Abstract
Human and animal studies suggest adolescence is a period of heightened sensitivity to adverse cognitive sequelae of alcohol exposure. The current study assessed the effects of intermittent binge ethanol intoxication during the periadolescent period of Wistar rats on subsequent performance in a Morris water maze spatial navigation task. For 4 weeks (P32–56), rats were exposed to ethanol or air 3 days/week via vapor inhalation chambers. Acquisition of spatial navigation was assessed beginning 5 days after the final day of exposure, with 3 days of training in the Morris Water maze (4 trials per day spaced at 90 sec inter-trial intervals [ITI]). Rats were placed into the water maze at one of 4 positions along the perimeter, with a different release position to begin each trial. A probe trial assessed retention of platform location on the day after the final set of training trials. Four days after this probe trial, rats entered a working memory phase in which the platform was in a new location each day and a variable ITI of 1, 2, or 4 hr was inserted between Trials 1 and 2; Trials 3 and 4 followed at 90 sec intervals after Trial 2 on each day. The “savings” in latency to find the platform and distance traveled before finding it from Trial 1 to Trial 2 on each day served as an index of working memory. Ethanol-exposed rats showed similar acquisition of spatial navigation as control rats during training, as well as similar retention of platform location during the probe trial. However, rats exposed to average BAL >200 mg% showed accelerated forgetting, with decreased retention of platform location at the 2 hr ITI (p<.05), compared to control rats. Therefore, a 4-week history of intermittent ethanol exposure at BAL in excess of 200 mg% during periadolescence led to a working memory deficit in young adult rats, demonstrated by accelerated forgetting of novel information. These behavioral data are consistent with findings from adolescent human studies, indicating that binge-style alcohol exposure during the periadolescent stage of development is associated with deficits in retention of information.
Keywords: alcohol, spatial working memory, adolescence, binge drinking
Introduction
Alcohol is the most widely consumed intoxicant among adolescents in the U.S., with about 80% of high school students reporting prior alcohol use, and over 30% of 12th graders reporting having gotten drunk in the past month (Johnston et al., 2007). While adults typically drink more frequently, adolescents consume more per occasion, and are more likely than adults to engage in heavy episodic or “binge” drinking (Substance Abuse and Mental Health Services Administration, 2003), defined as consuming 5 or more drinks on an occasion. Given the prevalence of heavy alcohol use during adolescence, its effects on neuromaturation and behavior are of great interest.
Evidence from human and animal studies suggests that adolescence may be a period of heightened sensitivity to the adverse cognitive sequelae of alcohol exposure, perhaps because neuromaturation continues throughout this stage of life (Giedd et al., 1999; Giedd et al., 1996; Jernigan and Gamst, 2005; Lenroot and Giedd, 2006; Paus, 2001; Sowell et al., 2004). In human adolescents with histories of heavy alcohol use, hippocampal (De Bellis et al., 2000; Medina et al., 2008; Medina et al., 2007; Nagel et al., 2005) and prefrontal cortex (De Bellis et al., 2005; Medina et al., 2008) volumes appear smaller, and poorer performances are observed on tests requiring verbal and non-verbal retrieval (Brown et al., 2000), attention (Marlatt and Tapert, 1993), visuospatial functioning (Giancola et al., 1998; Sher et al., 1997; Tapert et al., 2002), verbal working memory (Tapert et al., 2002), and spatial working memory (Tapert et al., 2001). Brain function abnormalities have also been detected: despite similar performance on a spatial working memory task, adolescents with histories of heavy drinking showed less prefrontal cortex and cerebellar activation to complete the task as compared to non-drinking controls, with a greater degree of abnormality linked to lifetime alcohol hangover and withdrawal symptoms (Tapert et al., 2004). Thus, although adolescents have short lifetime drinking durations, heavy alcohol use is associated with abnormalities in brain structure and function.
Animal models have supported that periadolescence (postnatal day [PD] 20–60; Spear, 2000) is a period of particular vulnerability to the acute effects of alcohol on spatial working memory, with rats showing significant impairment in acquisition of spatial navigation in a Morris water maze when pretreated on PD30 with doses of ethanol (1–2 g/kg) that did not disrupt performance in young adult rats (PD65) (Markwiese et al., 1998). Such increased sensitivity to the acute amnestic effects of ethanol in periadolescent rats extends beyond spatial navigation tasks, with 0.5 - 1.0 g/kg doses of ethanol administered post-training disrupting retention of an odor discrimination task in early adolescent (PD28) rats treated but not adult rats (PD 100–120) (Land and Spear, 2004). Finally, ethanol disrupts long-term potentiation in the hippocampus of periadolescent rats (PD15–30) at concentrations that do not disrupt this electrophysiological measure of plasticity in adult rats (PD70–100) (Pyapali et al., 1999; Swartzwelder et al., 1995a, b).
