectomy using an upper midline incision with reconstruction of the gastrointestinal tract via a Roux-en-Y esophago-jejunostomy. The complete removal of all gastric mucosa intraoperatively was documented and therein it was confirmed that only esophageal mucosa remained at the proximal specimen margin. Each of the CDH1-positive patients underwent 150 to 250 tissue block examinations and therein all showed microscopic foci of cancer, frequently at multiple sites. Importantly, the overlying normal gastric mucosa was identified. It was concluded that, “… familial gastric cancer is a new disease for which prophylactic surgery must be considered. The morbidity of this operation is much higher than that for other genetic diseases, but the alternative is a mortality risk of more than 80% at a young age.”
Subsequently, Norton et al. [16] studied a large HDGC family containing the CDH1 cancer-causing mutation. Six of the patients were identified as CDH1 mutation carriers (two men and four women) with a mean age of 54 years (range 51–57 years), wherein each underwent comprehensive cancer screening which included stool occult blood testing, standard upper gastrointestinal endoscopy with random gastric biopsies, high magnification endoscopy, endoscopic ultrasonography, CT, and PET scans for evaluation of the stomach for occult cancer. This was followed by “… total gastrectomy with D-2 node dissection and Roux-en-y esophagojejunostomy. The stomach and resected lymph nodes were evaluated pathologically.”
None of these individuals showed signs or symptoms of gastric cancer. However, while preoperative gastric findings were normal in each patient, with normal gastric and adjacent lymph nodes at surgery, nevertheless, “… each patient (6 of 6, 100%) was found to have multiple foci of T1 invasive diffuse gastric adenocarcinoma (pure signet-ring cell type). No patient had lymph node or distant metastases. Each was staged as T1N0M0. Each patient recovered uneventfully without morbidity or mortality.” Importantly, the presence of the mutation identified patients manifesting cancer prior to detectable symptoms or signs of HDGC. These six unaffected mutation carriers came from an extended family in which 11 first cousins underwent prophylactic gastrectomy. In this family, the prophylactic surgical decision was eased in a major way for these cousins, each of whom harbored the CDH1 mutation, in that the majority had witnessed a parent die as a result of the progressive course of DGC. When told that there was an approximate 30% chance that they would not develop DGC, due to its reduced penetrance, this temporarily factored negatively into some of their decision making, as evidenced by such a statement as, “If they can beat it, why can’t I?” Others reasoned that this knowledge could help them delay the decision for surgery. Knowing, however, that they harbored the deleterious mutation, they knew that they had to resolve these cancer probabilities, which they clearly realized mathematically favored their eventual development of DGC. They also learned through our educational genetic counseling program and our family information service (FIS) [20], that available DGC screening procedures were wholly inadequate [21].
The DGCs detected in these and other prophylactic gastrectomies have been almost invariably minute with many measuring less than 1mm in diameter [22]. As the cancers are both minute and underlying normal gastric mucosa, their invisibility to endoscopy is understandable. The rate of progression of the microscopic lesions to metastatic and thus potentially lethal DGCs is not known and it is possible that some of these early DGC lesions could be indolent. The natural history and biology of these lesions is an area of active study [23]. Even if some of the microscopic DGCs detected in these gastrectomies have little metastatic potential, this should not affect decision making as the penetrance for invasive, clinically relevant gastric cancer in mutation carriers is 70%.
Foremost on the positive side of their decision-making process was the psychological support they provided to each other in the sense of a “group therapy” family commitment to help each other [20]. A very strong sense of solidarity emerged among these cousins about the importance of prophylactic gastrectomy; this influence of camaraderie became pervasive throughout our genetic counseling sessions dealing with these high-risk individuals [24].
Once armed with facts about the natural history of DGC, many family members told us that, in essence, the only decision left to them was when the prophylactic gastrectomy should be performed. Knowing that their prognosis would become extremely grave once DGC symptoms became manifest, all 11 of these first cousins decided to undergo prophylactic surgery, most within a couple of years of receiving their positive CDH1 mutation results [24]. Ten of the eleven manifested submucosal foci of DGC in their stomach specimens.
Newfoundland Experience with Prophylactic Surgery in HDGC
Perhaps the largest experience with total prophylactic gastrectomy in CDH1 mutation carriers from HDGC families performed at a single center, has taken place in Newfoundland (DAW and PCH). Several of the families with CDH1 mutations in that Canadian province have been previously documented [6]. To date, 17 affected individuals from these families have opted for prophylactic total gastrectomy. Ten patients were male and seven were female; average age was 45 years.
Preoperative evaluation
The process of considering prophylactic total gastrectomy (PTG) began with patients’ assessment in a medical genetics clinic. After appropriate counseling and confirmatory genetic testing, patients wishing to consider prophylactic gastrectomy were referred to one of two fellowship-trained surgical oncologists. The risks and benefits of surgery were discussed in detail. Patients must understand the potential complications including hemorrhage, anastomotic leak and/or stricture, cardiopulmonary concerns and the low, but present, risk of death that this procedure carries. With the assistance of a dietician, they were counseled on a post-gastrectomy diet, expected weight loss and potential metabolic consequences including B12, iron, thiamine and zinc deficiencies.
Pre-operative medical work-up began with a detailed history and physical examination with attention to any medical co-morbidities. All patients to date have been in good health. Patients then underwent esophagogastroduodenoscopy (EGD) with at least 15 random biopsies of the antrum, incisura, fundus and body, given that there have been no gross mucosal abnormalities noted. Delineation of the gastroesophageal junction and presence/absence of hiatal herniation was also noted. Random biopsies revealed a microscopic foci of adenocarcinoma in one patient. Fourteen patients had colonoscopy and pre-operative abdominal CT scans; none had evidence of malignancy. Patients were then sent to the pre-admission clinic for anesthesia consultation and routine pre-operative labwork.
Operative details
All patients were admitted to hospital on the day of surgery. The average surgical time was 244 minutes (range 120 – 580 minutes). Estimated blood loss was 1000 mL (range 400 – 3000 mL), with three patients requiring blood transfusion of 1–2 units of red blood cells. One patient had gastric mucosa that extended into the chest on frozen section evaluation of the proximal gastric margin. This required conversion to a thoraco-abdominal approach. One patient had an intra-operative complication, requiring right hemicolectomy for a devascularized colon. Post-operatively, 15 patients went to the general surgical floor and 2 went to the intensive care unit.
