Abstract
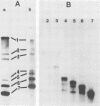
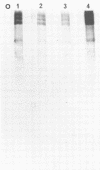
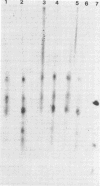
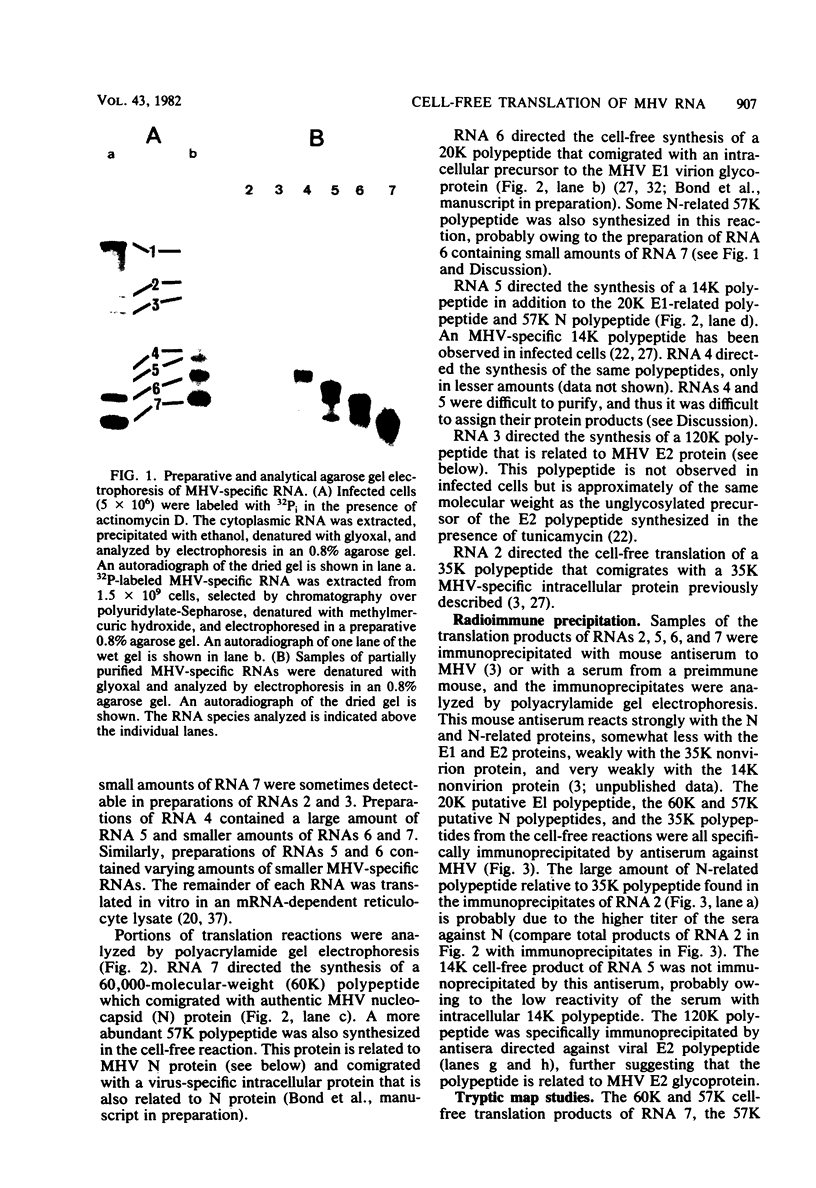
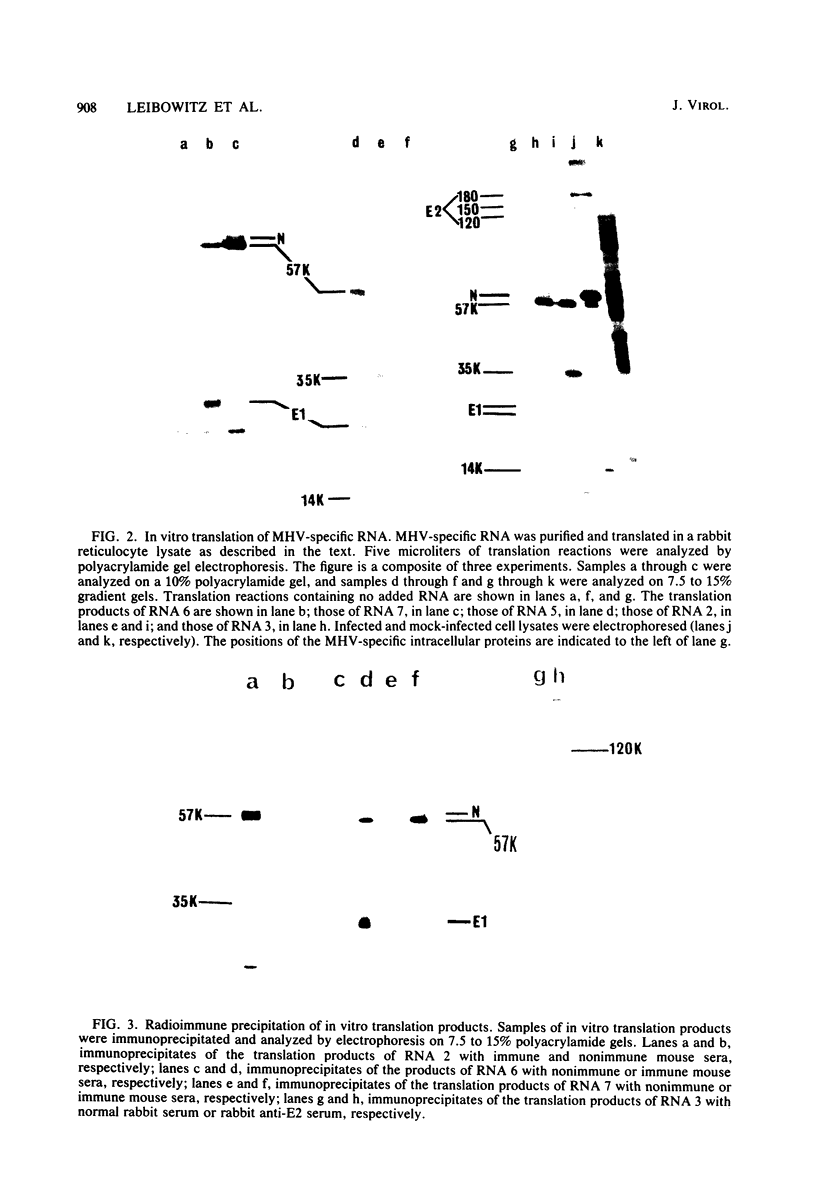
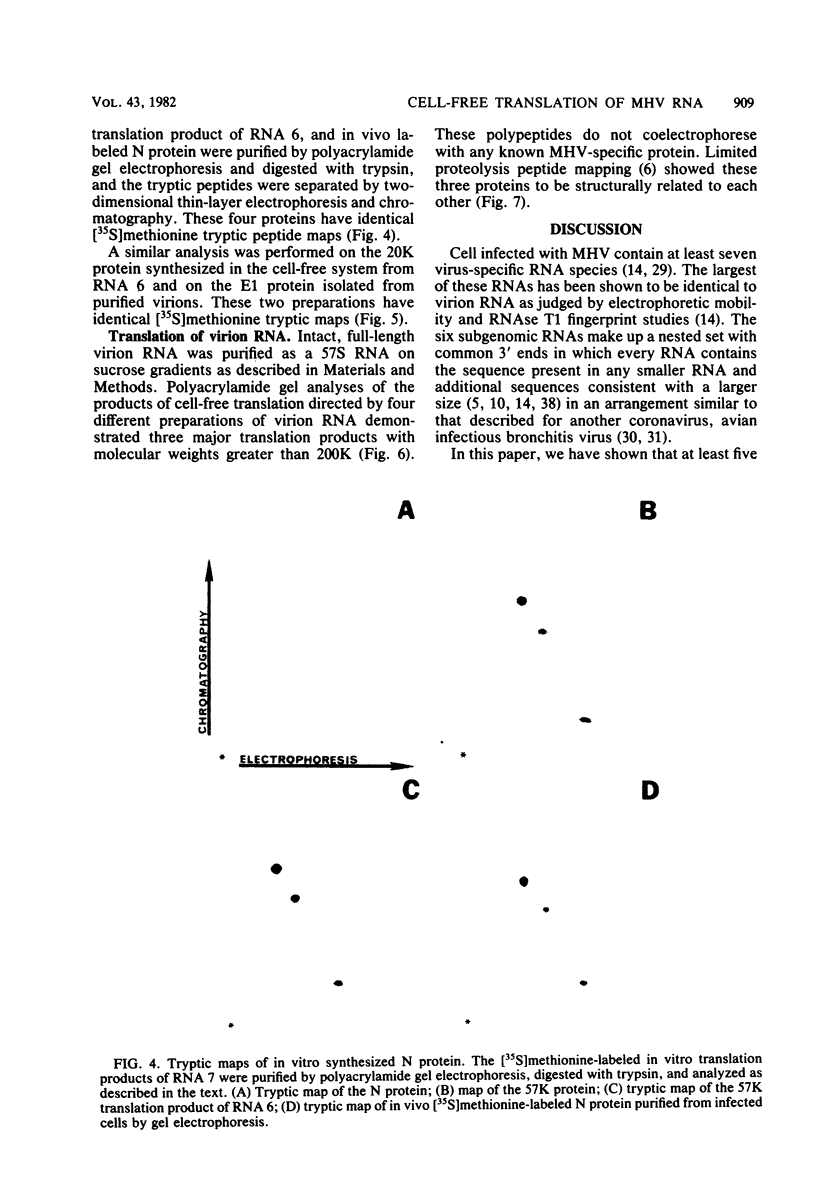
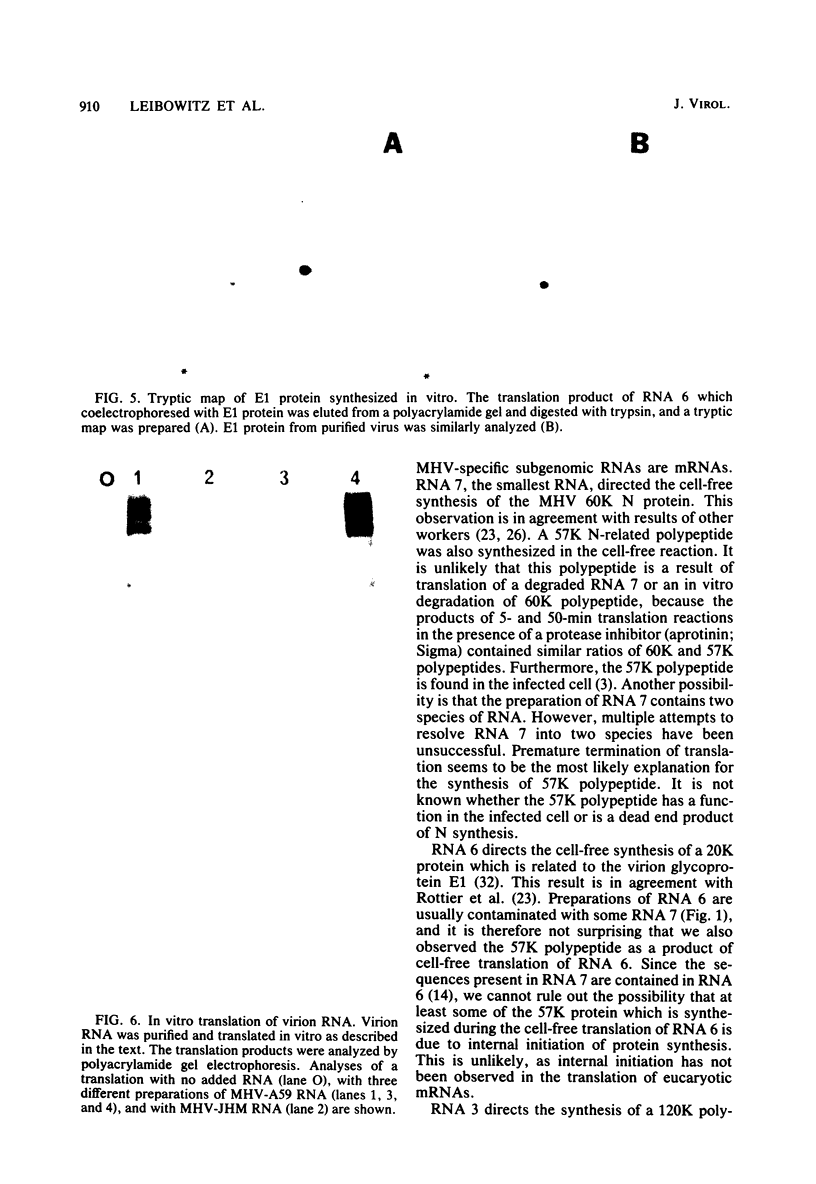
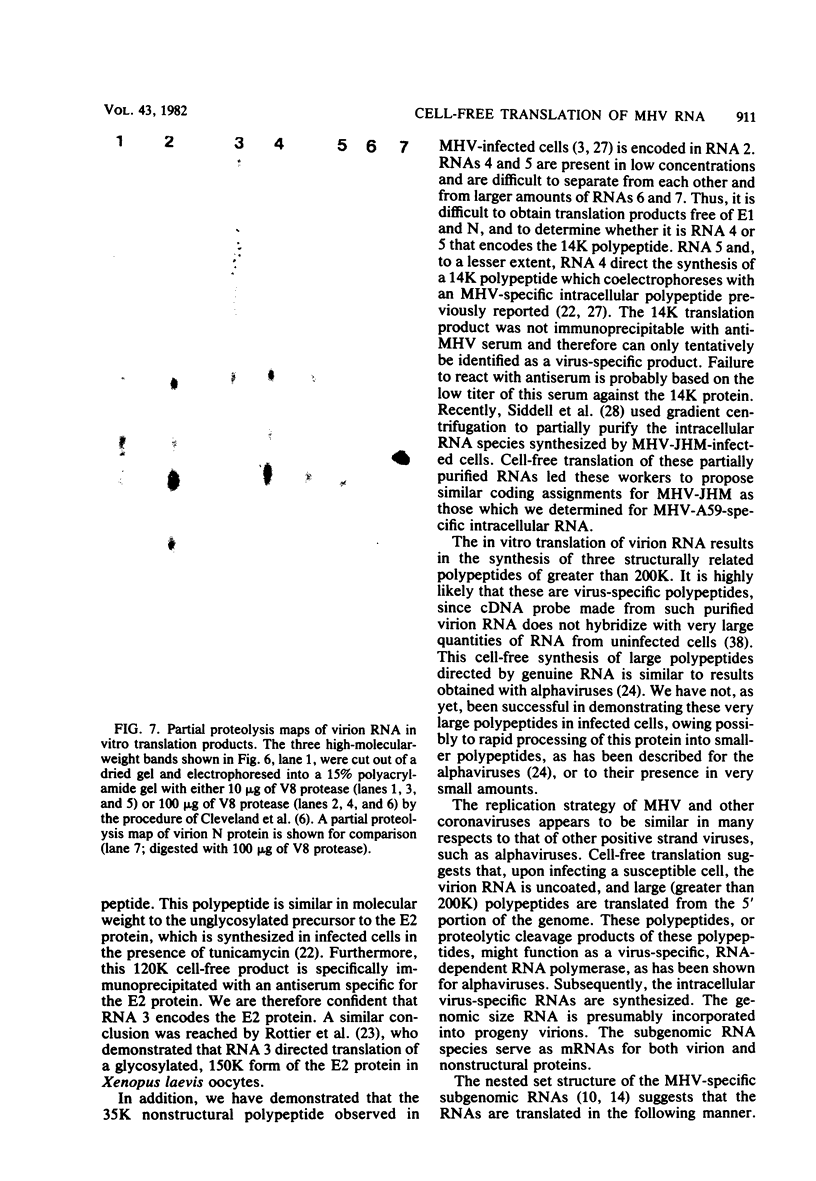
The coding assignments of the intracellular murine hepatitis virus-specific subgenomic RNA species and murine hepatitis virion RNA have been investigated by cell-free translation. The six murine hepatitis virus-specific subgenomic RNAs were partially purified by agarose gel electrophoresis and translated in an mRNA-dependent rabbit reticulocyte lysate, and the cell-free translation products were characterized by gel electrophoresis, immunoprecipitation, and tryptic peptide mapping. These studies have shown that RNA 7 codes for the nucleocapsid protein, RNA 6 codes for the E1 protein, RNA 3 codes for the E2 protein, and RNA 2 codes for a 35,000-dalton nonstructural protein. Genomic RNA directs the cell-free synthesis of three structurally related polypeptides of greater than 200,000 in molecular weight.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Cheley S., Haworth-Hatherell E. Comparison of polypeptides of two strains of murine hepatitis virus. Virology. 