Chronic treatment with high doses of ethanol results in increased damage to certain cortical regions in adolescent (PD35) versus adult rats (PD80–90) (Crews et al., 2000; Monti et al., 2005), and also leads to lasting deficits in spatial memory that outlast the period of ethanol treatment (Tomlinson et al., 1998; White et al., 2000). However, in the latter studies reporting such lasting deficits the range of ethanol doses employed (4–6 g/kg) produce very high blood alcohol levels (BAL) and loss of righting reflex for 5 hr or more, with BAL still in excess of 300 mg% upon return of righting reflex (Silveri and Spear, 1998; Swartzwelder et al., 1998). In addition, differential weight gain between ethanol and control rats when ethanol is repeatedly administered across the periadolescent period (PD30–50) is seen with these doses of ethanol (Silvers et al., 2003; Silvers et al., 2006). In the current study, we sought to develop a model of lasting spatial memory deficits produced by alcohol exposure in periadolescent rats, where the peak BAL achieved would more closely approximate levels achieved by heavy binge drinking youth. Therefore, we exposed Wistar rats to intermittent ethanol (3 days/week for 10 hr/day, from PD 32 to 56) via vapor inhalation, with an average BAL of 200–300 mg% targeted, representing a high but not uncommon BAL achieved during binge drinking. Rats were tested in the Morris water maze for the first time as young adults (PD 60–72). Based on prior findings, it was hypothesized that rats exposed to ethanol during periadolescence in a paradigm akin to binge drinking (i.e., intermittent high doses) would demonstrate lasting deficits in the acquisition and retention of spatial information, as compared to a placebo-exposed group. Because a prior study has also reported that repeated weekly dosing with ethanol (albeit at a high dose of 5 g/kg) during periadolescence produces increased anxiety-like behavior in a novelty exploration task (Popovic et al., 2004), we also examined a cohort of ethanol-exposed and control rats in the elevated plus maze model of anxiety.
Materials and Methods
Animals
Male Wistar rats (n = 63, Harlan Labs, Indianapolis, IN) arriving from the vendor at PD-26 to PD-28 were used as experimental subjects. All rats were pair-housed in a temperature- and humidity-controlled room with a 12 hour light/12 hour dark cycle (lights ON at 6:00 AM). Rats had ad libitum access to food and water at all times. All experimental procedures were approved by the Subcommittee on Animal Studies of the VA San Diego Healthcare System, an AAALAC-accredited facility, and are in strict accordance with the “Guide for the Care and Use of Laboratory Animals” (revised 1996).
Ethanol Vapor Delivery
The apparatus from La Jolla Alcohol Research (La Jolla, CA) consisted of 8 rat shoebox cages with lids equipped to form a tight seal with the cage. Each cage was divided in half across the long axis of the cage to create two square compartments with a Plexiglas wall that had multiple holes drilled into it; this permitted pairs of cage mates to be exposed to ethanol vapor in the same cage, while keeping them separated to avoid one from lying on top of the other while both are intoxicated. Each lid was equipped with two built-in reservoirs with a lick spout protruding from the bottom to provide water ad libitum, and each side of the cage contained a stainless steel food bowl with standard rat chow pellets also available ad libitum. Separate inlet and outlet ports delivered ethanol-vapor-containing air to each chamber and scavenged air from the chamber into exhaust venting in the room at an equal rate to avoid excess pressure build-up in the vapor chambers.
Ethanol vapor was created by dripping 95% ethanol into a large spherical vacuum flask kept at 50° C on a temperature-controlled heating unit. Air was blown over the bottom of the flask at a rate of 11–15 liters/min. Concentrations of the ethanol vapor were adjusted by varying the rate at which ethanol is pumped into the flask. Separate outlet ports delivered air to each ethanol chamber through tubing connected to the inlet ports of each chamber. Two separate pump, vapor flask, and heating units permitted independent delivery of ethanol to two sets of 4 chambers each, permitting groups of vapor-exposed (ETOH) and control (AIR) rats to be exposed and tested simultaneously. AIR controls were treated identically to ETOH counterparts, except the air delivered to their chambers did not contain ethanol vapor because the pumps were not active.
Cohorts of rats were exposed to ETOH (n=36) or AIR (n=27) conditions for 10 hr/day, 3 consecutive days/week, beginning at PD 32–34, and continuing for four consecutive weeks. These timing parameters were based on earlier work (Obernier et al., 2002; Popovic et al., 2004) which reported lasting consequences of binge alcohol exposure in 3–4 day epochs during periadolescence or young adulthood. Pilot experiments determined proper ethanol delivery rates to achieve blood alcohol levels (BAL) between 200–350 mg% for each rat at different stages in maturation. Ethanol was delivered continuously for the first 4 hr of each exposure session, to ensure a rapid rise to near-peak BAL, and that BAL was subsequently maintained for the duration of the 10 hr session by cycling the pumps off and on at hourly intervals. Pilot work demonstrated that this vapor exposure regimen resulted in similar BAL at 4 hr and 10 hr. As the periadolescent rats were rapidly increasing in size (more than doubling in weight across the 4-week test interval), 15–20% increases in initial delivery rate of ethanol were required from week to week to maintain stable BAL across weeks for each rat. Within each given week, rates were increased about 2–3% per day to ensure stable BAL across days for each rat.
Blood Alcohol Level (BAL) Determination
In keeping with blood volume collection standard operating procedures of the VA San Diego Healthcare System’s Veterinary Medical Unit (no more than 10% total volume every 2 weeks), blood samples (0.1–0.2 ml) were collected from the tail vein into heparinized 1.5 ml Eppendorf tubes after one of the three binge alcohol sessions each week. The day of exposure on which samples were taken varied from week to week for a given rat, and no more than one sample was ever taken in a week for each rat. Samples were spun for about 5 minutes at 3200 RPM and the plasma was removed and assayed for ethanol content using an ANALOX model AM1 blood analyzer (Analox Instruments, Lunenburg, MA). The enzymatic oxidation of ethanol in the sample to acetaldehyde was catalyzed by adding the enzyme AOD (alcohol: oxygen oxidoreductase). Required oxygen was abstracted from solution and the resultant oxygen change was measured by an oxygen sensor (pO2 electrode). Under conditions of the assay, the alcohol concentration is directly proportional to and can be calculated from the rate of oxygen consumption. The analyzer was calibrated to a reference standard of 100 mg% ethanol.