Post-operative details
Median length of hospital stay was 13 days (mean 25 days, range 10 – 107 days). There were no peri-operative deaths and no cardiovascular complications.
There were two anastomotic leaks with intra-abdominal abscesses. Both patients required operative drainage for definitive treatment. Three other patients had subclinical anastomotic leaks found on routine gastrograffin swallow performed on or about post-operative day 7. All were treated conservatively by maintaining their NPO status, with J-tube supplementation for 5 – 15 additional days.
One patient was re-admitted to hospital on post-operative day 19 with an intra-abdominal abscess from an infected hematoma. This patient was treated with percutaneous drainage of the abscess and antibiotics. He was discharged 6 days later.
One patient had a prolonged small bowel obstruction and returned to the OR on post-operative day 21. An internal hernia was found and repaired.
Finally, three patients had pulmonary emboli despite prophylactic heparin. One of these patients also had splenic infarction secondary to splenic vein thrombosis. All three patients were from the same family. Standard hypercoagulopathy screen was negative, although a familial predisposition for clotting is suspected.
Discussion
The stomach is a heavily stereotyped organ, which is impacted by numerous emotional and cultural overtones. For example, in some cultures, happiness is attributed in a major way by food offerings, and thereby its contribution to a “full stomach.” In contrast, an “empty stomach,” where people have experienced hunger in the presence of food shortages and, in the extreme, famine, with accompanying “gnawing pains in the stomach,” calls attention to this unfavorable side of the “empty stomach.”
The prophylactic gastrectomy decision may become particularly troublesome when patients are completely asymptomatic; under those circumstances, they may become bewildered and wonder why there is a need to undergo this prophylactic surgical procedure. Such a surgical decision dilemma may exist when patients have been fully informed about the long-term sequelae of DGC; but their anxiety and apprehension may be alleviated to a variable extent by knowing of family members who have adjusted to the morbidity of prophylactic gastrectomy but, not surprisingly, it may be extremely heightened by learning about those who have not done as well following surgery.
A special problem may be attributable to the known reduced penetrance of the CDH1 mutation when the patient is aware of one or more close relatives who are known to be CDH1 mutation carriers, but yet have never been symptomatic and may be thriving in their 70s or 80s, having been completely spared of any evidence of DGC. Their CDH1 mutation carrier brethren may then wonder whether they themselves will remain forever cancer free due to reduced penetrance of this mutation. They may also wonder about possible interaction with unknown environmental protective factors should they forego prophylactic gastrectomy. Clearly, these concerns will require intensive empathetic counseling by the surgeon.
Genetic Counseling
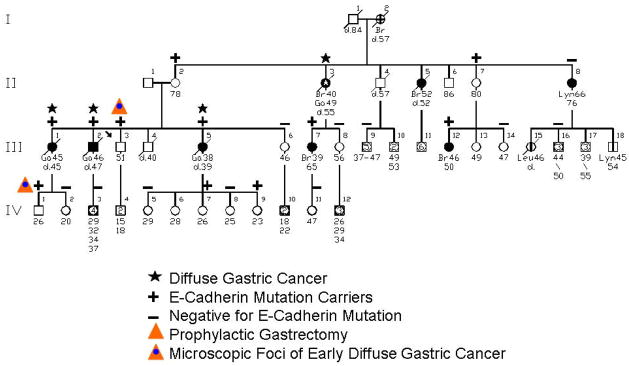
The first experience with genetic counseling for HDGC in concert with the CDH1 mutation involved a family (see Figure 1) wherein the proband (III-3 on Figure 1) consulted us because of his intense concern about the fact that three of his siblings died of DGC within a timeframe of only 18 months [21]. However, he became extremely well informed about the natural history of HDGC in general and his family in particular. Following lengthy discussions about the option of prophylactic gastrectomy, he continued to undergo periodic gastroduodenoscopy screening by his gastroenterologist. Contact was kept with him through telephone communication every six months over a period of three years, during which time he was reminded of the option of prophylactic gastrectomy. He slowly began considering this option, decided that this procedure was the right one for him, and he underwent the procedure. His surgical specimen showed no visible evidence of cancer (Figure 2), and he recovered uneventfully. However, the gastric pathology showed significant submucosal involvement of DGC (Figure 3). There was no evidence of regional or distal spread of DGC post surgically nor in the five years following this procedure, and he continues to do extremely well. Indeed, he became a strong spokesman for a repeat FIS in the interest of educating, testing, making appropriate recommendations for prophylactic gastrectomy to members of his family, and we obliged. The first family member to follow this advice for prophylactic gastrectomy was the proband’s nephew (IV-1 on Figure 1), who upon gastrectomy did show microscopic foci of DGC in the absence of local or regional spread.
Figure 1.
Pedigree of family harboring a CDH1 (E-cadherin) germline mutation. CSU = cancer site unknown; Lym = lymphoma; St = stomach cancer; Sk = skin cancer; Br = breast cancer. [Updated with permission from “Gastric Cancer: New Genetic Developments,” Lynch H, Grady W, Suriano G, Huntsman D. Journal of Surgical Oncology (Seminars) 90(3):114–133. Copyright © 2005 Wiley-Liss, Inc., A Wiley Company. All rights reserved]
Figure 2.
