Abstract
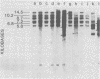
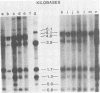
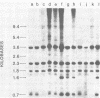
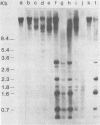
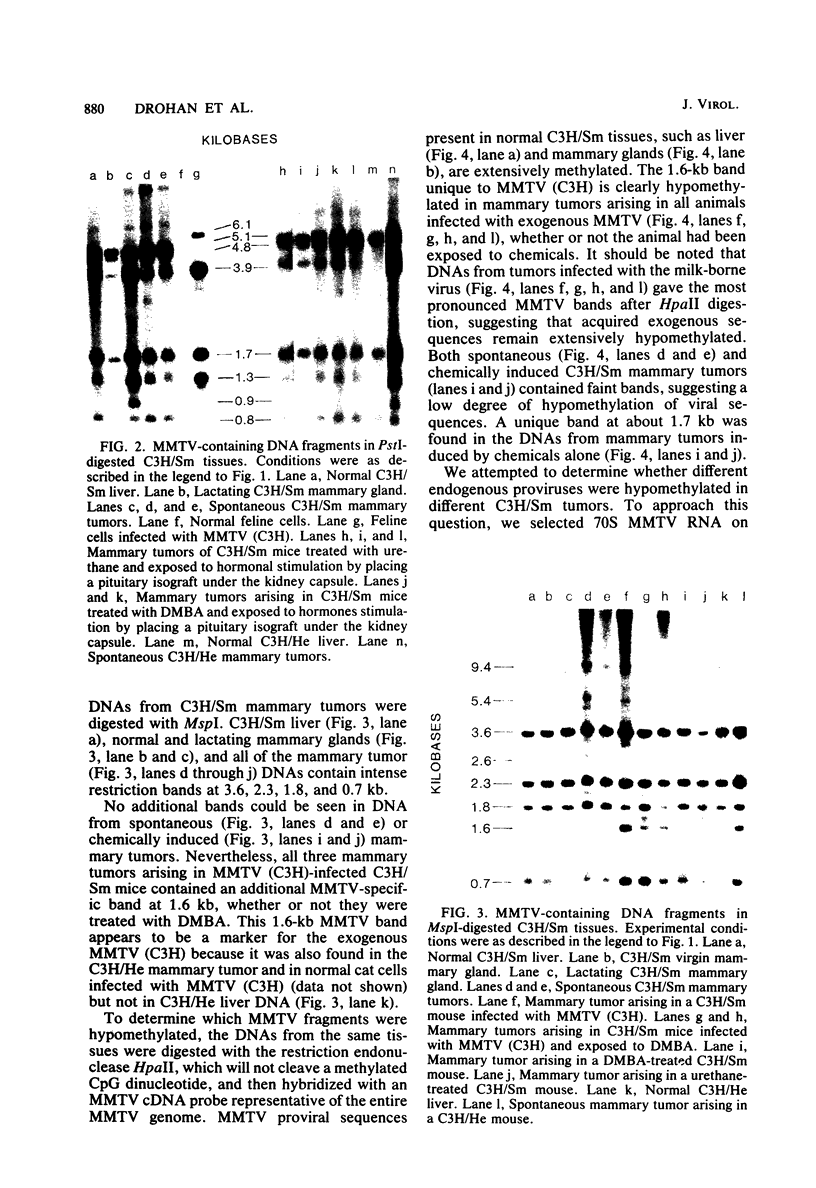
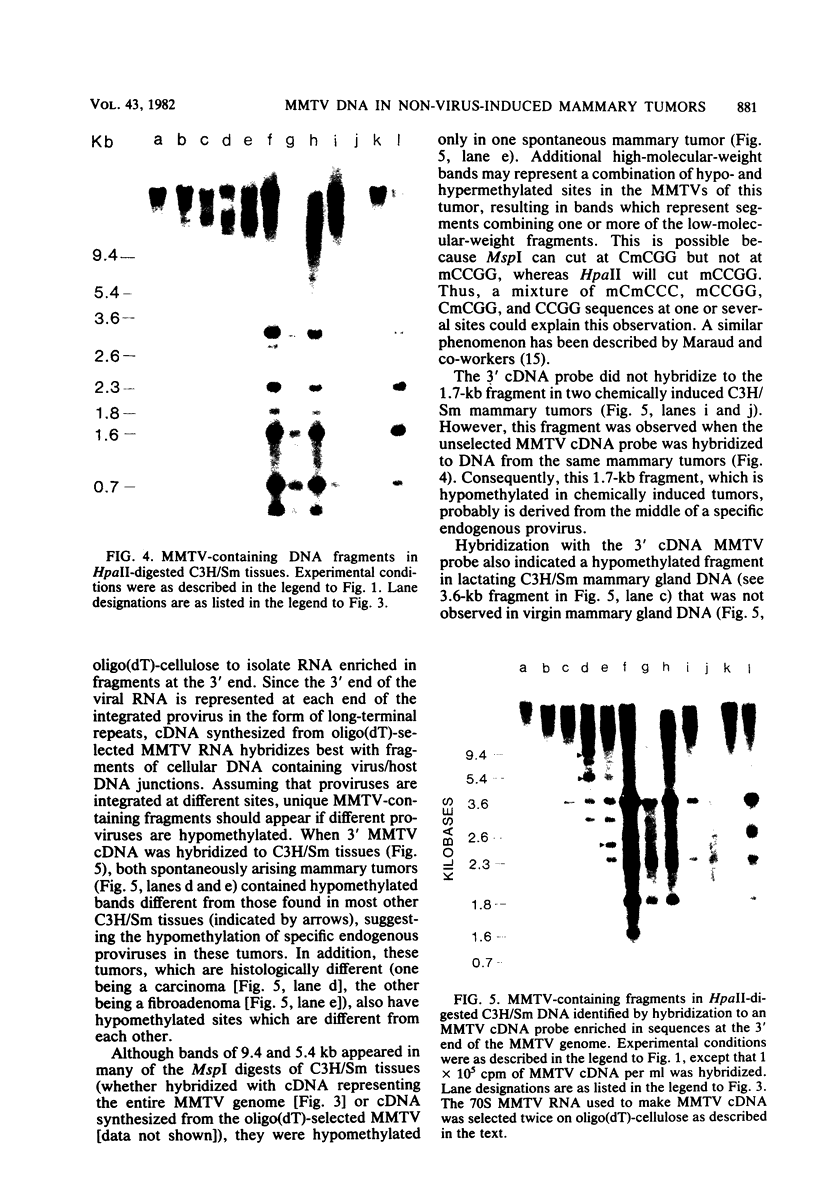
C3H/Sm mice have lost the exogenous milk-borne mouse mammary tumor virus (MMTV) characteristic of the C3H strain and have a very low (1.5%) incidence of spontaneous mammary tumors, yet they are highly susceptible to mammary carcinogenesis by either chemical carcinogens or infection with the milk-borne virus. We have analyzed the MMTV proviral DNA content of normal tissues and of spontaneous, virus-induced, and chemically induced mammary tumors by restriction endonuclease digestion and Southern blot analysis. Although the results clearly showed additional MMTV sequences in the virus-induced tumor which are not present in normal liver DNA, none of the spontaneous or chemically induced tumors could be shown to contain either newly acquired exogenous or amplified endogenous MMTV sequences. Interestingly, mammary tumors arising in C3H/Sm mice treated simultaneously with infectious MMTV (C3H) and dimethylbenz[a]anthracene (DMBA) possessed new exogenous MMTV DNA even though no quantitative change in tumor production was observed when these mice were compared with C3H/Sm mice treated with DMBA alone (Smith et al., Int. J. Cancer 26:373-379, 1980). Our data indicate that the endogenous MMTV proviral units are extensively methylated in normal tissues, such as livers and normal nonlactating mammary glands. In the absence of MMTV (C3H), we found that in the rare, spontaneously occurring C3H/Sm mammary tumors, certain endogenous MMTV sequences were specifically hypomethylated. Hypomethylation of endogenous MMTV sequences was also noted in the chemically induced mammary tumors, even though radioimmune competition assays for MMTV gp52 and p28 are negative (Smith et al., Int. J. Cancer 27:81-86, 1981). Our results support the conclusion that amplification of endogenous MMTV sequences is not intrinsic to C3H/Sm mouse mammary tumors arising spontaneously or after induction by chemicals. On the other hand, integration of exogenous MMTV DNA into the genome was a constant feature of mammary tumors developing in MMTV (C3H)-infected C3H/Sm mice, even when DMBA was used as the carcinogen. Hypomethylation of some endogenous MMTV sequences is characteristic of C3H/Sm