Morris Water Maze Procedure
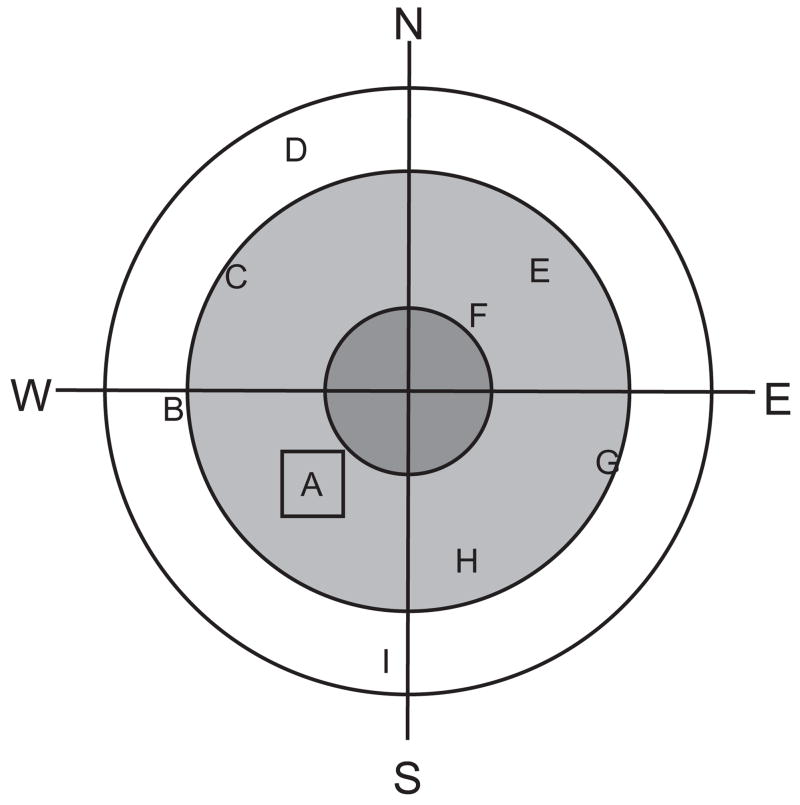
The apparatus consisted of a black circular pool, 1.52 m in diameter, filled with tap water adjusted to 20 ± 0.5° C temperature, and a black escape platform submerged 3 cm below the water surface. The room was dimly lit, with a 40 W bulb providing indirect light pointed at only one wall of the room to serve as a spatial cue; additional cues in the room were a clock on one wall, a door painted a different color from the wall, and a hose on a rack. The white rat’s position in the black pool was recorded by a video tracking system connected to a computer running Chromotrack software (San Diego Instruments, San Diego, CA USA). The computer recorded swim path, distance, and latency to find the platform. A map of the maze configured with concentric rings separating the outer rim of the maze, inner ring where the target platform was located during training, and center circle of the maze allowed the software to calculate relative time in each ring; similarly, division of the maze into four equal quadrants (NE, SE, SW, NW) allowed calculation of time spent in each quadrant, particular the target quadrant where the platform was located (see Figure 1).
Figure 1.
Schematic diagram of the Morris water maze. Position A represents the platform location during the Acquisition Phase of testing. Positions B through I represent possible platform locations during the Working Memory Phase of testing, from which 3 positions were semi-randomly selected for each rat for the 1 hr, 2 hr, and 4 hr ITI test days. To prevent swim strategies for identifying platform locations independent of visuospatial cues, the platform was located in a different quadrant and at a different radius from the center from its location on the preceding day. Thus, a combination of H-F-D would be permissible, but H-C-G would not.
Acquisition Phase
The acquisition phase began 3–5 days after the final exposure to ETOH or AIR vapor, and consisted of 3 consecutive days of training, with 4 trials per day separated by 90 sec intertrial intervals (ITI). The platform was located in a fixed position in the NW quadrant throughout training (location A in Figure 1). To begin each trial, a rat was gently released into the pool facing the wall at one of 4 defined release points (N, S, E, W) that were varied semi-randomly for each trial, so that each day there was one release from each point with varying order between rats tested on that day, and with varying order across days for each given rat. Once released, the rat was given 90 sec to find the platform, and if it failed to find it within the allotted time, it was gently guided to the platform by the investigator. After locating and climbing onto the platform, the rat was allowed to remain on it for 30 sec to attend to visuospatial cues in the environment, then was removed for a 90 sec ITI during which the rat was dried gently with a towel and placed under a heat lamp. This was repeated 3 additional times per training day.
Probe Trial
One day after the final training day, the rats were placed back into the pool for a single 90-sec trial in which the platform was removed, and the relative time spent in each quadrant was recorded to estimate the spatial memory of the rat for the previous platform location (Stewart and Morris, 1993). This probe trial also served the purpose of partially extinguishing the memory of previous platform location in preparation for the working memory phase of the experiment, which began 4 days later.
