Abstract
Work from our lab suggests that retinoic acid (RA) influences neuron development in the postnatal olfactory epithelium (OE). The studies reported here were carried out to identify and localize retinaldehyde dehydrogenase (RALDH) expression in postnatal rat OE to gain a better understanding of potential in vivo RA synthesis sites in this continuously regenerating tissue. RALDH 1, 2, and 3 mRNAs were detected in postnatal rat olfactory tissue by RT-PCR analysis, but RALDH 1 and 2 transcripts were predominant. RALDH 1 immunoreactivity was localized to sustentacular cells in the OE and to Bowman's gland cells, and GFAP+/p75− olfactory ensheathing cells (OECs) in the underlying lamina propria (LP). RALDH 2 did not colocalize with RALDH 1, but appeared to be expressed in GFAP−/RALDH 1− OECs, as well as in unidentified structures in the LP. Cellular RA binding protein (CRABP II) colocalized with RALDH 1. Cellular retinol/retinaldehyde binding protein (CRBP I) was localized to RALDH 1+ sites in the OE and LP, and RALDH 2+ sites, primarily surrounding nerve fiber bundles in the LP. Vitamin A deficiency altered RALDH 1, but not RALDH 2 protein expression. The isozymes and binding proteins exhibited random variability in levels and areas of expression both within and between animals. These findings support the hypothesis that RA is synthesized in the postnatal OE (catalyzed by RALDH 1) and underlying LP (differentially catalyzed by RALDH 1 and RALDH 2) at sites that could influence the development, maturation, targeting, and/or turnover of olfactory receptor neurons throughout the olfactory organ.
Keywords: RALDH 1, RALDH 2, RALDH 3, Vitamin A, olfactory epithelium, olfactory ensheathing cells
INTRODUCTION
Retinoic acid is an essential factor for growth and developmental processes during embryogenesis (reviewed in Clagett-Dame and DeLuca, 2002). The effects of retinoic acid are mediated by nuclear, retinoic acid receptor (RAR), complexes which bind to promoter elements in target genes to regulate gene expression (reviewed in Giguere, 1994; Lefebvre et al., 2005). The potential physiological role(s) that retinoic acid may be serving in adult animals has not been fully elucidated, but three lines of evidence support a role for retinoic acid in the maintenance of tissue homeostasis and in the regulation of cellular proliferation and differentiation in adult epithelia and other regenerative tissues throughout life. First, the presence of proteins with the potential to promote the synthesis, transport, and use of retinoic acid has been demonstrated in adult respiratory, urogenital, reproductive, digestive, liver, bone, skin, sensory, and brain tissues (Rajan et al., 1990; Napoli et al., 1991; Rajan et al., 1991; Elder et al., 1992; Ong et al., 1994; Ylikoski et al., 1994; Harada et al., 1995; Zetterstrom et al., 1999; Hind et al., 2002b; Everts et al., 2005; Lefebvre et al., 2005). Second, a continued physiological requirement for retinoic acid during postnatal and adult life is suggested by studies showing that animals deficient in vitamin A (a precursor for retinoic acid) develop multiple pathologic symptoms, some of which can be corrected by repletion with retinoic acid (Chun et al., 1992; Zhao and Ross, 1995; McGowan et al., 2004). Third, the therapeutic efficacy of retinoic acid in enhancing recovery or inducing regeneration of injured and diseased postnatal tissues suggests that retinoic acid-responsive signaling systems are maintained in the adult (Yee and Rawson, 2000; Maden and Hind, 2003).
Available evidence indicates that retinoic acid is produced in vivo via a two-step enzymatic pathway that oxidizes retinols, such as vitamin A (all-trans-retinol), first to retinaldehyde (catalyzed by specific cytosolic alcohol or microsomal retinol dehydrogenases), and then to retinoic acid. The second step of the pathway is catalyzed by one of several cytosolic retinaldehyde dehydrogenases (Duester et al, 2004; Napoli et al, 1991). Retinaldehyde dehydrogenases (RALDHs) in the superfamily of NAD(P) dependent aldehyde dehydrogenases have been categorized as cytosolic Class I aldehyde dehydrogenases (ALDH, EC 1.2.1.3), and in one case (RALDH 4), as cytosolic Class 8 aldehyde dehydrogenases (EC number not available) based on DNA sequence similarity (Sophos and Vasiliou, 2003). RALDH 1, RALDH 2, and RALDH 3 (formally classified as ALDH 1A1, ALDH 1A2, and ALDH 1A3) exhibit broad substrate specificity (Table 1), catalyzing oxidation of aldehydes to corresponding acids. These Class I ALDHs are distinguished from other Class I ALDHs, such as the phenobarbital-inducible isozyme, ALDH-PB (ALDH 1A7), by their capability of efficiently catalyzing retinoic acid synthesis in vitro (Table 1A) and ex vivo as demonstrated in experiments using transfected Xenopus oocytes and cultured cell reporter systems (Haselbeck et al., 1999; Suzuki et al., 2000). RALDH 4 also catalyzes synthesis of retinoic acid in vitro showing greater activity with 9-cis retinaldehyde than with all-trans retinaldehyde as substrate (Lin et al., 2003). All four RALDHs exhibit different spatial and temporal patterns of expression throughout the life of vertebrates. Of the four known RALDHs, RALDH 2 is expressed during early periods of mouse embryogenesis, and inactivation of this gene leads to death of embryos due to a lack of retinoic acid, which is required for morphogenesis, growth and differentiation of trunk, hindbrain and heart (Niederreither et al., 1999). Expression of RALDHs 1, 3, and 4, in addition to expression of RALDH 2, is observed at later stages of embryonic development and in postnatal animals throughout life in non-overlapping areas of the same tissues and/or in discrete locations within the developing fetus or adult (Haselbeck et al., 1999; Grun et al., 2000; Li et al., 2000; Zhai et al., 2001; Wagner et al., 2002; Niederreither et al., 2002a; Hind et al., 2002a; Lin et al., 2003; Duester et al., 2003).
Table 1A.
Comparison of in vitro kinetic constants for RALDHs using all-trans retinaldehyde as substrate.
| Enzyme |
Form |
Km (μM) |
Vmax / Km |
References |
|---|---|---|---|---|
| Rat RALDH 1 |
Native-Crude Protein | 0.7 − 9.8 | 5.4 | (Posch et al., 1992; Labrecque et al., 1993; Penzes et al., 1997; Kathmann et al., 2000; Montplaisir et al., 2002) |
| Native-Purified Protein | 0.8−10 | 27−331 | ||
| Recombinant |
0.6 − 10 |
0.32 − 37.1 |
|
|
| Rat RALDH 2 |
Recombinant |
0.4 |
49 |
(Wang et al., 1996) |
| Rat ALDH-PB |
Recombinant |
NAa |
------- |
(Kathmann et al., 2000; Montplaisir et al., 2002) |
| Mouse RALDH 1 |
Recombinant |
11.6 |
7.4 |
(Gagnon et al., 2003) |
| Mouse RALDH 2 |
Recombinant |
0.66 |
6.5 |
(Gagnon et al., 2002) |
| Mouse RALDH 3 |
Recombinant |
0.33 |
176 |
(Grun et al., 2000) |
| Chick RALDH 3 | Recombinant | 0.26 | 186 | (Grun et al., 2000) |
No activity was detected with retinal as substrate
The mammalian olfactory epithelium continues to regenerate neurons throughout adult life (Graziadei and Graziadei, 1979a; Graziadei and Graziadei, 1979b; Schwob, 2002) and thus, offers a unique system for investigating the potential involvement of retinoic acid in postnatal regenerative processes. The olfactory epithelium is a pseudostratified epithelium composed of non-neuronal cells and neuronal cells in different stages of development. Sustentacular cells are non-neuronal, glial-like, supporting cells with cell bodies positioned in a characteristic layer in the apical aspect of the epithelium (supranuclear layer). Sustentacular cells have a single apical process and a single basal process that terminates in end feet positioned along the basal lamina. The cell bodies of olfactory neurons are centrally located in the epithelium, with more developed neurons positioned in layers above those of less differentiated neuronal precursor cell bodies. Immature and mature neurons are bipolar, with a single dendritic process that extends from the neuron cell body to the apical edge of the epithelium and an axon that extends down through the basal lamina, where it merges with other axons in nerve fiber bundles in the lamina propria. The nerves extend to the olfactory bulb, where axons of mature olfactory neurons synapse with second order neurons in the bulb (Farbman, 2000; Schwob, 2002).