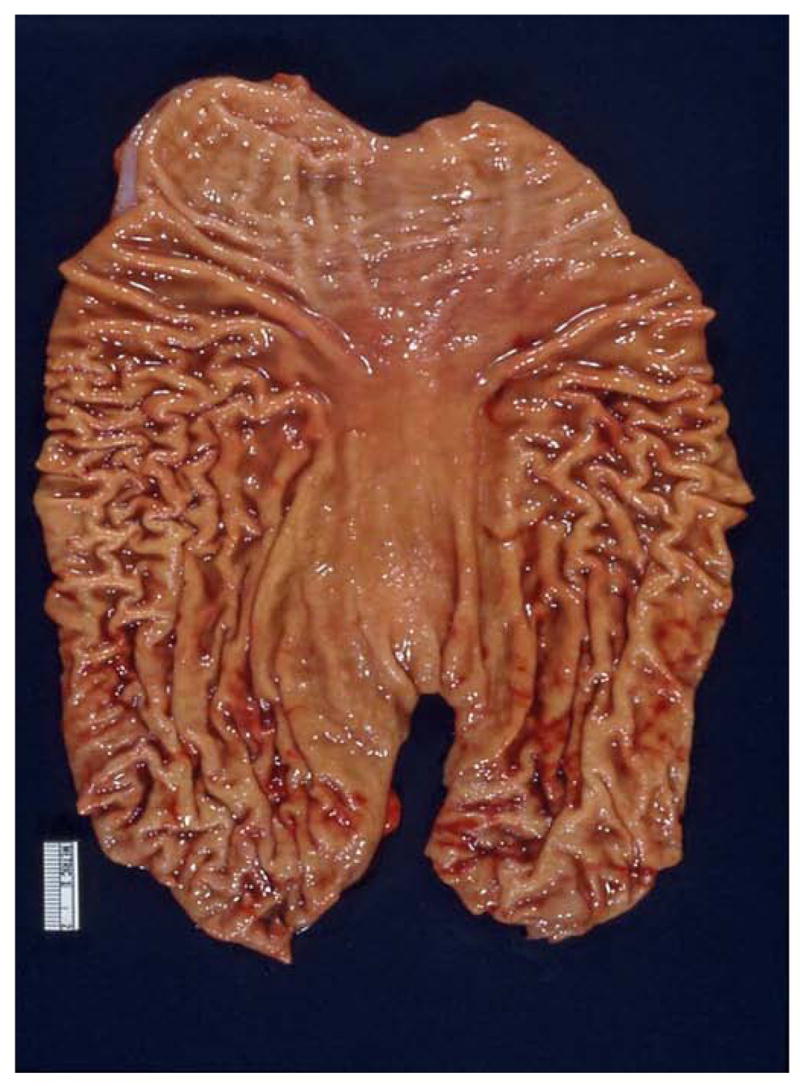
Photograph of the prophylactic gastrectomy specimen from the proband of the family shown in Figure 1, showing no visible abnormalities in the gastric mucosa. [Republished with permission from “Gastric Cancer: New Genetic Developments,” Lynch H, Grady W, Suriano G, Huntsman D. Journal of Surgical Oncology (Seminars) 90(3):114–133. Copyright © 2005 Wiley-Liss, Inc., A Wiley Company. All rights reserved]
Figure 3.
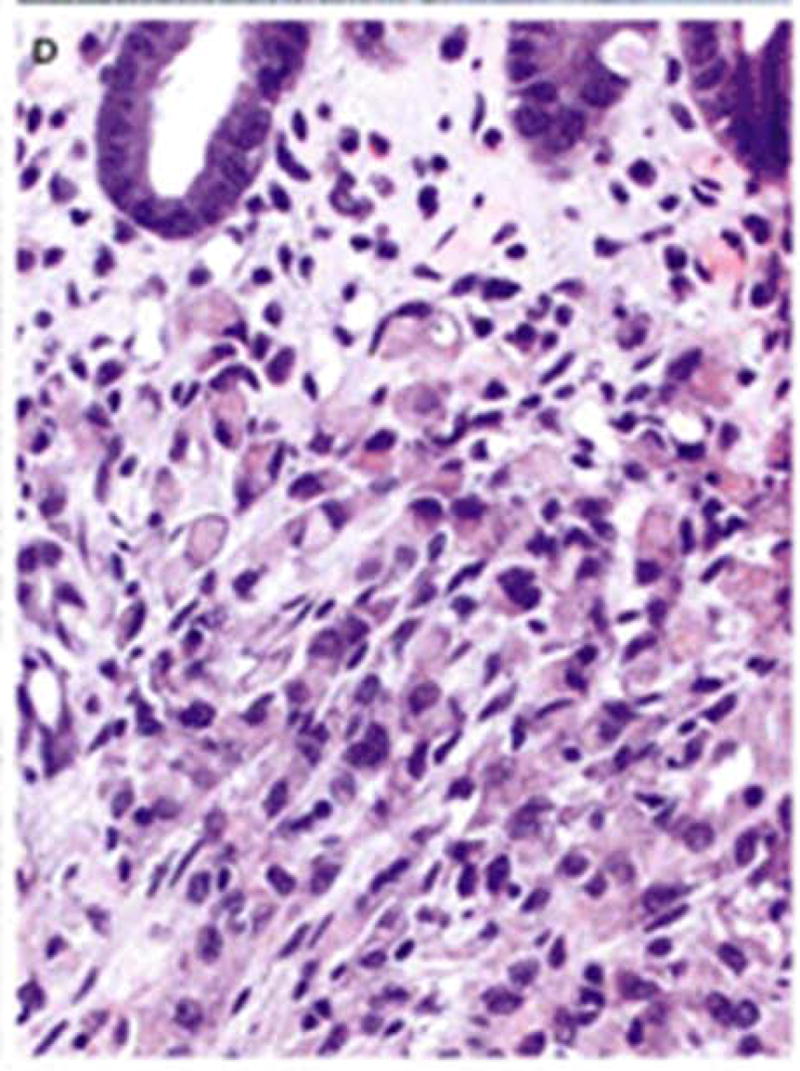
Photomicrograph of a focus of occult diffuse gastric cancer from the specimen shown in Figure 2. The cancer was stage 1A and the gastrectomy was presumed curative. [Republished with permission from “Gastric Cancer: New Genetic Developments,” Lynch H, Grady W, Suriano G, Huntsman D. Journal of Surgical Oncology (Seminars) 90(3):114–133. Copyright © 2005 Wiley-Liss, Inc., A Wiley Company. All rights reserved]
Because of evidence of DGC as early as the age of 14 by Gayther et al.[25], we tested a 16-year-old female family member who, unfortunately, turned out to be positive for the CDH1 mutation. She had a strong emotional response, cried uncontrollably, and expressed her concern as to whether she would ever be able to have children; she immediately wanted to know more about prophylactic gastrectomy. We then advised her that the penetrance of the mutation is not complete, but we also let her know that she was still a candidate for prophylactic gastrectomy and that this would be an option that she could consider, perhaps later in life once she married and had children.