Working Memory Phase
During this phase which began 10–12 days after the final vapor exposure session, the platform location was varied each day for each cohort of rats among 8 possible locations, all different from the fixed platform location used during acquisition (see Figure 1 locations B–I). Separate cohorts of rats had different combinations of platform locations across days, with platform locations varied by quadrant and radius from center each day, to preclude swim strategies in which rats could rapidly find subsequent locations by swimming a fixed radius within the pool. On Trial 1 each day, the rat had no knowledge of where the platform would be located, and after release from one of the four defined start points, it had 90 sec to explore the maze and identify the new target location. If a rat failed to find the platform in 90 sec, it was guided to it by the investigator. Once on the platform, the rat was given 30 sec to collect visuospatial information, then was removed for a 1, 2, or 4 hr ITI that varied semi-randomly across consecutive days of working memory testing. After the ITI for that given day, the rat was returned to the maze and released from a different starting point for its second trial. Trials 3 and 4 followed at 90 sec ITI, using the same procedure as described for the Acquisition Phase, again from different starting points such that each day the rat was released from each of the 4 starting points once. The “savings” in latency to find the platform and distance traveled before finding it from Trial 1 to Trial 2 on each day served as an index of working memory, the ability of the animal to retain the information on platform location on each given day across the extended ITI imposed between Trials 1 and 2. Trials 3 and 4 served as an index that any deficits observed in “savings” from Trial 1 to Trial 2 could be attributed to the extended ITI between those trials, and not a general disruption in performance or reference memory for how to solve the maze and escape from the water.
Elevated Plus Maze Apparatus and Procedure
The elevated plus maze apparatus and procedure has been described in detail elsewhere (Zhang et al., 2007; Zhang and Schulteis, 2008). The automated maze (Kinder Scientific, Poway, CA) consisted of two open arms (Length: 50 cm, Width 10.8 cm) with 4-mm-high ledges on the sides and at the end of the arms, and two perpendicular closed arms of equal length and width but with 33.5 cm high walls on all sides except the entrance to the open center of the maze (10.8 x 10.8 cm) which connected all 4 arms at 90° relative to each adjacent arm. The maze floor was elevated 85 cm from the floor of the testing room. All surfaces of the open and closed arms were constructed from black Plexiglas. Position of the rat in the maze was continuously tracked by photo beam arrays.
On PD 63–65, 8 ETOH and 8 AIR control rats were tested on the plus maze for 5 min. The rats were handled gently one day prior to testing (for about 5 min/rat). Testing was conducted in a quiet room with a white noise generator providing approximately 65 dB background noise. The testing room was illuminated only by two 25-W light bulbs in clip-on fixtures that were attached to the legs of the enclosed arms and positioned to direct light against the walls of the testing room behind each closed arm. These conditions ensure a high baseline exploration of the open arms (about 30–45% time spent and entries into the open arms), readily permitting detection of anxiety-like behavior elicited by experimental treatments (Zhang et al., 2007; Zhang and Schulteis, 2008). To begin a test session, rats were placed in the center of the maze facing towards one of the enclosed arms and allowed to freely explore the entire apparatus for 5 min. Between each trial, the maze was cleaned with a damp sponge and dried with paper towels.
Data were collected and analyzed by a Windows-XP-driven computer using MotorMonitor Software (Kinder Scientific, Poway CA). From the computer-recorded data, the following measures were computed for each rat: 1) time spent in the open arms as a percentage of the total time spent exploring both the open and closed arms (Percent Time); and 2) number of entries into the open arms as a percentage of the total number of entries into both open and closed arms (Percent Entries). These two measures consistently have been shown in factor analyses to have the highest loading on the factor representing the “anxiety” dimension (Cruz et al., 1994; Rodgers and Dalvi, 1997; Wall and Messier, 2001).
Statistical Analysis
Data from the Acquisition, Probe Trial, and Working Memory phases of Morris water maze testing were analyzed separately. Distance to platform, latency to escape onto platform, swim speed, and percent distance traveled near outer walls (a measure of perseveration of most common initial behavior in the maze as rats attempt to escape from the edges) served as dependent variables for the Acquisition and Working Memory Phases. For the Acquisition Phase, each dependent variable was analyzed in a mixed factor ANOVA with training day (1–3) and trial within each day (1–4) as within-subjects factors and treatment condition (ETOH, AIR) as a between-subjects factor. For the Working Memory Phase, difference for each dependent variable from Trial 1 to each of Trials 2, 3 and 4 was calculated, and this difference measure was analyzed in a mixed factor ANOVA with ITI (1, 2, 4 hr) and Retention Trial (2, 3, and 4) as within-subjects factors and treatment condition (ETOH, AIR) as a between-subjects factor. Follow-up comparisons consisted of interaction contrasts or simple main effects as dictated by the outcome of the overall ANOVA, followed by individual means comparisons as appropriate (Newman-Keuls, significance set at p< .05 two-tailed). Probe Trial data were expressed as percent of time (90 sec total) spent in each quadrant, and time spent in each quadrant by the ETOH and AIR groups was compared by between-subjects ANOVA. Finally, Percent Time in and Percent Entries onto the open arms of the elevated plus maze were compared between ETOH and AIR groups using Student’s t-tests.
Results
Blood Alcohol Levels (BAL)
Among 36 rats exposed to ethanol, 30 had a BAL of at least 200 mg% on three or more of the weekly BAL determinations, an average BAL across all four determinations above 200 mg%, and no more than 25% variation from this average BAL of any single BAL determination across exposure weeks. The average BAL in these 30 subjects, of which 22 were entered into Morris water maze testing and 8 into elevated plus maze testing, was 241 + 7.5 mg% (range of 202 - 314 mg%). The remaining 6 ETOH rats showed significant variability in BAL across weeks despite identical ethanol exposure conditions, with BAL ranging from as low as 97 mg% on some days to over 240 mg% on others for the same rat, and all 6 rats had individual BAL readings for a given week of exposure that differed by 25–50% from their average BAL across all 4 weeks (e.g. Rat #07–360 had an average BAL of 170 mg%, but individual BAL determinations of 158, 185, 241, and 97 mg%). This cohort of 6 variable rats was excluded from further study as a consequence of their unpredictable BAL across weeks of the periadolescent treatment period. Nineteen AIR control rats were tested in the Morris water maze, and 8 were tested in the elevated plus maze. Rats in the ETOH and AIR groups showed comparable weights at the beginning (127 ± 3 g versus 121 ± 4) and conclusion (263 ± 11 g versus 271 ± 9 g) of the 4-week vapor chamber exposure period.