1979 Sep;97(2):492–494. doi: 10.1016/0042-6822(79)90363-5. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bond C. W., Leibowitz J. L., Robb J. A. Pathogenic murine coronaviruses. II. Characterization of virus-specific proteins of murine coronaviruses JHMV and A59V. Virology. 1979 Apr 30;94(2):371–384. doi: 10.1016/0042-6822(79)90468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cheley S., Anderson R., Cupples M. J., Chan E. C., Morris V. L. Intracellular murine hepatitis virus-specific RNAs contain common sequences. Virology. 1981 Jul 30;112(2):596–604. doi: 10.1016/0042-6822(81)90305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Guy J. S., Brian D. A. Bovine coronavirus genome. J Virol. 1979 Jan;29(1):293–300. doi: 10.1128/jvi.29.1.293-300.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Lai M. M., Brayton P. R., Armen R. C., Patton C. D., Pugh C., Stohlman S. A. Mouse hepatitis virus A59: mRNA structure and genetic localization of the sequence divergence from hepatotropic strain MHV-3. J Virol. 1981 Sep;39(3):823–834. doi: 10.1128/jvi.39.3.823-834.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Stohlman S. A. RNA of mouse hepatitis virus. J Virol. 1978 May;26(2):236–242. doi: 10.1128/jvi.26.2.236-242.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Leibowitz J. L., Weiss S. R. Murine coronavirus RNA. Adv Exp Med Biol. 1981;142:227–243. doi: 10.1007/978-1-4757-0456-3_19. [DOI] [PubMed] [Google Scholar]
- Leibowitz J. L., Wilhelmsen K. C., Bond C. W. The virus-specific intracellular RNA species of two murine coronaviruses: MHV-a59 and MHV-JHM. Virology. 1981 Oct 15;114(1):39–51. doi: 10.1016/0042-6822(81)90250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B. Biological properties of avian coronavirus RNA. J Gen Virol. 1977 Sep;36(3):531–533. doi: 10.1099/0022-1317-36-3-531. [DOI] [PubMed] [Google Scholar]
- Lomniczi B., Kennedy I. Genome of infectious bronchitis virus. J Virol. 1977 Oct;24(1):99–107. doi: 10.1128/jvi.24.1.99-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughton M. R., Madge M. H. The genome of human coronavirus strain 229E. J Gen Virol. 1978 Jun;39(3):497–504. doi: 10.1099/0022-1317-39-3-497. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Bond C. W. Pathogenic murine coronaviruses. I. Characterization of biological behavior in vitro and virus-specific intracellular RNA of strongly neurotropic JHMV and weakly neurotropic A59V viruses. Virology. 