The lamina propria underlying the sensory epithelium is composed of a loose connective tissue framework that encompasses the fila olfactoria (bundles of unsheathed individual axons that are ensheathed by the overlapping processes of one or more olfactory ensheathing cells), Bowman's glands, blood vessels, fibroblasts, lymphocytes and other immune cells. This network of supporting cells and extracellular matrix is also referred to as stroma. Olfactory ensheathing cells are nucleated glial cells with two structurally and functionally distinct surfaces. The outer surface of the processes surrounding the axons faces a collagen-containing extracellular space and is completely covered by a basal lamina. The inner surface of the encircling olfactory ensheathing cell processes send out a ramification of processes that subdivide the nerve bundle into interconnected compartments of axons. One to six olfactory nerve fibroblast processes form an outer boundary layer separated from the olfactory ensheathing cells by the collagen-containing space (Field et al., 2003).
Previous studies from our laboratory and others have provided evidence that the cytosolic retinoic acid binding proteins (CRABP I and CRABP II) and the nuclear retinoic acid binding protein (RAR-α) are present in the postnatal rodent olfactory epithelium (Gustafson et al., 1999; Asson-Batres et al., 2003a; Yee and Rawson, 2005). CRBP I, a protein that avidly binds vitamin A retinol and retinaldehyde, the precursors of retinoic acid, has been localized to the stroma underlying postnatal mouse olfactory epithelium (Gustafson et al., 1999), and the distribution of RALDH mRNA in the olfactory organ of three to six week old mice has been demonstrated by in situ hybridization (Norlin et al., 2001; Niederreither et al., 2002a; Kawauchi et al., 2004). To date, this is the first formal report describing immunolocalization of RALDHs in postnatal olfactory epithelium. Some of these results were reported in abstract form at Annual Meetings of the Association for Chemoreception Sciences (Asson-Batres, 2002 Chem Senses 27:662; Asson-Batres and Smith, 2003 Chem Senses 28:560; Asson-Batres and Smith, 2005 Chem Senses 30:A89).
Available data support an involvement of retinoic acid in the development of the olfactory system during embryogenesis (Anchan et al., 1997; Whitesides et al., 1998; Grun et al., 2000; Li et al., 2000; Suzuki et al., 2000; Niederreither et al., 2002b), and an involvement in the development of olfactory receptor neurons throughout adult life (Asson-Batres et al., 2003b). In previous studies, we have noted a concomitant increase in the number of proliferating basal cells and a decrease in the number of mature olfactory receptor neurons throughout the olfactory epithelium of frankly vitamin A-deficient postnatal rats (Asson-Batres et al., 2003b). These results support the notion that vitamin A and/or its derivative, retinoic acid, are playing an essential role in regulating cell proliferation and differentiation events in the postnatal olfactory epithelium. Specifically, we hypothesize that retinoic acid is regulating the development and maturation of olfactory receptor neurons throughout the postnatal olfactory epithelium. With this in mind, the present study was carried out to identify and localize RALDH protein expression in the postnatal olfactory organ and to characterize in greater detail the localization of the retinol/retinaldehyde binding protein, CRBP I, and the retinoic acid binding protein, CRABP II, in order to more precisely define potential cellular sites of synthesis and, as such, sources of retinoic acid in this sensory tissue. Our results indicate that RALDH 1 and RALDH 2 exhibit high levels of expression in different cell types throughout the postnatal rat olfactory organ and are found in close association with proteins that bind and transport retinol/retinaldehyde substrates and retinoic acid products utilized or produced by RALDH-catalyzed reactions.
MATERIALS AND METHODS
Animals
Chow-fed, male Hsd Blue Long-Evans Rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN and Charles River Laboratories, Wilmington, MA ), approximately 200−300 g, 50−79 days of age, were used in these studies. Tissue from at least 3 different rats was used to localize expression of each RALDH; tissue from 4 different rats was used to investigate whether gradients of RALDH 2 expression were present; and tissue from 3 vitamin A-sufficient and 3 vitamin A-deficient rats was used to evaluate effects of vitamin A status on the expression of RALDH 1 and RALDH 2. Animals used in the studies were boarded and cared for at the Division of Animal Care at Vanderbilt University, Nashville, Tennessee. All procedures for animal handling, anesthesia, and euthanasia were approved by the Vanderbilt University Institutional Animal Use Committee. Rats were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Antibodies
The RALDH 1 polyclonal antiserum used in experiments reported here has been well-characterized (Kathmann et al., 2000) and was kindly provided by James Lipsky (Mayo Clinic and Foundation, Rochester, NY). The RALDH 1 antiserum was raised against a keyhole limpet hemocyanin-conjugated peptide (AQPAVPAPLANL) corresponding to residues 5−16 of the rat RALDH 1 deduced amino acid sequence. These residues share 100% identity with residues 5−16 in the rat ALDH-PB deduced amino acid sequence; 33% identity with residues 2−13 of the RALDH 2 deduced amino acid sequence (4 out of 12 matching residues); and 50% identity with residues residues 16−27 of the RALDH 3 deduced amino acid sequence (6 out of 12 matching residues). The RALDH 1 antiserum exhibits positive immunoreactivity with purified, recombinant RALDH 1 and ALDH-PB protein, but not purified, recombinant RALDH 2 protein on immunoblots (Kathmann et al., 2000).
The RALDH 2 and 3 polyclonal antisera were prepared against recombinant mouse RALDH 2 and RALDH 3 protein, respectively, and have been characterized and used in studies with chick embryos and mouse brain (Berggren et al., 1999; Wagner et al., 2002; Luo et al, 2004), and were kindly provided by Ursula Drager (E. Kennedy Shriver Center, Waltham, MA). The RALDH 2 and 3 antisera have been reported to show pronounced specificity for their respective RALDH antigens on (1) western blots of embryonic tissue lysates separated by isoelectric focusing (to separate the similar molecular weight RALDHs) and (2) on embryonic tissue sections as shown by comparison of neighboring sections reacted with anti-RALDH 2 antisera or RALDH 2 riboprobes and RALDH 3 antisera or RALDH 3 riboprobes. Weak cross-reactivity of the RALDH 2 and 3 antisera with RALDH 1, 2, and 3 antigen (due to the high sequence similarity of the RALDHs) was observed, but it was noted that this non-specific reactivity could be minimized by using the antisera at high dilutions (Berggren et al, 1999; Luo et al, 2004).