Another relative had been under severe stress with insomnia for at least a week prior to DNA test disclosure, fearing that she would be positive for the mutation. When told that she did not inherit the mutation, she became greatly relieved.
Prophylactic Total Gastrectomy: Benefits and Complications
Prophylactic surgical procedures carry a known risk of complications, but this risk should be weighed against the risk of performing the same surgery in the future on a patient who has become debilitated or compromised. If the screening criteria are very sensitive they can be used to proceed with prophylactic surgery in the healthy patient with acceptable morbidity and mortality, particularly when the potential for curative intervention is negligible once the patient becomes symptomatic from the cancer. Of all the potential malignant disorders amenable to preventive screening and surgical prophylaxis, gastric cancer has emerged recently [26] as the one in which therapeutic intervention at the time of diagnosis in a symptomatic patient is still accompanied by the worst and nearly universally fatal outcome. Though less virulent cancers, a similar argument for early surgical prophylaxis can be made for ovarian cancer [27], medullary carcinoma of the thyroid [28], colon cancer in familial adenomatous polyposis (FAP) and Lynch syndrome [10,29,30], as well as breast cancer in BRCA mutation carriers [31–35]. All of these have proportionately (as listed) increasing potential for survival through therapeutic advances, including adjuvant systemic therapy. (See Guillem et al. [36] for a review.)
Of all of these hereditary cancer disorders, perhaps none has yielded molecular screening methodology as sensitive as that for the RET mutation, which predisposes to medullary thyroid cancer (MCT) in multiple endocrine neoplasia (MEN) kindreds [28]. With studies documenting a 93% incidence of MTC in RET positive members of MEN kindreds, the decision to undergo a prophylactic, relatively low-risk procedure such as total thyroidectomy is less difficult. Reports showing an increased survival advantage in RET mutation carriers undergoing prophylactic total thyroidectomy well before age 8 [28] permit the completion of the operation in a clinically normal neck thus decreasing the morbidity associated with the same operation in the affected patient in whom the cancer may be fixed to vital structures (recurrent laryngeal nerve, trachea) in the operative field.
Recent advances in the molecular characterization of the CDH1 germline mutation (E-cadherin, epithelial cadherin) and its association with the onset of HDGC is reminiscent of the RET/MTC story [5,6,37,38]. CDH1 germline mutations have approximately a 70% penetrance and the resulting HDGC phenotype for CDH1 mutation carriers has an almost 100% mortality when discovered in the symptomatic patient. As with ovarian cancer in the BRCA carrier, current screening modalities for HDGC in the CDH1 mutation carrier are very poor. Given that HDGC has a higher penetrance than does ovarian cancer when the respective mutations are present, that HDGC has a higher mortality when symptomatic, that there is currently no reliable systemic chemotherapy for symptomatic HDGC patients, it clearly stands to reason that curative surgical intervention should be adopted for the asymptomatic CDH1 carrier.
Although current understanding of the relationship between CDH1 and HDGC is more vexing than in the example of MEN2, both syndromes raise similar questions regarding prophylactic surgery: 1) At what age should surgical intervention occur? 2) What is the associated mortality and morbidity of prophylactic total gastrectomy? 3) Are there any curative options for the symptomatic HDGC patient with a known CDH1 mutation?
Similar questions can also be proposed for the management of colon cancer [30] and breast cancer [34,35] in known FAP or hereditary breast-ovarian cancer (HBOC) germline mutation carriers for whom many additional effective adjuvant systemic therapies exist to treat the patient in the post operative setting. However, such options are absent in the case of the symptomatic HDGC patient and therefore what remains is a straight forward assessment of the risk and benefit of an operation, namely, total gastrectomy and reconstruction, which understandably has a more serious mortality and morbidity profile than any of the prophylactic surgical procedures contemplated by a patient in a MEN2, HBOC, or FAP cancer-prone pedigree.
Prophylactic Total Gastrectomy: Surgical Complications
In the absence of complications associated with all gastrointestinal elective surgical resections, such as bleeding, infection, and anesthetic misadventures, the most significant complication of a prophylactic total gastrectomy results from a potential leak at the single critical anastomosis, the esophago-jejunostomy. Randomized controlled trials have shown that the incidence of an anastomotic leak, stenosis, morbidity and length of hospitalization are not statistically different when a stapled versus a hand-sewn anastomosis is performed [39,40]. A recent report from Japan in patients with cancer showed a leak rate of 0.5% (2/390) [41]. Overall, mortality in 14 controlled randomized trials of gastric pouch reconstruction after total gastrectomy ranges from 0% to 22% [42]. However, the great majority of these trials show mortality figures less than 4%. The latter figure should be expected of any team of surgical oncologists and other specialists attending to the management of the patient with HDGC.
Morbidity estimates for total gastrectomy interposition (TGI) and reconstruction are more germane to our discussion given the nearly 100% mortality of the untreated HDGC patient in whom a 0% to 4% intra-operative mortality can be quoted, although 2% should be a more reasonable estimate for a trained surgical oncologist. This is not an exaggerated figure given that all of the documented CDH1 carriers are likely to be young and otherwise healthy candidates unlike the physiologically and nutritionally compromised patient from the general population with gastric cancer from whom the above quoted mortality and morbidity figures are derived. In patients undergoing total gastrectomy with reconstruction for cancer, morbidity estimates of 60% are alarming [42]. It is likely that these numbers would be lessened in the younger, healthy asymptomatic CDH1 mutation carrier treated by an experienced surgeon. Nevertheless, the scope of the problems responsible for the reported morbidity rates remains the same in the CDH1 carrier.