Acquisition Phase
As confirmed by mixed design ANOVA both ETOH and AIR groups showed significant decreases in distance traveled and latency to find the escape platform (see Figure 2, Panels A and B) as a function of both training day and trials within each day (Main Effects of Trial: F [3,123] = 101 and 85.2, respectively, p’s <.0001; Main Effects of Day: F [2,82] = 120 and 110, respectively, p’s< .0001; Trial x Day Interaction: F [6,246] = 9.69 and 9.95, respectively, p’s<.001). Lack of a significant main effect of treatment condition or any interaction with this factor indicated that there were no significant differences across treatment groups for either measure of acquisition (all F’s<2.16, p’s>.10). Thus, both ETOH and AIR groups showed comparable learning of the spatial navigation strategy to a fixed platform location within the water maze across training days in the Acquisition Phase.
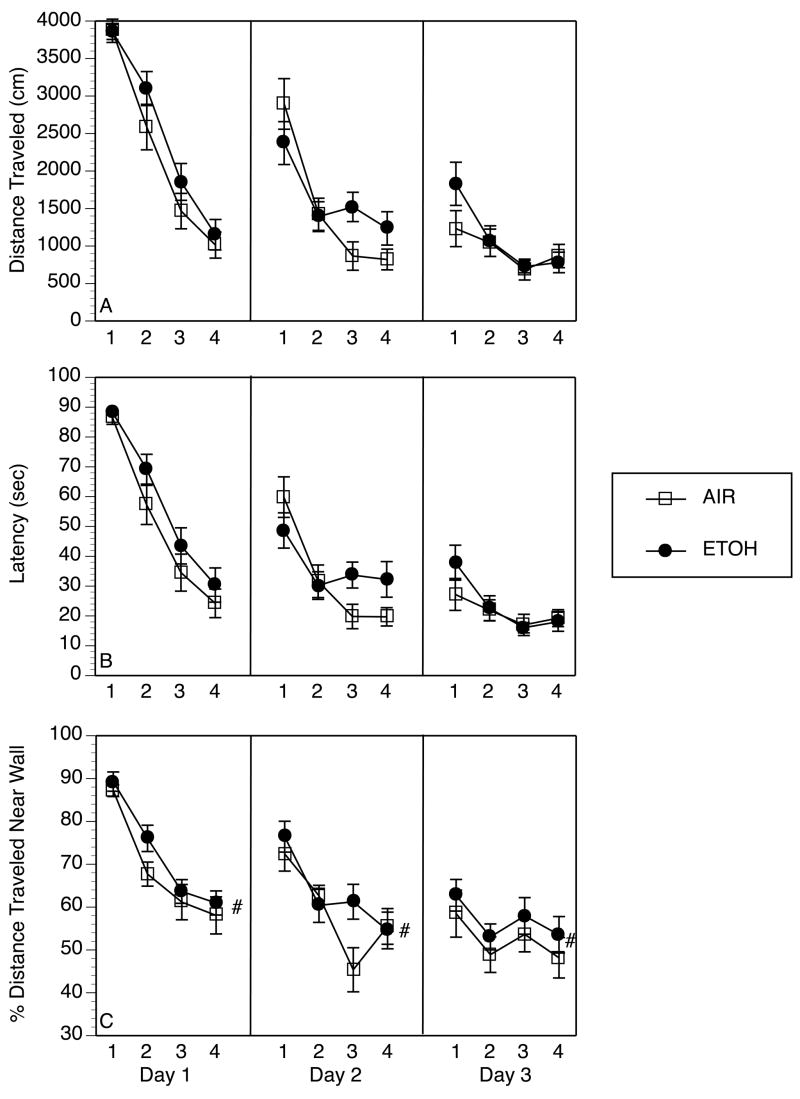
Figure 2.
Acquisition of spatial navigation in the Morris water maze was not impaired by prior exposure to ethanol (ETOH). Each panel represents the average performance of ethanol-exposed (ETOH, n= 22) and control (AIR, n= 19) rats across 3 days of testing, with 4 trials/day, beginning 3–5 days after the final exposure to ethanol. Data represent mean ± SEM for each dependent variable. A) Distance traveled in cm from start position until platform was located; B) Latency in sec to find and escape onto the platform; C) Distance traveled along the outer rim of the maze (white-shaded region in Figure 1) as a percent of total distance traveled on a given trial (#p =.062, Main Effect of Treatment Condition, a trend towards significant increase in time spent hugging the walls of the maze in ETOH rats versus AIR controls).
Swim speed showed a significant main effect of trial (F [3,123] = 20.4, p<0.0001), but no other main effect or interaction for this measure achieved significance (all F’s<2.26, p’s>.10). Thus, swim speed significantly decreased across trials within each day, but this did not vary across days of training or between ETOH and AIR groups (data not shown).
Percent distance traveled along the outer wall significantly differed by day (Main Effect: F [3,123] = 32.1, p<.0001) and trial (Main Effect: F[2,82] = 46.1, p<.0001), and a day x trial interaction (F [6,246] = 4.12, p<.01) revealed that distance along the outer walls decreased systematically across trials within a day and across days of training, as would be expected given the location of the platform away from the outer edges (see Figure 2, Panel D). The main effect of treatment condition (ETOH, AIR) also approached significance (F [1,41] = 3.68, p = .062), suggesting a trend towards increased time spent along the outer walls in the ETOH group (most prominent on Trial 2 of Day 1 and Trial 3 of Day 2). There was no significant interaction of treatment condition with any other factor (F’s<1.13, p’s>.10).