Antibodies for localization studies were kindly provided by the following investigators: CRBP I and CRABP II (David Ong, Vanderbilt University, Nashville, TN); OMP (Frank Margolis, University of Maryland, Baltimore, MD); and SUS 4 (James Schwob, Tufts University, Boston, MA), or were obtained from commercial vendors, as indicated in Table 2. The CRABP II antibody was produced in rabbits immunized with a conjugated peptide (CEQRLLKGEGPKTS) corresponding to residues 97−110 of the rat CRABP II deduced amino acid sequence, and the IgG fraction of immunized rabbit antisera showing specificity for CRABP II protein was purified on an affinity column prepared with recombinant rat CRABP II protein produced by a bacterial expression system (David Ong, personal communication). We tested the specificity of the affinity-purified CRABP II antibody on western blots prepared with purified, recombinant CRABP II and CRABP I (kindly provided by David Ong), and with rat olfactory tissue lysates. The antibody was specific for CRABP II, and reacted with a single band that comigrated with CRABP II on SDS polyacrylamide gels (apparent molecular weight, approximately 15 kDa; Ahmad, 2001). Specificity of the CRBP I and GAP-43 antibody preparations for their respective, purified protein antigens has been reported (Zheng and Ong, 1998; Liang et al, 2002). The GFAP antibody was solid-phase absorbed with human and cow serum proteins, and recognizes one distinct precipitate (GFAP) in cow brain extract; in indirect ELISA, the GFAP antibody shows no reaction with human plasma or cow serum. GFAP exhibits 90−95% homology between species, and, as demonstrated by immunocytochemistry, cross-reacts with GFAP in rat. The OMP antisera has been used in numerous studies as a marker for mature olfactory receptor neurons in wild type mice and rats, and has been shown to have no positive immunoreactivity in the olfactory epithelium of OMP knockout mice (Buiakova et al, 1996). The p75 antibody specifically binds to p75 receptors in binding assays (Chandler et al, 1984). The selectivity of the SUS-4 antibody preparation for sustentacular cells and cells of Bowman's glands is based on morphologic analysis of olfactory tissue sections reacted with the antibody (Goldstein and Schwob, 1996).
TABLE 2.
Primary antibodies and immunohistochemical techniques.
| Antigen | Antibody Format | Immunogen | Tissue Prep | Dilution | Source |
|---|---|---|---|---|---|
| ABC-TSA-METHOD* | |||||
| CRABP II | Polyclonal rabbit 530 - affinity purified | Peptide based on rat deduced amino acid sequence, residues 97−110 | Paraffin | 1/250 | D Ong |
| CRBP 1 | Polyclonal rabbit 110 - affinity purified | Human CRBP purified on rat CRBP affinity column | Cryosection | 1/5000 | D Ong1 |
| GAP-43 | Mouse monoclonal Clone 9−1E12 - purified IgG | Rat GAP-43 purified from brain | Paraffin | 1/100 | Chemicon #MAB347 |
| GFAP | Polyclonal rabbit antiserum - purified IgG | Cow GFAP isolated from spinal cord - cross reacts with rat protein | Paraffin | 1/100000 | DAKO #Z 0334 |
| OMP | Polyclonal goat #255 antiserum | Rodent OMP - cross reacts with rat and mouse protein | Paraffin | 1/20000 | F Margolis2 |
| p75 low affinity Nerve Growth Factor-Receptor | Mouse monoclonal 192 IgG — purified IgG fraction | n-octoglucoside stabilized proteins containing rat-specific p75, isolated from PC-12 plasma membranes | Paraffin Cryosection | 1/5000 1/10000 | Chemicon # MAB3653 |
| RALDH 1 | Polyclonal rabbit antiserum | Peptide based on rat deduced amino acid sequence, residues 5−16 | Paraffin Cryosection | 1/500 1/500 | J Lipsky4 |
| RALDH 2 | Polyclonal rabbit antiserum | Recombinant chick RALDH 2 derived from bacculovirus expression system | Paraffin Cryosection | 1/50000 1/20000 | U Drager5 |
| RALDH 3 | Polyclonal rabbit antiserum | Recombinant mouse RALDH 3 derived from bacterial expression system | Cryosection | 1/10000 | U Drager6 |
| SUS 4 | Mouse monoclonal — purified IgG from hybridoma supernates | Regenerating rat olfactory mucosa containing sustentacular cell-specific proteins | Cryosection | 1/500 | J Schwob7 |
| RALDH 2 and RALDH 1 | As above | As above | Paraffin | 1/2000 1/500 | U Drager J Lipsky |
| INDIRECT FLUORESCENCE METHOD | |||||
| RALDH 1 and GAP-43 | As above | As above | Paraffin | 1/200 1/100 | As above |
| RALDH 1 and GFAP | As above | As above | Cryosection | 1/200 1/500 | As above |
Vectastain ABC Kit followed by TSA-Cyanine 3 (CY3) or TSA-Fluorescein (FITC) System
RT-PCR
Total RNA was isolated from rat olfactory tissue using guanidine isothiocyanate-cesium chloride gradient centrifugation (Wilkinson, 1991). RNA integrity was checked by agarose gel electrophoresis and concentration was determined using spectrophotometry. The RNA was treated with DNase I according to the manufacturer's directions (ZYMO Research, Orange, CA). The quality of the DNAse-treated RNA was checked by gel electrophoresis, and concentration was determined by spectrophotometry. cDNA was synthesized (SuperScript First Strand Synthesis Kit, Invitrogen, Carlsbad, CA), with oligo dT primers. Non-intron spanning, PCR primers, designed to specifically amplify up ALDH-PB, RALDH 1, 2, and 3 (Table 3), were synthesized by BioSource Intl (Camarillo, CA). Preliminary reactions were run to optimize PCR conditions for each primer set, and reaction products were cleaned and concentrated (ZYMO Research Kit, Orange, CA), submitted for preparation of sequence reactions using AmpliTaq™ DNA Polymerase dye terminator cycle sequencing chemistry (Perkin Elmer Applied Biosystems Division, Foster City, CA) and automated DNA sequencing (GenePass, Nashville, TN). For a qualitative comparison of the relative levels of expression of the ALDH/RALDH transcripts, PCR reactions for each transcript were prepared from 100 ng olfactory cDNA and all reactions were run simultaneously. Reaction mixes were prepared using Hot Start™ 100 Tubes (Promega, Madison, WN) according to the manufacturer's instructions. Each reaction mix contained 2 μl cDNA, 200 nM each primer, 200 μM each dNTP in TAQ polymerase buffer with 1.5 mM MgCl2, 0.5 μl TAQ polymerase (Promega, Madison, WN) in a final volume of 100 μl. A control reaction was prepared without cDNA and with primer sets for all of the ALDH/RALDH transcripts. Cycling conditions were 95°C × 5 min × 1 cycle; 95°C × 1 min; 65°C × 1 min; 72°C × 1.5 min for 30 cycles; 72°C × 10 min × 1 cycle using a Gene Amp PCR System 2400 (Perkin Elmer Life Sciences, Boston, MA). An additional reaction mix with primers for RALDH 3 was included in this experiment and was amplified for 40 cycles. PCR reactions were also prepared from 100 ng rat genomic DNA (rat liver genomic DNA #D4434149, BioChain Institute, Hayward, CA) using primer sets for RALDH 1 and ALDH-PB. Cycling conditions were the same as those used to amplify up olfactory cDNA, with the exception that the annealing temperature was 60°C.