1979 Apr 30;94(2):352–370. doi: 10.1016/0042-6822(79)90467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Horzinek M. C., van der Zeijst B. A. Viral protein synthesis in mouse hepatitis virus strain A59-infected cells: effect of tunicamycin. J Virol. 1981 Nov;40(2):350–357. doi: 10.1128/jvi.40.2.350-357.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Spaan W. J., Horzinek M. C., van der Zeijst B. A. Translation of three mouse hepatitis virus strain A59 subgenomic RNAs in Xenopus laevis oocytes. J Virol. 1981 Apr;38(1):20–26. doi: 10.1128/jvi.38.1.20-26.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Stevens R. H., Simpson R. W. Presence of infectious polyadenylated RNA in coronavirus avian bronchitis virus. Virology. 1977 Apr;77(2):772–782. doi: 10.1016/0042-6822(77)90498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: cell-free synthesis of structural protein p60. J Virol. 1980 Jan;33(1):10–17. doi: 10.1128/jvi.33.1.10-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: intracellular protein synthesis. J Gen Virol. 1981 Mar;53(Pt 1):145–155. doi: 10.1099/0022-1317-53-1-145. [DOI] [PubMed] [Google Scholar]
- Siddell S., Wege H., Barthel A., ter Meulen V. Intracellular protein synthesis and the in vitro translation of coronavirus JHM mRNA. Adv Exp Med Biol. 1981;142:193–207. doi: 10.1007/978-1-4757-0456-3_17. [DOI] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. I. Identification and characterization of virus-specified RNA. J Virol. 1980 Jun;34(3):665–674. doi: 10.1128/jvi.34.3.665-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. II. Mapping the avian infectious bronchitis virus intracellular RNA species to the genome. J Virol. 1980 Nov;36(2):440–449. doi: 10.1128/jvi.36.2.440-449.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V., Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980 Jan;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S. I. Structural proteins: effects of preparative conditions on the migration of protein in polyacrylamide gels. Virology. 1977 Apr;77(2):637–649. doi: 10.1016/0042-6822(77)90488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Takemoto K. K. Enhanced growth of a murine coronavirus in transformed mouse cells. Infect Immun. 1972 Oct;6(4):501–507. doi: 10.1128/iai.6.4.501-507.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Alexander D. J., Almeida J. D., Cunningham C. H., Easterday B. C., Garwes D. J., Hierholzer J. C., Kapikian A., Macnaughton M. R., McIntosh K. Coronaviridae: second report. Intervirology. 1978;10(6):321–328. doi: 10.1159/000148996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Müller A., ter Meulen V. Genomic RNA of the murine coronavirus JHM. J Gen Virol. 1978 Nov;41(2):217–227. doi: 10.1099/0022-1317-41-2-217. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Hackett P. B., Oppermann H., Ullrich A., Levintow L., Bishop J. M. Cell-free translation of avian sarcoma virus RNA: suppression of the gag termination codon does not augment synthesis of the joint gag/pol product. Cell. 1978 Oct;15(2):607–614. doi: 10.1016/0092-8674(78)90029-6. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Leibowitz J. L. Comparison of the RNAs of murine and human coronaviruses. Adv Exp Med Biol. 1981;142:245–259. doi: 10.1007/978-1-4757-0456-3_20. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Sefton B. M., Esch F. S. Amino-terminal sequence analysis of alphavirus polypeptides. J Virol. 1981 Jun;38(3):968–972. doi: 10.1128/jvi.38.3.968-972.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilt F. H. The dynamics of maternal poly(A)-containing mRNA in fertilized sea urchin eggs. Cell. 1977 Jul;11(3):673–681. doi: 10.1016/0092-8674(77)90084-8. [DOI] [PubMed] [Google Scholar]