The surgical oncologist, genetic counselor, and nutrition team need to fully discuss with the patient all of these factors, as well as the reported mortality data, in preoperative conferences. The morbidity of a TGI and reconstruction and its impact on the quality of life (QOL) encompass several areas affecting mechanical and metabolic consequences of the operation. Absent the stomach, a patient can expect a decrease in eating capacity at one sitting as well as increased transit time due to the absence of the pyloric sphincter. Although historically many bemoan the malnutrition seen after TGI, it is now understood that the potential accompanying malnutrition can, in fact, be corrected or prevented by a measured increase in daily caloric intake [43]. Other expected metabolic deficits thought to be derived from a decrease in vitamin B12 absorption, malabsorption of protein, and bacterial overgrowth due to loss of parietal and chief cells of the stomach, appear to be less serious and can be corrected by modest lifestyle adjustments [44]. More significant QOL problems such as reflux, dumping, and weight loss are known to persist. Attempts to remedy these problems account for the large number of proposed reconstructive procedures performed to reestablish intestinal continuity after a prophylactic total gastrectomy. Many technical variations for interposition have been reported and none appear to be universally better than the traditional R-Y esophago-jejunostomy in eliminating all of the QOL issues mentioned above [45].
Nearly all of the variations in reconstructive techniques involve the use and the length of a side to side stapled jejunal pouch in the manner of a Hunt-Lawrence interposition [45]. These approaches were enacted under the proposition that loss of the gastric reservoir is responsible for most of the postoperative problems with weight loss and malnutrition seen after total gastrectomy. Due to the fact that nearly all of the randomized trials comparing total gastrectomy with and without a pouch for interposition did not account for simple variations in total caloric intake after surgery, and relied primarily on the patient’s perception of what they could eat before and after surgery, little can be concluded about the impact of the use of the pouch in restoring nutritional deficits. Even less can be reliably concluded about the impact of subtle changes in pouch length, length of the R-Y limb and inclusion or exclusion of the duodenum from the reconstructed alimentary limb when most of these randomized studies do not standardize the total caloric intake, or other more variable post-operative parameters such as exercise or level of physical activity. Still, among two studies with daily energy intake assessment, no advantage was noted when patients had a pouch interposition versus a straight R-Y esophago-jejunostomy [46,47]. This flaw may account for the observation that in these trials no significant differences were noted in QOL parameters or weight gain. Most of these studies suggest that pouch reconstruction may improve the ability of patients to eat in the immediate months after surgery. In most individuals the putative early benefit of the pouch becomes less apparent over time [42]. Recent data from Japan also suggests that a short 15cm. pouch may be more effective than a 20cm. pouch in controlling other QOL measures such as esophageal reflux [48]. The same group concluded that a short J-pouch although better at controlling reflux did not fare any better than standard R-Y esophago-jejunostomy in controlling dumping and other less frequent post-prandial complaints. Yet, in most cases and in agreement with current surgical approaches to minimizing bile reflux, a putative QOL benefit was seen in patients undergoing a short pouch interposition with the standard 40cm. distance between the esophago-jejunostomy and the R-Y jejuno-jejunostomy. Among those patients undergoing a prophylactic gastrectomy for HDGC it is important to note that the more elaborate the reconstruction the more potential exists for surgical complications. Although these are likely decreased by the good performance status of these individuals, other complications may result from additional surgical misadventures resulting from the performance of an extended lymphadenectomy (D2 node dissection) in the setting of a prophylactic gastrectomy.
Sentinel Node Mapping
It is true that HDGC has a significant propensity for nodal metastases and carcinomatosis. Yet, in the prophylactic setting these considerations may not warrant the additional surgical problems associated with a D2 lymphadenectomy. For example, the Dutch trial of limited D1 lymphadenectomy versus extended radical D2 lymphadenectomy as described by the Japanese led to marked increase in surgical morbidity and a three-fold increase in operative mortality (1.7% to 5.9%) with no improvement in survival.
Sentinel node mapping may seem an ideal alternative for the staging of nodal disease and an alternative to a D2 lymphadenectomy in the setting of a prophylactic total gastrectomy. It has been described using radiotracer with gamma probe localization in patients with T1N0 or T2N0 gastric cancer on preoperative staging [49,50]. The incidence of sentinel node metastases was much higher than that in non-sentinel node metastases (non-radioactive nodes). A sentinel node was found in 95.2% of the cases with metastases documented in 7.8% of the patients. Non-sentinel node metastases were seen in only 0.3% of the patients [49]. A similar report in comparably staged patients used blue dye and radionucleotide tracer by Hayashi demonstrated that a sentinel node could be identified in nearly 100% of the patients. The study included only 31 patients compared with the 145 patients in the previously quoted report by Kitagawa [50]. The use of two tracers is complimentary with some nodes appearing to be blue while others may appear to be radioactive or hot. Similar observations have been made in the use of sentinel node mapping for breast cancer and melanoma where the principle has shown exquisite sensitivity in identifying micro-metastases to otherwise normal appearing nodes initially examined by routine H&E but subsequently examined by step sections and RT-PCR technology. Thus, all nodes which are hot or blue are by definition considered to be sentinel nodes [51]. Perhaps, for this reason the combined use of radiotracer and blue dye by Hayashi revealed lymph node metastases in 7 of 31 (20%) patients studied with T1N0 or T2N0 gastric cancer compared to the 7.8% sentinel node positive rate in Kitagawa’s report with 145 patients of similar stage.
In these series, the smallest eligible patients had T1 lesions which were the target for the radiotracer injection. In the prophylactic setting a clear lesion is not often evident. Attempts at localization of any early abnormalities in the gastric wall with endoscopic ultrasonography or other sensitive radiologic approaches such as PET-CT may provide such a target area. Alternatively, before the application of SNM technology in this setting, concordance studies with injection of the radiotracer and blue dye may be necessary to delineate the potential drainage routes of tumors arising in different parts of the stomach. Similar studies in breast cancer patients ultimately disclosed that the lymphatic drainage of the entire breast ultimately led to the same sentinel nodes regardless of the location of the primary cancer or site of injection of the radiotracer or vital blue dye [52,53].