Probe Trial
On the Probe Trial, both ETOH and AIR groups showed a nearly identical distribution of percentage of distance traveled in each of the four quadrants. Both ETOH and AIR groups spent the majority of time in the SW target quadrant (39.45 ± 2.36 and 41.52 ± 2.44 percent time, respectively), and the least time in the opposite NE quadrant that shared no borders with the target quadrant (16.56 ± 1.24 and 15.58 ± 1.84 percent time, respectively). There was no statistically significant difference between ETOH and AIR groups in time allotted to any of the four quadrants (all F’s< 1.79, p’s > 0.10). The distribution of exploratory activity on the probe trial reflects significant retention of former platform location by both ETOH and AIR groups.
Working Memory
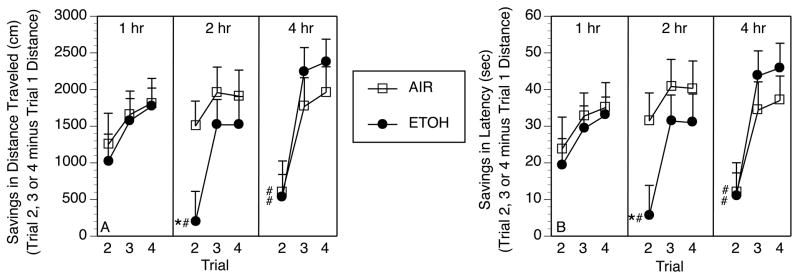
A comparison of performance on Trial 1 at each ITI (1, 2, 4 hr) in ETOH and AIR groups revealed no main effect of condition, ITI, or their interaction for distance traveled or latency to escape onto platform (all F’s<1.21, p’s >.10), indicating equivalent performance on Trial 1 across groups. This permitted subsequent evaluation of savings from Trial 1 to subsequent trials without concern over pre-existing group differences. As shown in Figure 3, ETOH and AIR groups showed comparable savings from Trial 1 of the working memory test conducted at the 1 hr ITI between Trials 1 and 2, with the ETOH group showing a significant deficit on Trial 2 at both the 2 and 4 hr ITI, and the AIR control group showing a similar deficit only at the 4 hr ITI. As revealed by the outcomes of the overall ANOVA analyses for each dependent measure of interest, significant interactions of Trial (2, 3, 4) x treatment condition (Latency: F [2,82] = 3.33, p<.05; Distance: F [2,82] = 3.42, p<.05) and Trial x ITI (1, 2, 4 hr) (Latency: F [4,164] = 3.97, p<.01; Distance: F[4,164] = 4.66, p<.01) suggested that this differential rate of onset of working memory deficit was restricted to Trial 2, with both groups showing marked improvement in locating the platform on Trials 3 and 4 regardless of ITI. Follow-up interaction contrasts revealed a significant interaction of condition and Trial only at the 2 hr ITI (F[2, 82] = 3.88 and 3.33 for distance and latency, respectively, p’s <.05). Similar analyses at the 1 hr and 4 hr ITI revealed significant main effects of Trial (F[2, 82] = 13.58 and 11.62 for distance and latency, respectively, p’s <.05), but no main effect of condition or condition x trial interaction, indicating identical patterns of performance across groups on these days. Swim speed did not differ across treatment groups under any ITI condition.
Figure 3.
Prior exposure to ethanol (ETOH) during adolescence leads to more rapid forgetting of platform location in the Working Memory Phase of water maze testing as compared to AIR controls. “Savings scores” were calculated for each rat as the difference in distance traveled (Panel A) or latency to escape onto platform (Panel B) between Trial 2, 3 or 4 and Trial 1 (e.g. Trial 2 distance minus Trial 1 distance, etc). For example, a savings score of “0” for Trial 2 would represent no difference in distance traveled on Trial 2 from distance traveled before finding the platform on Trial 1, whereas a savings score of “1500” would indicate that the rat traveled 1500 cm less on Trial 2 than on Trial 1 before finding the platform. Thus, AIR controls show good “savings” of spatial information acquired on Trial 1 at intertrial intervals (ITI) of 1 hr and 2 hr between Trial 1 and Trial 2, but showed significantly reduced savings at the 4 hr ITI in both distance traveled and latency to find the platform (#p < .05 vs. AIR at 1 hr ITI). ETOH animals show performance equivalent to AIR controls at the 1 hr ITI, but reduced savings at the 2 hr ITI and beyond (*p < .05 vs. AIR at 2 hr ITI, # p < .05 vs. ETOH at 1 hr ITI).
Elevated Plus Maze Behavior
ETOH and AIR groups tested on the elevated plus maze on PD63–65 did not differ in terms of Percent Time (33.89 ± 5.43 and 31.58 ± 3.78, respectively) or Percent Entries (35.14 ± 3.04 and 32.33 ± 2.87, respectively) measures of elevated plus-maze behavior (t’s < 0.97, p’s > .85), suggesting no differences in baseline anxiety-like behavior when tested at a time corresponding to the Acquisition phase of Morris maze testing.