TABLE 3.
Rat Aldehyde Dehydrogenase Nomenclature and Oligonucleotide Primer Sequences for RT-PCR.
|
Sequence Identifier PCR Product Length |
Accession Number - Sequence Name(s) |
Oligonucleotide Primer Sequences |
|---|---|---|
| ALDH-PB | M23995 - Phenobarbital-inducible rat aldehyde dehydrogenase1 | Fwd 5’-AGCCAGCAGAGTGACGAGA-3’ |
| 423 bp |
|
Rev 5’-AAGCATTTCCATTTGACAAGCAG-3’ |
| RALDH 1 | NM_022407 - Constitutively expressed rat cytosolic aldehyde dehydrogenase, Aldh1a12-4 | Fwd 5’-CGTCACCAGCAAAGTGTTGT-3’ |
| 301 bp |
|
Rev 5’-AGCAGTTCAAGGGGTCACAG 3’ |
| RALDH 2 | NM_053896 – Rat retinal dehydrogenase, Aldh1a25 | Fwd 5’-GCAGCAATAGCGTCTCACATT-3’ |
| 747 bp |
|
Rev 5’-AAGCCAAACTCACCCATTTCT-3’ |
| RALDH 3 | AF434845 - Rat aldehyde dehydrogenase 6, Aldh1a36 | Fwd 5’-CGATAAGCCCGATGTGGAC-3’ |
| 550 bp | Rev 5’-CTGTGGATGATAGGAGATTGC-3’ |
(Zheng,WL, et al, unpublished)
Agarose Gel Electrophoresis
The ALDH/RALDH olfactory RT-PCR products were separated on a 2% agarose/TBE gel (2 μl reaction mix + 3 μl TE + 1μl loading buffer per lane), and the genomic DNA PCR products were separated on a 0.8% agarose/TBE gel (18 μl reaction mix + 2 μl loading buffer) at 80V, constant. Gels weres stained with 1/10,000 SYBR Green I (Molecular Probes, Eugene, OR) for 30 min at room temperature. The products were visualized and quantified using an EpiChemi3 Image Analysis System (UVP, Upland, CA).
Tissue preparation
Rats were anesthetized with a cocktail of 70 mg/kg ketamine/14 mg/kg xylazine. When unresponsive to a pinch test, the chest cavity was opened, a cannula was inserted into the left ventricle of the heart, and the animal was perfused with 200 ml heparinized saline using a perfusion pump (15 ml/min), followed by perfusion with 200 ml 4% paraformaldehyde in Sorenson's phosphate buffer. An additional 100 ml fixative was perfused through the animal and allowed to recirculate for 20 min. Olfactory region sections were prepared from dissected heads that were trimmed of excess skin, muscle, and other tissue, postfixed overnight, and decalcified at 4°C with intermittent changes of Regular Cal Immuno (BBC Biochemical, Stanwood, WA) until bone could be easily cut with a razor blade (approximately 2 weeks). Some fixed skulls were dehydrated through a graded series of ethanol and xylene, and embedded in paraffin; some were cryoprotected with graded solutions of sucrose (10%, 20%, and 30% in Sorenson's phosphate buffer) and frozen in Tissue Tek O.C.T. (Sakura, Torrance, CA). Transverse sections, 5 μm, were cut from the blocks, and tissue sections were mounted on Superfrost/Plus (Fisher Scientific, Pittsburgh, PA) microscope slides.
Immunohistochemistry
Preliminary experiments were carried out to determine the optimal methodology and optimal dilutions for each primary antibody. Primary antibodies, dilutions, and sources and labeling methods are listed in Table 2. The optimal antibody dilutions for some primary antibodies varied depending on whether it was used in single-label or double-label reactions, or was used on deparaffinized or frozen tissue sections (Table 2). Negative control tissue sections were prepared by excluding the primary antibody (minus antibody control), by excluding the conjugated secondary antibody-fluorophore, by excluding the tyramide-fluorophore reagent, and, in the case of RALDH 1, including the peptide antigen used to generate the antibody in the primary antibody reaction. Positive immunoreactivity with the antibodies of interest was not observed on any of the negative control slides (data not shown).
Deparaffinized or frozen sections were rehydrated and pretreated with peroxide for 30 min (4.5 ml in 150 ml methanol) to block endogenous peroxidase activity. Pre-treatment with Proteinase K was required for optimal tissue reactivity with the GFAP antibody (#53020, DAKO Cytomation, Carpinteria, CA; reagent as supplied for 4 min at room temperature), but this pre-treatment could not be used in double-label procedures with RALDH 1 antiserum because it produced artifactual results. Substitution of DAKO Target Retrieval Solution (DAKO Target Retrieval Solution, #S1700; reagent as supplied for 30 min at 100°C; DAKO Cytomation, Carpinteria, CA) for Proteinase K corrected the problem with the RALDH 1 antiserum, while preserving GFAP reactivity on olfactory tissue sections. After pre-treatment, tissue sections were washed with phosphate buffered saline (PBS), blocked with normal serum in PBS, and reacted with primary antibody diluted in PBS (see Table 2 for primary antibody information) overnight at 4°C. Three methods of detection were used, (1) ABC-TSA , (2) Indirect Immunofluorescence, or (3), DAKO Cytomation Envision+ System HRP/DAB+.
Single immunolabeling with the ABC-TSA method was carried out with an appropriate biotinylated secondary antibody (1/200 Biotinylated goat anti-rabbit, Vector # BA 1000; 1/200 Biotinylated horse anti-mouse, Vector # BA 2001; 1/1000 Biotinylated donkey anti-goat, Jackson # 705 065 147) and ABC reagent according to manufacturer's directions (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA), substituting tyramide-cyanine 3 (CY3) or tyramide-fluorescein isothiocyanate (FITC) (Perkin Elmer Life Sciences, Boston, MA) for diaminobenzidene (DAB) to localize peroxidase activity. The tyramide-fluorophore reagent was prepared at a 1/50 dilution in the supplied amplification buffer and applied to the specimen for 5 min at room temperature. Coverslips were mounted with ProLong Anti-Fade (Molecular Probes, Eugene, OR).
Double immunolabeling using the ABC-TSA method was carried out as follows. The olfactory tissue sections were reacted with the first primary antibody (overnight at 4°C), biotinylated goat anti-rabbit, biotinylated horse anti-mouse, or biotinylated donkey anti-goat secondary antibody (source and dilution, as indicated above; 1 h at room temperature), ABC reagent (1 h at room temperature), and tyramide-CY3 reagent (1/50 dilution for 5 min at room temperature), with PBS washes between each step. Following exposure to tyramide-CY3, the sections were washed in PBS, and then were incubated in 1% hydrogen peroxide in PBS, pH 7.0, to deactivate remaining horseradish peroxidase activity. The tissue sections were washed with PBS, incubated in block, reacted with the second primary antibody overnight at 4°C, and processed for detection with the tyramide-FITC reagent. Coverslips were mounted with ProLong Anti-Fade. Preliminary experiments, using appropriate controls indicated that this technique was selective and showed no antibody or detection reagent carryover when one primary antibody was a rabbit polyclonal, and the other, was a mouse monoclonal antibody, or, when both primary antibodies were made in rabbits. The experiments reported here include internal controls which dismiss the possibility of false positive labeling in same-species double labeling experiments. It will be noted (see Results, below) that in some same-species, double-labeling results reported here, only discrete, non-overlapping signals (CY3-generated red-channel signal and FITC-generated green-channel signal) are observed. In these experiments, each primary antibody is presented in a rabbit antiserum background or is, itself, a rabbit IgG. There would be an overlapping, “orange” rather than “red” signal) if there was non-specific rabbit IgG binding to potentially open anti-rabbit secondary IgG sites from the first step of the immunolabeling procedure in these experiments, but we do not observe such a result. Thus, we interpret those cases showing colocalized signal as being representative of true positive results. This interpretation is substantiated by the further observation that signals produced by colocalized primary antibodies show similar labeling patterns in single label experiments.