The potential need for any lymphadenectomy more extensive than a D1 procedure can be inferred from the Japanese data documenting the frequency of nodal metastases in early gastric cancer treated with D2 procedures. The frequency of nodal metastases for patients with gastric cancer limited to the gastric mucosa is 5% while some 16% of patients with submucosal involvement had nodal metastases [50]. Therefore, in the setting of a prophylactic gastrectomy for HDGC the finding of nodal metastases by sentinel node mapping would potentially convert a preventive procedure into a therapeutic procedure in a small number of cases if nodal disease could be ascertained intra-operatively. Otherwise, the above rationale suggests that a D1 lymphadenectomy would suffice in this scenario. Obviously, should intra-operative sentinel node assessment, the standard of care in breast cancer management today, not be available for gastric cancer patients then the standard of care for the patient with a known CDH1 mutation who elects to undergo a prophylactic gastrectomy should be a D1 lymphadenectomy. It would provide the putative therapeutic advantage of a lymphadenectomy without incurring the additional complications with a D2 lymphadenectomy as described by the Dutch trial [26]. Patients with positive nodes would then be eligible for post-operative adjuvant chemo-radiation as described by Macdonald [54] or for participation in clinical trials.
Recently, detection of subclinical carcinomatosis in diffuse gastric cancer patients was reported by Kodera et al. [55] using peritoneal washings. Using RT-PCR probes to detect CEA mRNA in the washings, 80% of patients were found to have disseminated disease in the peritoneal cavity [55]. However, it would be very unlikely that asymptomatic patients undergoing a prophylactic gastrectomy for HDGC would have positive peritoneal washings. And even if they did the situation would be analogous to that of positive peritoneal washings in pancreatic cancer [51,56] where, despite positive cytology, patients who were resectable underwent a pancreatico-duodenectomy with curative intent. The presence of positive peritoneal washings in these patients was found to be a surrogate for early recurrence and marked these patients as candidates for aggressive adjuvant systemic therapy. Similar findings in prophylactic gastrectomy could make these patients eligible for clinical trials.
In the typical patient from a HDGC pedigree, who is found to be CDH1 positive, management of the gastric cancer risk is most pressing. As mentioned, the average age of onset in studied pedigrees is 38 years; however, afflicted CDH1 carriers from age 14 to 82 have been described [6]. Significantly, penetrance estimates reported for HDGC suggest that the risk of symptomatic HDGC arising in any individual carrier is 1% by age 20 [10]. The risk increases with advancing age and may be inferred from the age of the youngest affected member of a known HDGC pedigree.
Guilford and co-authors [37] suggest that once a known carrier is identified a prophylactic total gastrectomy should be performed if the individual is older than 20. At this age and beyond, the 1% quoted mortality for the operation is exceeded by the risk of development of the fatal HDGC phenotype.
Lobular Carcinoma of the Breast in HDGC Families
In known female CDH1 carriers the DGC risk in HDGC should be addressed first. However, these women remain at risk for developing invasive lobular carcinoma of the breast [7,8]. Although, penetrance estimates vary, current estimates as high as 39–52% have been reported [6]. Unfortunately, patient screening in this setting may be hindered by the same limitations that plague screening modalities in the hereditary breast and ovarian cancer syndrome (HBOC) [34]. Invasive lobular carcinoma is frequently missed by routine mammographic surveillance. Patients with very large tumors, even in older women whose breast density should be favorable for screening, can present with normal mammograms. These lesions can be spotted more readily with MRI imaging [57]. In patients under age 40 with mammographic exams of poor informative value, screening with MRI is preferable. Conversely, worrisome breast cancer pedigrees with an affected surviving patient afflicted with a lobular carcinoma of the breast should be tested for the CDH1 mutation if they test negative for a BRCA mutation. As in any of the hereditary breast cancer syndromes, only those patients who test negative for the known mutation (in this case, CDH1) co-inherited with the known malignant phenotype affecting their kindred can be excluded from further surveillance or prophylactic surgical intervention. In CDH1 afflicted pedigrees, prophylactic bilateral mastectomy, risk reduction strategies using tamoxifen, or surveillance every 6 months with MRI alternating with breast ultrasonography, may be reasonable approaches depending on the age of the patient, after addressing the more virulent HDGC risk. Management strategies and algorithms for care of the high risk pedigree patient with a recently diagnosed breast cancer have been reported by our group recently [33–35]. Our algorithm for management of these patients has been endorsed as the best approach for the management of the breast cancer patient at risk for carrying a germline mutation for breast cancer [58].
Future Directions
Although prophylactic gastrectomy may be the most prudent option for today’s adult unaffected mutation carriers, it is likely that through improvements in endoscopic and imaging modalities [59,60] other options will be available for their children. In addition, through the study of the early gastric cancers that have been detected in prophylactic gastrectomy specimens, biomarkers suitable for early detection may be identified [23].
In summary, it is clear that our current knowledge of molecular and genetic diagnostics calls for the addition of HDGC to the roster of malignant familial syndromes in which early counseling and preventive surgical intervention should become the standard of care.
Acknowledgments
Funding Support
This paper was supported by revenue from Nebraska cigarette taxes awarded to Creighton University by the Nebraska Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the State of Nebraska or the Nebraska Department of Health and Human Services.