mammary tumors, whether spontaneous or induced by chemicals, which suggests that these sequences are located in actively transcribing regions of the tumor cell genome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentvelzen P., Daams J. H., Hageman P., Calafat J. Genetic transmission of viruses that incite mammary tumor in mice. Proc Natl Acad Sci U S A. 1970 Sep;67(1):377–384. doi: 10.1073/pnas.67.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P., Taggart M. H., Smith B. A. Methylated and unmethylated DNA compartments in the sea urchin genome. Cell. 1979 Aug;17(4):889–901. doi: 10.1016/0092-8674(79)90329-5. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Proviruses of mouse mammary tumor virus in normal and neoplastic tissues from GR and C3Hf mouse strains. J Virol. 1980 Aug;35(2):298–305. doi: 10.1128/jvi.35.2.298-305.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drohan W., Schlom J. Diversity of mammary tumor viral genes within the genus Mus, the species Mus musculus, and the strain C3H. J Virol. 1979 Jul;31(1):53–62. doi: 10.1128/jvi.31.1.53-62.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drohan W., Teramoto Y. A., Medina D., Schlom J. Isolation and characterization of a new mouse mammary tumor virus from BALB/c mice. Virology. 1981 Oct 15;114(1):175–186. doi: 10.1016/0042-6822(81)90263-4. [DOI] [PubMed] [Google Scholar]
- Fanning T. G., Puma J. P., Cardiff R. D. Selective amplification of mouse mammary tumor virus in mammary tumors of GR mice. J Virol. 1980 Oct;36(1):109–114. doi: 10.1128/jvi.36.1.109-114.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Buetti E., Diggelmann H., Hynes N. E. Characterization of endogenous and exogenous mouse mammary tumor virus proviral DNA with site-specific molecular clones. J Virol. 1980 Dec;36(3):734–745. doi: 10.1128/jvi.36.3.734-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Long C. A., Dumaswala U. J., Tancin S. L., Vaidya A. B. Organization and expression of endogenous murine mammary tumor virus genes in mice congenic at the H-2 complex. Virology. 1980 May;103(1):167–177. doi: 10.1016/0042-6822(80)90135-x. [DOI] [PubMed] [Google Scholar]