Double immunolabeling with the indirect immunofluorescence method was carried out with a CY3- or FITC-coupled secondary antibody diluted in PBS (1/50 Donkey anti-rabbit-CY3, Jackson # 711 165 152; 1/50 Donkey anti-rabbit-FITC, Jackson # 711 095 152). Tissue sections were incubated with the antibody directed against GFAP (Table 2) overnight at 4°C, washed with PBS, and incubated with the secondary antibody for 1 h at room temperature. The sections were washed in PBS, incubated in 1% hydrogen peroxide in PBS, pH 7.0, to deactivate remaining horseradish peroxidase activity, incubated in block, and reacted with the second primary antibody (anti-RALDH 1) overnight at 4°C. After incubation of the specimen with the primary antibody, the tissue sections were washed with PBS, incubated with secondary antibody (1 h at room temperature), washed with PBS, and coverslips were mounted with Prolong Anti-Fade.
Regio olfactoria sections from levels III and IV in the rostro-caudal dimension (Young, 1981) were prepared from three vitamin A-sufficient (VAS) and three vitamin A-deficient (VAD) animals. Sections from each animal were visualized with DAB in at least two different experiments conducted on different days. Endogenous peroxidase was neutralized with 0.03% hydrogen peroxide, followed by treatment with a casein-based protein reagent (DAKO Cytomation, Carpinteria, CA) to block nonspecific staining. The sections were incubated with the RALDH 1 antiserum, diluted 1/500, for 30 min. Sections reacted with RALDH 1 antiserum plus peptide antigen served as negative controls. The DAKO Cytomation Envision+ System HRP/DAB+ (DAKO Cytomation, Carpenteria, CA) was used to produce localized, visible staining. The sections were lightly counterstained with Mayer's hematoxylin.
Fluorescent images were captured using Simple PCI, version 4.0 software using a Nikon PCM2000 dual laser scanning confocal system. FITC was activated with an argon laser (excitation wavelength, 488 nm) and CY3 was activated with a helium-neon (HeNe) laser (excitation wavelength, 543 nm). Brightfield images and epifluorescent images showing full-sized olfactory region tissue sections were acquired with a QImaging Retiga 1300i Digital CCD camera attached to a Leica DM 4000D Microscope. Image sets collected to demonstrate animal to animal variability or experimental treatment effects on a particular parameter were collected using the same gain and intensity settings for all specimens in the image set. Digitized images were imported into Adobe Photoshop version 6.0.1 for editing, formatting, and, where necessary, background, contrast and brightness adjustments. All final, edited, images were evaluated for their accuracy in portraying either the original specimen as viewed under brightfield or epifluorescent microscopy or the original digitized output acquired using dual laser confocal microscopy.
RESULTS
Presence of RALDH mRNA in Postnatal Rat Olfactory Tissue
Single products of expected sizes were amplified in RT-PCR reactions prepared with olfactory cDNA and primers for RALDH 1 (Figure 1 A, lane 2, 300 bp) or RALDH 2 (Figure 1A, lane 4, 750 bp). The identities of the gel isolated RALDH 1 and RALDH 2 RT-PCR products were verified by DNA sequence analysis.
Figure 1.

PCR reactions with primers specific for RALDH 1, 2, and 3 and ALDH-PB were carried out as described in Materials and Methods using either 100 ng postnatal rat olfactory cDNA (A) or 100 ng genomic DNA (B). A. mRNAs encoding RALDH 1, 2, and 3 are expressed in postnatal rat olfactory tissue. Shown is an image of the gel-separated, RT-PCR products from reactions containing cDNA prepared from DNAse-I treated olfactory tissue RNA. Bands were visualized with SYBR Green I. Lane 1, 100 bp ladder; lane 2, RALDH 1; lane 3, ALDH-PB; lane 4, RALDH 2; lane 5, RALDH 3; lane 6, minus cDNA control reaction containing primers for all ALDH-PB / RALDH transcripts. B. ALDH-PB mRNA was not detected in olfactory tissue, but the ALDH-PB primers amplify authentic gene products from rat genomic DNA. Shown is an image of gel-separated, PCR products from reactions containing rat genomic DNA. Bands were visualized with SYBR Green I. Lane 1, RALDH 1; lane 2, ALDH-PB. Markers designating migration points of 300 and 400 bp ladder transcripts that were run on the gel are shown to the left of the image.
An RALDH 3 transcript was amplified from olfactory cDNA, but with difficulty. One of three primer sets designed to amplify this target sequence produced RT-PCR products from olfactory cDNA that included two bands of approximately 550 bp (Figure 1A, lane 5). DNA extracted from the upper band produced a very good DNA sequence read that was confirmed to encode RALDH 3. DNA extracted from the lower band produced a poor sequence read, suggesting the presence of more than one transcript in the reaction mix, with the predominant species showing homology to alpha-2u globulin PGCL4 (odorant binding protein 3), a transcript lacking sequence homology with RALDH 3.
An ALDH-PB product was not observed when the RT-PCR conditions that were used to amplify RALDH mRNAs from olfactory cDNA were used to amplify ALDH-PB mRNA (Figure 1A, lane 3). To evaluate the efficacy and efficiency of the ALDH-PB primer set, PCR reactions were carried out using rat genomic DNA and ALDH-PB and RALDH 1 primer sets. A single band of approximately 420 bp, verified to be ALDH-PB by DNA sequence analysis, was amplified up from rat genomic DNA (Figure 1B, lane 2). Levels of amplified ALDH-PB DNA were relatively low in comparison with those of amplified RALDH 1 DNA (Figure 1B, lane 1).
Relative Abundance of RALDH Transcripts in Postnatal Rat Olfactory Tissue
The results in Figure 1A provide a qualitative comparison of the relative levels of expression of the RALDH 1, 2, and 3 transcripts in postnatal rat olfactory tissue. A reaction cocktail containing all components of the PCR mix with the exception of primers specific for each mRNA was prepared and added to Hot Start™ tubes (Promega, Madison, WN) containing the primer sets. After 30 cycles of amplification, equal aliquots of the reaction products were separated on a 2% agarose/TBE gel, and the relative intensities of the SYBR I-green stained bands produced in the RALDH 1, 2, and 3 reactions were compared. The RALDH 1 (4.96 × 106 arbitrary units, AU, of intensity) and RALDH 2 (3.83 × 106 AU) transcripts were highly amplified, reaching threshold levels of transcript accumulation under these conditions. The band for the RALDH 3 transcript was faint after 30 cycles of amplification (0.84 × 106 AU; upper band, Figure 1, lane 5), but reached threshold levels by 40 cycles of PCR amplification (data not shown).