Discussion
The results of the present study indicate that exposure to repeated binges of ethanol (BAL ≥200 mg %) throughout the adolescent period of Wistar rats results in significant deficits in working memory for a spatial navigation water maze task 10–15 days after the final day of exposure. The deficit is revealed as a more rapid forgetting of spatial information from Trial 1 to Trial 2 of a given working memory test as a function of increasing ITI between those trials. Thus, whilst both ETOH rats and AIR controls show good savings from Trial 1 to Trial 2 with a 1 hr ITI (1000–1200 cm less distance traveled and 20–22 sec decreased latency), the ethanol-exposed rats shows a significant savings deficit from Trial 1 to Trial 2 relative to controls at the 2 hr ITI. These data indicate that adolescent binge ethanol exposure for 3 days/week, in which BAL is rapidly increased to 200–350 mg% over 2–4 hr then maintained for 6 hr, results in lasting deficits in capacity to hold information in a working memory buffer for periods of 2 hr or longer.
These results are consistent with a prior study (Sircar and Sircar, 2005) which demonstrated that 5 daily treatments beginning at PD-30 with 2 g/kg ethanol, a dose that produces peak BAL around 200–300 mg% (Schulteis and Liu, 2006), disrupted spatial navigation in a Morris water maze for at least 25 days post-treatment. Sircar and Sircar, however, trained their rats in the water maze under the influence of ethanol on PD-30 to PD-34, and then subsequently tested ethanol-free, making it unclear whether subsequent impaired performance was due at least in part to disrupted acquisition produced by acute ethanol during initial training. Yttri and colleagues (Yttri et al., 2004) found that 4 treatments at 48 hr intervals from PD-28 to PD-32 with a slightly higher ethanol dose (2.5 g/kg) could impair trace conditioning in a conditioned fear paradigm when rats were trained and tested ethanol-free beginning on PD-40. Our current findings similarly indicate that lasting consequences of periadolescent ethanol exposure can be observed with all training and testing taking place after the ethanol exposure period has concluded.
Thus, the deficit in spatial working memory induced by periadolescent ethanol exposure in the current study cannot be attributed to residual intoxication with ethanol, since the deficit was observed at least 10 days after the final exposure to ethanol. One might argue instead that the effect was due to ethanol withdrawal, but behavioral consequences of ethanol withdrawal usually peak within the first 48–72 hr post-ethanol, even with sustained durations of ethanol exposure of 2 weeks or more (Roberts et al., 1996; Roberts et al., 2000; Schulteis et al., 1995; Valdez et al., 2002). Therefore, the unimpaired acquisition of spatial navigation in the first week after exposure and retention of spatial memory for location 6 days after exposure argue against the deficits emerging later still during the Working Memory Phase as being due to withdrawal.
At first glance it might appear somewhat surprising that rats exposed to ethanol were not impaired in their acquisition of the spatial memory task. However, this finding is not unique, as it has previously been reported that acquisition of Morris water maze performance is not impaired when rats are trained during the ETOH exposure phase, with training occurring on non-treatment days between successive treatments with high dose ethanol (5 g/kg) once every 48 hr from PD30 to PD50 (Silvers et al., 2003; Silvers et al., 2006). In addition, young adult rats treated with intragastric ethanol for a 4 day “binge” showed normal acquisition of Morris water maze spatial navigation when training began 5 days after the final ethanol treatment (Obernier et al., 2002). In addition, as shown in Figure 3 ETOH rats in the current study did not differ from controls in retention of platform location in a short-term memory buffer for periods of at least 1 hr. Instead, evidence of an ethanol-dependent deficit required ITI of 2 hr or greater. Because the training trials during the Acquisition Phase consisted of 4 massed trials per day with only a 90 sec ITI, and earlier work reporting no deficit in acquisition of reference memory also employed massed training trials with 60–90 sec ITI (Obernier et al., 2002; Silvers et al., 2003; Silvers et al., 2006), it is likely that these training conditions were sufficient to permit normal retention of information acquired on the previous trial, and therefore improved performance on the subsequent trials. Had the Acquisition Phase consisted of fewer trials per day and/or with longer intervals between subsequent trials, our findings of a spatial working memory deficit in ETOH rats at ITI of 2 hr and greater indicates that deficits in acquisition may have been observed. However, this was not the primary intention of the current study, as we wished to specifically model working memory deficits observed in human adolescent binge drinkers, and a priority was to train ETOH and AIR animals to equivalent levels of performance prior to assessment of such potential deficits.
There was a trend towards increased time near the walls of the water maze in ETOH-treated versus AIR control rats that approached significance (p = 0.062, see Figure 2D). Wall-hugging behavior or thigmotaxis can be interpreted as anxiety-like behavior in animals exploring a novel environment (Prut and Belzung, 2003), and thigmotaxis produced by cocaine pretreatment prior to Morris maze training has been interpreted as increased anxiety-like behavior (Mendez et al., 2008). Popovic et al., (2004) reported that repeated binges of 3 daily treatments with 5 g/kg ethanol for 4 weeks during the periadolescent period produced behaviors consistent with increased anxiety in a novelty exploration task administered to the rats as young adults. As described above, this dose of ethanol produces severe intoxication and loss of righting for several hours post-injection (Silveri and Spear, 1998; Swartzwelder et al., 1998). In contrast, under the ethanol exposure conditions employed herein which produce BAL more consistent with those that might typically be achieved by an adolescent drinker, we found that ETOH rats were not different from AIR controls in their exploration of the elevated plus maze, a model well-validated as an index of anxiety-like behavior in rodents (Cruz et al., 1994; Rodgers and Dalvi, 1997; Wall and Messier, 2001).