Support was also given by the National Institutes of Health through grant #1U01 CA 86389. Dr. Henry Lynch’s work is partially funded through the Charles F. and Mary C. Heider Chair in Cancer Research, which he holds at Creighton University. Dr. David Huntsman’s work is funded by the Canadian Cancer Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Alberts SR, Cervantes A, van De Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14(Suppl 2):ii31–ii36. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 4.Jones EG. Familial gastric cancer. NZ Med J. 1964;63:287–96. [PubMed] [Google Scholar]
- 5.Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–5. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 6.Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA. 2007;297:2360–72. doi: 10.1001/jama.297.21.2360. [DOI] [PubMed] [Google Scholar]
- 7.Keller G, Vogelsang H, Becker I, et al. Diffuse type gastric and lobular breast carcinoma in a familial gastric cancer patient with an E-cadherin germline mutation. Am J Pathol. 1999;155:337–42. doi: 10.1016/S0002-9440(10)65129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrader KA, Masciari S, Boyd N, et al. Hereditary diffuse gastric cancer: association with lobular breast cancer. Fam Cancer. 2008;7:73–82. doi: 10.1007/s10689-007-9172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suriano G, Oliveira C, Ferreira P, et al. Identification of CDH1 germline missense mutations associated with functional inactivation of the E-cadherin protein in young gastric cancer probands. Hum Mol Genet. 2003;12:575–82. doi: 10.1093/hmg/ddg048. [DOI] [PubMed] [Google Scholar]
- 10.Pharoah PDP, Guilford P, Caldas C, et al. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348–53. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald DJ, Lessick M. Hereditary cancers in children and ethical and psychosocial implications. J Pediatr Nurs. 2000;15:217–25. doi: 10.1053/jpdn.2000.8044. [DOI] [PubMed] [Google Scholar]
- 12.Caldas C, Carneiro F, Lynch HT, et al. Familial gastric cancer: overview and guidelines for management. J Med Genet. 1999;36:873–80. [PMC free article] [PubMed] [Google Scholar]
- 13.Suriano G, Yew S, Ferreira P, et al. Characterization of a recurrent germ line mutation of the E-cadherin gene: implications for genetic testing and clinical management. Clin Cancer Res. 2005;11:5401–9. doi: 10.1158/1078-0432.CCR-05-0247. [DOI] [PubMed] [Google Scholar]
- 14.Brooks-Wilson AR, Kaurah P, Suriano G, et al. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet. 2004;41:508–17. doi: 10.1136/jmg.2004.018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullins F, Dietz L, Lay M, et al. Identification of an intronic single nucleotide polymorphism leading to allele dropout during validation of a CDH1 sequencing assay: implications for designing polymerase chain reaction-based assays. Genet Med. 2007;9:752–60. doi: 10.1097/gim.0b013e318159a369. [DOI] [PubMed] [Google Scholar]
- 16.Norton JA, Ham CM, Van Dam J, et al. CDH1 truncating mutations in the E-cadherin gene: an indication for total gastrectomy to treat hereditary diffuse gastric cancer. Ann Surg. 2007;245:873–9. doi: 10.1097/01.sla.0000254370.29893.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis FR, Mellinger JD, Hayashi A, et al. Prophylactic total gastrectomy for familial gastric cancer. Surgery. 2001;130:612–7. doi: 10.1067/msy.2001.117099. [DOI] [PubMed] [Google Scholar]
- 18.Bacani JT, Soares M, Zwingerman R, et al. CDH1/E-cadherin germline mutations in early onset gastric cancer. J Med Genet. 2006;43:867–72. doi: 10.1136/jmg.2006.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huntsman DG, Carneiro F, Lewis FR, et al. Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N Engl J Med. 2001;344:1904–9. doi: 10.1056/NEJM200106213442504. [DOI] [PubMed] [Google Scholar]
- 20.Lynch HT. Family Information Service and hereditary cancer. Cancer. 2001;91:625–8. doi: 10.1002/1097-0142(20010215)91:4<625::aid-cncr1044>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Lynch HT, Grady W, Lynch JF, et al. E-cadherin mutation-based genetic counseling and hereditary diffuse gastric carcinoma. Cancer Genet Cytogenet. 2000;122:1–6. doi: 10.1016/s0165-4608(00)00273-9. [DOI] [PubMed] [Google Scholar]
- 22.Carneiro F, Huntsman DG, Smyrk TC, et al. Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J Pathol. 2004;203:681–7. doi: 10.1002/path.1564. [DOI] [PubMed] [Google Scholar]
- 23.Humar B, Fukuzawa R, Blair V, et al. Destabilized adhesion in the gastric proliferative zone and c-Src kinase activation mark the development of early diffuse gastric cancer. Cancer Res. 2007;67:2480–9. doi: 10.1158/0008-5472.CAN-06-3021. [DOI] [PubMed] [Google Scholar]
- 24.Lynch HT, Kaurah P, Wirtzfeld D, Rubinstein WS, Weissman S, Lynch JF, Grady W, Wiyrick S, Senz J, Huntsman D. Hereditary diffuse gastric cancer: diagnosis, genetic counseling, and prophylactic total gastrectomy. Cancer. 2008 doi: 10.1002/cncr.23501. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gayther SA, Gorringe KL, Ramus SJ, et al. Identification of germ-line E-cadherin mutations in gastric cancer families of European origin. Cancer Res. 1998;58:4086–9. [PubMed] [Google Scholar]
- 26.Hartgrink HH, van De Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–77. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–22. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 28.National Cancer Institute. [Accessed March 11, 08];Genetics of medullary thyroid cancer (PDQ): Health Professional Version. Available at: http://www.cancer.gov/cancertopics/pdq/genetics/medullarythyroid/HealthProfessional/allpages.
- 29.Schmeler KM, Lynch HT, Chen L-M, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–9. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 30.Lynch HT. Is there a role for prophylactic subtotal colectomy among hereditary nonpolyposis colorectal cancer germline mutation carriers? Dis Colon Rectum. 1996;39:109–10. doi: 10.1007/BF02048279. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. 2001;93:1633–7. doi: 10.1093/jnci/93.21.1633. [DOI] [PubMed] [Google Scholar]
- 32.Rebbeck TR, Levin AM, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475–9. doi: 10.1093/jnci/91.17.1475. [DOI] [PubMed] [Google Scholar]