Presence of RALDH Protein in Postnatal Rat Olfactory Tissue
Immunoblots
Polyclonal rabbit antisera directed against RALDH 1, 2, and 3 were tested on immunoblots prepared from total rat olfactory protein extracts separated on sodium dodecyl sulfate polyacrylamide gels. The RALDH 1 and RALDH 3 antisera each reacted with a single band of approximately 55 kD (the theoretical molecular weights of RALDH 1, 2, and 3 as calculated from their predicted amino acid sequences are 54459, 54740, and 56171, respectively). The RALDH 2 antiserum reacted strongly with a band of the expected size and weakly with two bands of relatively higher and one band of relatively lower molecular weight (data not shown). Reactivity with the RALDH 1 antiserum was completely blocked when immunoblots were incubated with antiserum pre-absorbed with the RALDH 1 peptide immunogen (data not shown).
Olfactory Tissue Immunoreactivity with the RALDH Antisera
All three RALDH antisera showed reactivity with olfactory region tissue sections from postnatal rats (Figure 2). The RALDH I antiserum labeled cells and cell processes in both the olfactory epithelium and the underlying lamina propria (Figure 2A). This reactivity was completely blocked when RALDH 1 antiserum was pre-absorbed with the RALDH 1 peptide immunogen (data not shown). The RALDH 2 antiserum displayed a very different labeling pattern than the RALDH 1 antiserum. Unlike RALDH 1, RALDH 2 immunoreactivity was not observed in the epithelium (Figure 2B). In the underlying lamina propria, the RALDH 2 antiserum labeled a fibrous network with a morphology characteristic of interconnected cellular processes and/or connective tissue structures (Figure 2B). Similar to RALDH 1, the RALDH 3 antiserum stained olfactory epithelium and lamina propria throughout tissue sections. The RALDH 3 antiserum labeled cells and cell processes in the epithelium and the underlying lamina propria (Figure 2C). Double-immunolabeling studies using the RALDH 1 and RALDH 3 antisera indicated colocalization of the RALDH 1 and RALDH 3 isozymes in olfactory epithelium and underlying stroma (data not shown, but see Supplementary Figure One). All three antisera were highly reactive with respiratory epithelium (data not shown).
Figure 2. RALDH 1, 2, and 3 proteins are present in postnatal rat olfactory tissue.
Olfactory region paraffin sections, prepared from postnatal rats, were single-labeled with RALDH 1 (plate A), RALDH 2 (plate B), or RALDH 3 (plate C) antisera. Reactivity was visualized using tyramide-FITC (RALDH 1 and 2) or tyramide-CY3 (RALDH 3) using the ABC-TSA method as described in Materials and Methods. Shown are single plane, confocal, images. Arrows designate location of oe, olfactory epithelium and lp, lamina propria . Scale bars represents 50 μm.
RALDH 1 and 2 isozymes do not display gradients of expression in postnatal rat epithelium and underlying lamina propria
Norlin et al (2001) reported that RALDH 2 mRNA is expressed in a gradient across the olfactory organ of postnatal mice, with areas of high and low expression mapping to zones in the olfactory organ previously shown to express different subsets of olfactory receptor neurons (Ressler et al., 1993; Vassar et al., 1993). We questioned whether RALDH 1 or 2 proteins exhibit expression gradients in the postnatal rat olfactory organ.
Reactivity with the RALDH 1 antiserum varied throughout olfactory region tissue sections, but this variable pattern of expression was random and did not suggest a gradient (data not shown here, but see Figure Nine, below). The RALDH 1 antiserum exhibited intense labeling of cell processes in the apical and/or basal aspects of the epithelium in some areas of olfactory region tissue sections, whereas in other areas, RALDH 1 immunoreactivity was relatively weak or absent in the epithelium. Some areas of tissue sections, exhibited intense labeling of dense aggregates of cellular structures in the lamina propria with a morphology characteristic of Bowman's glands; while staining in other areas of the lamina propria revealed few or no stained Bowman's glands, but a more apparent labeling of cellular processes in fila olfactoria.
Figure 9. RALDH 1 expression is altered by vitamin A deficiency in the postnatal rat olfactory organ.
Postnatal rats were made vitamin A deficient (VAD) by feeding them a diet lacking a source of vitamin A from before weaning to approximately postnatal day 90. Control rats (VAS) were fed the same diet supplemented with a source of vitamin A. Skulls from three age-matched VAS and three VAD animals were used to prepare paraffin-embedded olfactory region tissue sections. Tissue sections from different regions across the rostro-caudal dimension were reacted with RALDH 1 antiserum. Sections from all six animals were used in at least two different experiments conducted on different days. Positive immunoreactivity was visualized using detection with diaminobenzadine (DAB) as described in Materials and Methods. Representative results from all six animals are shown. A-D. Low magnification, brightfield images of olfactory region sections from VAS animals 1 and 2 (plates A and C, respectively) and VAD animals 1 and 2 (plates B and D, respectively). Scale bar in plate A = 500μm. Note the elevated intensities of RALDH 1 signal in VAD tissues as compared with VAS tissues in plates B and D versus A and C. E-F. Higher magnification, brightfield images of olfactory region sections from VAS animal 3 (plate E) and VAD animal 3 (plate F) reacted with RALDH1. Scale bars in plates E and F = 100 μm. Note the variability in RALDH 1 expression in different areas of both VAS and VAD tissue sections in plates E and F. Sus/ecp-labeled arrows depict areas with RALDH 1-labeled sustentacular (sus) cells in the olfactory epithelium and ensheathing cell processes (ecp) in fila olfactoria in the lamina propria, but relatively few RALDH 1-labeled Bowman's glands in the lamina propria. Bg-labeled arrows depict areas with dense aggregates of RALDH 1-stained Bowman's glands in the lamina propria and relatively few RALDH 1-stained sustentacular cells in the olfactory epithelium or ensheathing cells in the lamina propria. The staining intensity of RALDH 1 is elevated in these areas in VAD tissue as compared with VAS tissue, but particularly so in areas of dense Bowman's gland staining (compare the image in plate F with the image in plate E).
We observed differences in the RALDH 2 staining intensities in different regions of the olfactory organ with antiserum dilutions found optimal for characterizing the immunoreactive properties of olfactory tissue (Figure 3). We questioned whether a gradient might be masked by these antibody levels, since areas with high RALDH 2 immunoreactivity may produce fluorescent signals exceeding threshold levels of detection. Thus, decreasing concentrations of RALDH 2 antiserum were reacted with olfactory region tissue sections to determine whether lowered levels of antibody might reveal gradients of expression. At the highest concentrations of RALDH 2 antiserum, RALDH 2 protein was observed in fibrous networks in all areas of the olfactory organ lined with sensory tissue. Lower concentrations of RALDH 2 antiserum revealed consistently high levels of staining in the dorsal meatus and the septum-facing aspect of the ventral arm of endoturbinate 2 and otherwise variable staining intensities in other parts of the lamina propria (Figure 3). Careful inspection of results from four animals, at different levels of resolution, failed to reveal a gradient of RALDH 2 protein expression in postnatal rat olfactory tissue.
Figure 3. RALDH 2 does not exhibit a gradient in postnatal rat olfactory sensory tissue.
Olfactory region frozen sections, prepared from four different postnatal rats (plates 1−4), were single-labeled with RALDH 2 antiserum and tyramide-FITC using the ABC-TSA method as described in Materials and Methods. Shown are digital epifluorescent images acquired using a 1.25× objective.