Increased time and distance traveled near the walls of the Morris maze also could be interpreted as a tendency towards perseveration of the initial response strategy of most rats in a water maze, which is to swim near the walls and attempt to “climb” from the apparatus (Stewart and Morris, 1993). This would be consistent with earlier reports that binge ethanol exposure in young adult rats can elicit perseverative responding or cognitive inflexibility as measured in a reversal learning paradigm when tested several weeks after ethanol exposure (Obernier et al., 2002). Therefore, the trend towards increased thigmotaxis on days 2 and 3 of the Acquisition phase of testing might most parsimoniously be explained as perseveration of initial escape strategy.
In summary, the results from our animal model parallel human findings of subtle but reliable decrements in retention and working memory in individuals who drank alcohol heavily during adolescence (Brown et al., 2000; Tapert et al., 2001; Tapert et al., 2002). For example, alcohol dependent adolescents were tested on learning and memory of verbal and visuospatial material after a 3-week detoxification period. While they initially learned the material adequately, they retrieved 10% less verbal and non-verbal material after a delay period than demographically similar non-drinking youth (Brown et al., 2000). The current model should therefore serve as a useful tool for analysis of neurobiological substrates mediating impaired working memory resulting from occasional heavy binge alcohol intoxication during the critical adolescent period.
Acknowledgments
This work was supported by grants R01 AA12800 (Schulteis), R01 MH64729 (Frank), and R01 AA13419 (Tapert). The authors express their deep gratitude to Dr. Donald Pizzo and the late Dr. Leon J. Thal for their gracious donation of time in their Morris water maze testing facility for completion of the study reported herein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–171. [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Mezzich AC, Tarter RE. Disruptive, delinquent and aggressive behavior in female adolescents with a psychoactive substance use disorder: Relation to executive cognitive functioning. J Stud Alcohol. 1998;59:560–567. doi: 10.15288/jsa.1998.59.560. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Jernigan T, Gamst A. Changes in volume with age: Consistency and interpretation of observed effects. Neurobiol Aging. 2005;26:1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use, 1975–2006: Volume 1, secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2007. [Google Scholar]
- Land C, Spear NE. Ethanol impairs memory of a simple discrimination in adolescent rats at doses that leave adult memory unaffected. Neurobiol Learn Mem. 2004;81:75–81. doi: 10.1016/j.nlm.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Marlatt GA, Tapert SF. Harm reduction: Reducing the risks of addictive behaviors. In: Baer JS, Marlatt GA, McMahon RJ, editors. Addictive behaviors across the life span: Prevention, treatment, and policy issues. Newbury Park, CA: Sage; 1993. pp. 243–273. [Google Scholar]
- Medina K, McQueeny T, Nagel B, Hanson K, Schweinsburg A, SF T. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Montgomery KS, LaSarge CL, Simon NW, Bizon JL, Setlow B. Long-term effects of prior cocaine exposure on Morris water maze performance. Neurobiol Learn Mem. 2008;89:185–191. doi: 10.1016/j.nlm.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Miranda R, Jr, Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, White A, Crews FT. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. 2005;29:207–220. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol Biochem Behav. 2002;72:521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Popovic M, Caballero-Bleda M, Puelles L, Guerri C. Multiple binge alcohol consumption during rat adolescence increases anxiety but does not impair retention in the passive avoidance task. Neurosci Lett. 2004;357:79–82. doi: 10.1016/j.neulet.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose dependent effects of ethanol on the induction of hippocampal long term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Liu J. Brain reward deficits accompany withdrawal (hangover) from acute ethanol in rats. Alcohol. 2006;39:21–28. doi: 10.1016/j.alcohol.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Martin ED, Wood PK, Rutledge PC. Alcohol use disorders and neuropsychological functioning in first-year undergraduates. Exp Clin Psychopharmacol. 1997;5:304–315. doi: 10.1037//1064-1297.5.3.304. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcohol Clin Exp Res. 2003;27:1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, O’Buckley T, Morrow AL, Matthews DB. Chronic intermittent ethanol exposure during adolescence reduces the effect of ethanol challenge on hippocampal allopregnanolone levels and Morris water maze task performance. Alcohol. 2006;39:151–158. doi: 10.1016/j.alcohol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res. 2005;29:1402–1410. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CA, Morris RGM. The Watermaze. In: Sahgal A, editor. Behavioral Neuroscience: A Practical Approach. Oxford: Oxford University Press; 1993. pp. 107–122. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health, 2002. Bethesda, MD: Office of Applied Studies; 2003. [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995a;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995b;19:320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann S, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tomlinson D, Wilce P, Bedi KS. Spatial learning ability of rats following differing levels of exposure to alcohol during early postnatal life. Physiol Behav. 1998;63:205–211. doi: 10.1016/s0031-9384(97)00424-1. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci Biobehav Rev. 2001;25:275–286. doi: 10.1016/s0149-7634(01)00013-6. [DOI] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–1256. [PubMed] [Google Scholar]
- Yttri EA, Burk JA, Hunt PS. Intermittent ethanol exposure in adolescent rats: dose dependent impairments in trace conditioning. Alcohol Clin Exp Res. 2004;28:1433–1436. doi: 10.1097/01.alc.0000147657.51745.a7. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Morse AC, Koob GF, Schulteis G. Dose- and time-dependent expression of anxiety-like behavior in the elevated plus-maze during withdrawal from acute and repeated intermittent ethanol intoxication in rats. Alcohol Clin Exp Res. 2007;31:1811–1819. doi: 10.1111/j.1530-0277.2007.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Schulteis G. Withdrawal from acute morphine dependence is accompanied by increased anxiety-like behavior in the elevated plus maze. Pharmacol Biochem Behav. 2008;89:392–403. doi: 10.1016/j.pbb.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]