- 33.Lynch HT, Silva E, Snyder C, et al. Hereditary breast cancer: part I. Diagnosing hereditary breast cancer syndromes. Breast J. 2008;14:3–13. doi: 10.1111/j.1524-4741.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 34.Silva E, Gatalica Z, Snyder C, et al. Hereditary breast cancer: part II. Management of hereditary breast cancer: implications of molecular genetics and pathology. Breast J. 2008;14:14–24. doi: 10.1111/j.1524-4741.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 35.Silva E. Genetic counseling and clinical management of newly diagnosed breast cancer patients at genetic risk for BRCA germline mutations: perspective of a surgical oncologist. Fam Cancer. 2008;7:91–5. doi: 10.1007/s10689-007-9167-3. [DOI] [PubMed] [Google Scholar]
- 36.Guillem JG, Wood WC, Moley JF, et al. ASCO/SSO review of current role of risk-reducing surgery in common hereditary cancer syndromes. J Clin Oncol. 2006;24:4642–60. doi: 10.1200/JCO.2005.04.5260. [DOI] [PubMed] [Google Scholar]
- 37.Guilford P, Blair V, More H, et al. A short guide to hereditary diffuse gastric cancer. Hereditary Cancer in Clinical Practice. 2007;5:183–94. doi: 10.1186/1897-4287-5-4-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch HT, Grady W, Suriano G, et al. Gastric cancer: New genetic developments. J Surg Oncol. 2005;90:114–33. doi: 10.1002/jso.20214. [DOI] [PubMed] [Google Scholar]
- 39.Seufert RM, Schmidt-Matthiesen A, Beyer A. Total gastrectomy and oesophagojejunostomy - a prospective randomized trial of hand-sutured versus mechanically stapled anastomoses. Br J Surg. 1990;77:50–2. doi: 10.1002/bjs.1800770118. [DOI] [PubMed] [Google Scholar]
- 40.Fujimoto S, Takahashi M, Endoh F, et al. Stapled or manual suturing in esophagojejunostomy after total gastrectomy: a comparison of outcome in 379 patients. Am J Surg. 1991;162:256–9. doi: 10.1016/0002-9610(91)90081-n. [DOI] [PubMed] [Google Scholar]
- 41.Hyodo M, Hosoya Y, Hirashima Y, et al. Minimum leakage rate (0.5%) of stapled esophagojejunostomy with sacrifice of a small part of the jejunum after total gastrectomy in 390 consecutive patients. Dig Surg. 2007;24:169–72. doi: 10.1159/000102100. [DOI] [PubMed] [Google Scholar]
- 42.Lehnert T, Buhl K. Techniques of reconstruction after total gastrectomy for cancer. Br J Surg. 2004;91:528–39. doi: 10.1002/bjs.4512. [DOI] [PubMed] [Google Scholar]
- 43.Braga M, Zuliani W, Foppa L, et al. Food intake and nutritional status after total gastrectomy: results of a nutritional follow-up. Br J Surg. 1988;75:477–80. [PubMed] [Google Scholar]
- 44.Buhl K, Lehnert T, Schlag P, et al. Reconstruction after gastrectomy and quality of life. World J Surg. 1995;19:558–64. doi: 10.1007/BF00294722. [DOI] [PubMed] [Google Scholar]
- 45.Sharma D. Choice of digestive tract reconstructive procedure following total gastrectomy: a critical reappraisal. Indian J Surg. 2004;66:270–6. [Google Scholar]
- 46.Liedman B, Bosaeus I, Hugosson I, et al. Long-term beneficial effects of a gastric reservoir on weight control after total gastrectomy: a study of potential mechanisms. Br J Surg. 1998;85:542–7. doi: 10.1046/j.1365-2168.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 47.Bozzetti F, Bonfanti G, Castellani R, et al. Comparing reconstruction with Roux-en-Y to a pouch following total gastrectomy. J Am Coll Surg. 1996;183:243–8. [PubMed] [Google Scholar]
- 48.Tanaka T, Fujiwara Y, Nakagawa K, et al. Reflux esophagitis after total gastrectomy with jejunal pouch reconstruction: comparison of long and short pouches. Am J Gastroenterol. 1997;92:821–4. [PubMed] [Google Scholar]
- 49.Kitagawa Y, Fujii H, Mukai M, et al. Radio-guided sentinel node detection for gastric cancer. Br J Surg. 2002;89:604–8. doi: 10.1046/j.1365-2168.2002.02065.x. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi H, Ochiai T, Mori M, et al. Sentinel lymph node mapping for gastric cancer using a dual procedure with dye- and gamma probe-guided techniques. J Am Coll Surg. 2003;196:68–74. doi: 10.1016/s1072-7515(02)01594-6. [DOI] [PubMed] [Google Scholar]
- 51.Beitsch PD, Clifford E, Whitworth P, et al. Improved lymphatic mapping technique for breast cancer. Breast J. 2001;7:219–23. doi: 10.1046/j.1524-4741.2001.20120.x. [DOI] [PubMed] [Google Scholar]
- 52.Chagpar A, Martin RC, III, Chao C, et al. Validation of subareolar and periareolar injection techniques for breast sentinel lymph node biopsy. Arch Surg. 2004;139:614–8. doi: 10.1001/archsurg.139.6.614. [DOI] [PubMed] [Google Scholar]
- 53.McMasters KM, Wong SL, Martin RC, II, et al. Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multiinstitutional study. Ann Surg. 2001;233:676–87. doi: 10.1097/00000658-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macdonald JS. Role of post-operative chemoradiation in resected gastric cancer. J Surg Oncol. 2005;90:166–70. doi: 10.1002/jso.20223. [DOI] [PubMed] [Google Scholar]
- 55.Kodera Y, Nakanishi H, Ito S, et al. Detection of disseminated cancer cells in linitis plastica-type gastric carcinoma. Jpn J Clin Oncol. 2004;34:525–31. doi: 10.1093/jjco/hyh097. [DOI] [PubMed] [Google Scholar]
- 56.Yachida S, Fukushima N, Sakamoto M, et al. Implications of peritoneal washing cytology in patients with potentially resectable pancreatic cancer. Br J Surg. 2002;89:573–8. doi: 10.1046/j.1365-2168.2002.02061.x. [DOI] [PubMed] [Google Scholar]
- 57.Francis A, England DW, Rowlands DC, et al. The diagnosis of invasive lobular breast carcinoma. Does MRI have a role? Breast. 2001;10:38–40. doi: 10.1054/brst.2000.0183. [DOI] [PubMed] [Google Scholar]
- 58.Chung MA, Cady B. Re: Genetic counseling and management of newly diagnosed breast cancer patients at genetic risk for BRCA germline mutations. Breast J. 2006;12:282–3. doi: 10.1111/j.1075-122X.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 59.van Kouwen MC, Drenth JP, Oyen WJ, et al. [18F]Fluoro-2-deoxy-D-glucose positron emission tomography detects gastric carcinoma in an early stage in an asymptomatic E-cadherin mutation carrier. Clin Cancer Res. 2004;10:6456–9. doi: 10.1158/1078-0432.CCR-04-0599. [DOI] [PubMed] [Google Scholar]
- 60.Shaw D, Blair V, Framp A, et al. Chromoendoscopic surveillance in hereditary diffuse gastric cancer: an alternative to prophylactic gastrectomy? Gut. 2005;54:461–8. doi: 10.1136/gut.2004.049171. [DOI] [PMC free article] [PubMed] [Google Scholar]