Localization of RALDH 1 Protein in Postnatal Rat Olfactory Sensory Tissue
Double immunolabeling experiments were carried out to more specifically identify areas of RALDH 1 expression in the postnatal olfactory epithelium and underlying lamina propria. The following phenotypic markers were used: Olfactory Marker Protein (OMP), a marker for mature olfactory neurons (Verhaagen et al., 1990); growth associated protein (GAP-43), a marker for immature olfactory neurons (Verhaagen et al., 1990); SUS 4, a marker for sustentacular cells and Bowman's gland cells (Goldstein and Schwob, 1996); glial fibrillary acidic protein (GFAP) and the low-affinity nerve growth factor receptor (p75), markers for olfactory ensheathing cells (Gong et al., 1994; Kafitz and Greer, 1999; Tisay and Key, 1999; Au and Roskams, 2003).
The RALDH 1 antiserum did not label mature or immature olfactory receptor neuron cell bodies or processes in the olfactory epithelium (Figure 4 A-4 F) or axons in fila olfactoria in the lamina propria underlying the olfactory epithelium (Figure 4B, 4C, 4E, 4F). The labeling patterns of RALDH 1 and SUS 4 were similar (compare Figure 4G with 4H), and RALDH 1 colocalized with SUS 4 in the olfactory epithelium, where it labeled sustentacular cell bodies in the supranuclear zone of the epithelium, sustentacular cell processes extending to the apical edge of the epithelium, and sustentacular cell processes terminating in bulbous endfeet along the basal lamina (Figure 4G and 4I). RALDH 1 labeling was observed in Bowman's gland cells as indicated by morphologic identification (Figure 4A and 4C) and by colocalization with SUS 4 (Figure 4I). RALDH 1 labeling was observed in OMP− / GAP-43− cell processes that encircled and subdivided the axon bundle space in fila olfactoria in the lamina propria (Figure 4A, 4C, 4D, and 4F). This labeling pattern is characteristic of olfactory ensheathing cell processes (Field et al., 2003). Consistent with this morphologic identification, RALDH 1 colocalized with the olfactory ensheathing cell marker, GFAP, in these processes (Figure 4J-4L).
Figure 4. Localization of RALDH 1 immunoreactivity in postnatal rat olfactory sensory tissue.
Olfactory region sections were prepared from postnatal rats and processed for reactivity using either the ABC-TSA method (plates A-I) or the indirect immunofluorescence method (plates J-L). See Materials and Methods section for details. Non-overlapping CY3 and FITC-signals are pseudocolored red and green, respectively; and co-localized CY3 and FITC-signals are pseudocolored yellow in images showing merged signals (plates C, F, I, and L). A-C. Colocalization with anti-RALDH 1 (FITC) and OMP (CY3), a marker for mature olfactory neurons. Plates A-C show the same deparaffinized, tissue section with RALDH 1-FITC signal only (plate A), OMP-CY3 signal only (plate B), or merged signals (plate C). D-F. Colocalization with RALDH 1(FITC) and GAP-43 (CY3), a marker for immature olfactory neurons. Plates D-F show the same deparaffinized, tissue section with RALDH 1-FITC signal only (plate D), GAP-43-CY3 signal only (plate E), or merged signals (plate F). G-I. Colocalization with RALDH 1 (FITC) and SUS 4 (CY3), a marker for sustentacular cells and Bowman's Glands. Plates G-I show three different tissue sections: frozen section single-labeled with anti-RALDH 1 (plate G), frozen section single-labeled with anti-SUS 4 (plate H), and frozen section double-labeled with anti-RALDH 1 and anti-SUS 4 (plate I). J-L. Co-localization with RALDH 1(FITC) and GFAP(CY3), a marker for olfactory ensheathing cells. Plattes J-L show the same double-labeled frozen section with RALDH 1-FITC signal only (plate J), GFAP-CY3 signal only (plate K), or merged signals (plate L). Scale bars represent 20 μm. Single-plane images were acquired using dual laser confocal microscopy. –S, sustentacular cell body; -mn, mature neuron cell body; -in, immature neuron cell body; -bg, Bowman's Gland cell; -ecp, ensheathing cell process in fila olfactoria; -ax, axons in fila olfactoria.
Localization of RALDH 2 Protein in Postnatal Rat Olfactory Sensory Tissue
RALDH 2 labeling was observed in processes that encircle and subdivide the axon bundle space in fila olfactoria in the lamina propria (Figure 5 A-5 B; see also Figure 5G and 8A, below), but RALDH 2 did not colocalize with either RALDH 1 (Figure 5A) or GFAP (Figure 5B). RALDH 2 labeling was also observed in cell processes / basement membrane-type structures along the basal lamina and throughout the lamina propria (Figure 5A-B).
Figure 5. RALDH 1 and RALDH 2 do not colocalize in the postnatal rat olfactory organ.
Olfactory region sections were prepared from postnatal rats and processed for reactivity with antibodies as described in Materials and Methods. Shown are confocal images, with FITC signals pseudocolored green and CY3 signals pseudocolored red. A. RALDH 1 and RALDH 2 do not colocalize in postnatal olfactory tissue. Deparaffinized tissue section double-labeled with RALDH 1 (FITC) and RALDH 2 (CY3) antisera. Note RALDH 1-stained sustentacular cells (S-labeled arrow) in the olfactory epithelium and ensheathing cell processes in fila olfactoria (FO-labeled arrow) in the lamina propria. RALDH 2 labeling is not present in the olfactory epithelium, but is seen in processes located on the periphery of the fila olfactoria, as well as throughout the lamina propria and along the basal lamina (arrowheads). B. RALDH 2 and GFAP do not colocalize in postnatal olfactory tissue. Deparaffinized tissue section double-labeled with RALDH 2 (FITC) and RALDH 2 (CY3) antisera. Note GFAP-stained ensheathing cell processes in fila olfactoria (FO-labeled arrow) in the lamina propria. RALDH 2-stained processes encircle the fila olfactoria, and are also present along the basal lamina (arrowheads). C-D. p75 labels processes in the lamina propria underlying the olfactory epithelium of postnatal rats. Frozen section single-labeled with anti-p75 (CY3) shown at low (plate C) and high (plate D) magnification. E. RALDH 1 (FITC) does not colocalize with p75 (CY3). Deparaffinized tissue section double-labeled with RALDH 1 (FITC) antiserum and p75 (CY3) antibody. Note RALDH 1-stained sustentacular cells in the olfactory epithelium (S-labeled arrow) and ensheathing cell processes in fila olfactoria (FO-labeled arrow) in the lamina propria. p75-stained processes encircle the fila olfactoria. F-H. RALDH 2 and p75 labeling patterns follow similar trajectories, but do not colocalize. Frozen tissue sections double-labeled with RALDH 2 (FITC) antiserum and p75 (CY3) antibody. F. Note p75 is present in ensheathing cell processes that subdivide the axon fiber bundles in fila olfactoria (arrows) in the lamina propria. G. Note that both p75 (arrow pointing to the right) and RALDH 2 (downward pointing arrow) label ensheathing cell processes that sub-divide the axon fiber bundles in fila olfactoria (FO). p75 also labels processes along the basal lamina (arrowheads). H. RALDH 2 labeling is evident in processes immediately adjacent to the exterior aspect of an axon fiber bundle (small arrows) found within fila olfactoria (FO). p75 labeling is also evident in processes immediately adjacent to the exterior aspect of the same axon fiber bundle (large arrows; note, in this case, the p75-labeled process is partially delaminated from the axon fiber bundle as a result of tissue processing). Scale bars represent 50 μm in plate C and 20 μm in plates A, B, and D-H. S, sustentacular cells; FO, fila olfactoria OE, olfactory epithelium; and LP, lamina propria.