- Marcaud L., Reynaud C. A., Therwath A., Scherrer K. Modification of the methylation pattern in the vicinity of the chicken globin genes in avian erythroblastosis virus transformed cells. Nucleic Acids Res. 1981 Apr 24;9(8):1841–1851. doi: 10.1093/nar/9.8.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nuss R. R., van Nie R. Identification of the Mtv-2 gene responsible for the early appearance of mammary tumors in the GR mouse by nucleic acid hybridization. Proc Natl Acad Sci U S A. 1978 May;75(5):2368–2372. doi: 10.1073/pnas.75.5.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., van Nie R., Nusse R., Hynes N. E., Groner B. Mammary tumor induction loci in GR and DBAf mice contain one provirus of the mouse mammary tumor virus. Cell. 1981 Jan;23(1):165–173. doi: 10.1016/0092-8674(81)90281-6. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Vlasschaert J. E., Beard C. L., Milazzo M. F., Bradbury W. C. Mammary tumors from BALB/c mice with a reported high mammary tumor incidence have acquired new mammary tumor virus DNA sequences. Virology. 1980 Jan 15;100(1):101–109. doi: 10.1016/0042-6822(80)90555-3. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Hackett A. J. Tissue culture studies of mouse mammary tumor cells and associated viruses. J Natl Cancer Inst. 1972 Nov;49(5):1321–1332. [PubMed] [Google Scholar]
- Sager R., Kitchin R. Selective silencing of eukaryotic DNA. Science. 1975 Aug 8;189(4201):426–433. [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. H., Arthur L. A., Medina D. Evidence of separate pathways for viral and chemical carcinogenesis in C3H/StWi mouse mammary glands. Int J Cancer. 1980 Sep 15;26(3):373–379. doi: 10.1002/ijc.2910260318. [DOI] [PubMed] [Google Scholar]
- Smith G. H., Henry T. J., Vlahakis G., Arthur L. O. Suppression of spontaneous mammary tumorigenesis despite Mtv-1 gene expression in hybrid and backcross C3H-AvyfB X C3H/Sm mice. Int J Cancer. 1982 May 15;29(5):591–598. doi: 10.1002/ijc.2910290517. [DOI] [PubMed] [Google Scholar]
- Smith G. H., Pauley R. J., Socher S. H., Medina D. Chemical carcinogenesis in C3H/StWi mice, a worthwhile experimental model for breast cancer. Cancer Res. 1978 Dec;38(12):4504–4509. [PubMed] [Google Scholar]
- Smith G. H. Role of the milk agent in disappearance of mammary cancer in C3H/StWi mice. J Natl Cancer Inst. 1966 Apr;36(4):685–701. doi: 10.1093/jnci/36.4.685. [DOI] [PubMed] [Google Scholar]
- Smith G. H., Teramoto Y. A., Medina D. Hormones, chemicals and proviral gene expression as contributing factors during mammary carcinogenesis in C3H/StWi mice. Int J Cancer. 1981 Jan 15;27(1):81–86. doi: 10.1002/ijc.2910270113. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]