Figure 8. RALDH 2 colocalizes with CRBP I in the lamina propria underlying the olfactory epithelium of postnatal rats.

A. RALDH 2 labels lamina propria (LP) but not olfactory epithelium (OE). Frozen tissue section single-labeled with RALDH 2 and visualized with tyramide-FITC. B. CRBP I labels OE and LP. Frozen tissue section single-labeled with CRBP 1 and visualized with tyramide-CY3. As in Figure 7, note CRBP I exhibits dispersed punctate labeling in the OE (arrowheads; see also plate D), as well as punctuate labeling of Bowman's gland ducts (arrow, BGD). CRBP I labels cell processes / basement membrane-type structures in the LP. C-D. Different regions of the same cryosection double-labeled with RALDH 2 (green) antiserum and CRBP 1 (red) antibody are shown at low (plate C) and high (plate D) powers of magnification. CRBP 1 and RALDH 2 are colocalized in some, but not all cell processes / basement membrane-type structures in the LP. Note, the cell processes / basement membrane-type structures that exhibit a yellow signal (arrows in plate D), particularly surrounding fila olfactoria (FO) and along the basal lamina (BL-labeled arrows in plate D), indicating colocalization of the proteins. Scale bars represent 50 μm in plate C and 20 μm in plates A, B, and D.
Low affinity nerve growth factor receptor/p75 had a very different staining pattern than GFAP in the postnatal rat olfactory organ. Whereas GFAP (and RALDH 1) were expressed in olfactory ensheathing cell processes that were primarily within fila olfactoria (compare Figures 4C and 4D with Figures 4B and 4A), p75 was expressed in processes exterior to those labeled by anti-GFAP and anti-RALDH 1 antibodies (Figure 5E). p75 staining was observed in cell processes / basement membrane-type structures surrounding nerve fascicles and nerve fibers (Figures 5F-5H) and also along the basal lamina (Figure 5G).
The labeling patterns of RALDH 2 and p75 were very similar, but there was no evidence for colocalization of their signals (Figures 5F-5H). Close examination of tissue sections suggested that the two proteins were expressed by similar cells (based on morphologic considerations), but that expression of one protein by a particular cell precluded expression of the other. For example, RALDH 2 and p75 were both intermittently expressed in cell processes / basement membrane-type structures along the basal lamina; however, the two proteins were never coexpressed at this site (Compare Figures 5A and 5B, where RALDH 2 is expressed along the basal lamina, with Figure 5G, where p75 is expressed along the basal lamina). Similarly, both proteins labeled cell processes surrounding or traversing fila olfactoria, but the signals for RALDH 2 and p75 did not overlap (Figure 5F-5H). Figure 5H shows an example of two cell processes, one expressing RALDH 2 (designated by small arrows) and the other expressing p75 (designated by large arrows), that encircle an axon fiber bundle. The RALDH 2- and p75- stained processes both share direct contact with the inner axon fiber space, but in different locations along the periphery of the bundle. Note that the p75-labeled cell process is partially delaminated from the axon fiber bundle as a consequence of tissue processing.
RALDH 1 colocalizes with CRABP II in Postnatal Rat Olfactory Sensory Tissue
RALDH 1 colocalizes with CRABP II in sustentacular cells in the olfactory epithelium. Punctate staining by the CRABP II antibody colocalizes with RALDH 1 around sustentacular cell nuclei (Figure 6 A-6 C), in sustentacular cell apical processes (Figure 6A-6C) and in sustentacular cell endfeet along the basal lamina (Figure 6A-6B). These findings confirm our earlier prediction, based on morphological evidence, that CRABP II might be localized in sustentacular cells (Asson-Batres et al., 2003b). Additionally, CRABP II is co-expressed with RALDH 1 in Bowman's gland cells in the lamina propria (Figure 6A-6B) and is present in areas of the olfactory epithelium where RALDH 1 labeling is not observed (Figure 6C-6D).
Figure 6. CRABP II colocalizes with RALDH 1 in Sustentacular cells and Bowman's gland cells in postnatal rat olfactory sensory tissue.
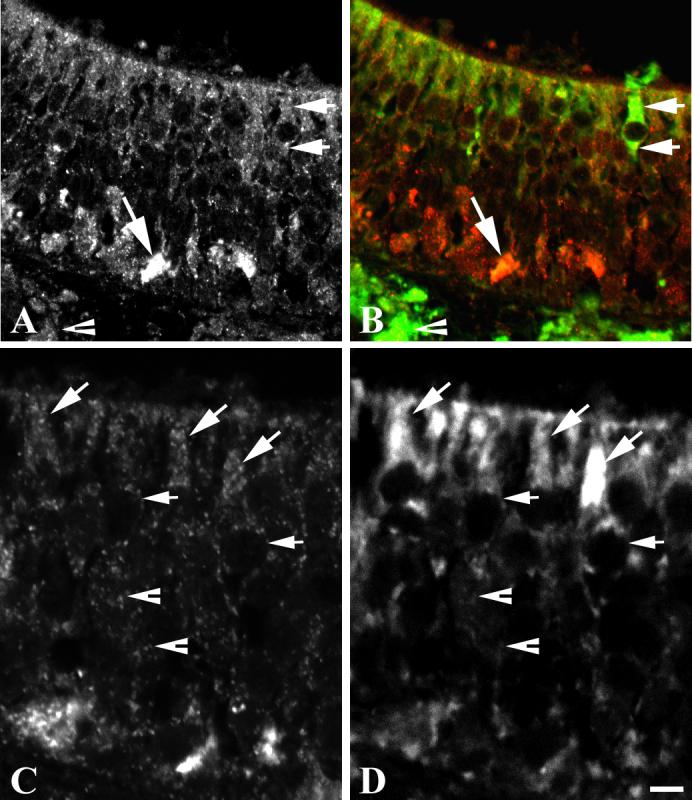
A-D. Images of a deparaffinized tissue section double-labeled with RALDH 1 (FITC) and CRABP II (CY3) were acquired at 600X (plates A and B) and 1000X (C and D) magnification. Shown are single-plane, confocal images of CY3 signal only (plates A and C), merged signals (plate B), and FITC signal only (plate D). Non-overlapping CY3 and FITC-signals are pseudocolored red and green, respectively; and co-localized CY3 and FITC-signals are pseudocolored yellow in the image showing merged signals (B). A-B. Two horizontal arrows point to an RALDH 1-labeled sustentacular cell that shows punctate labeling with CRABP II around the nucleus and apical and basal-directed processes of the cell (plates A and B). The diagonal arrow in plates A and B points to a sustentacular cell endfoot intensely labeled by both RALDH 1 antiserum and CRABP II antibodies (merged signal is yellow). Note, also, the yellow punctuate signal in Bowman's gland cells in the lamina propria (arrowheads in plates A and B), indicative of CRABP II-RALDH 1 colocalization in these cells. C-D. Diagonal arrows denote punctate staining with CRABP II antibody (plate C) and diffuse staining with RALDH 1 antiserum (plate D) in apical processes of sustentacular cells. Horizontal arrows denote punctate CRABP II labeling around the nucleus of sustentacular cells (plate C). Arrowheads denote punctate CRABP II labeling in areas of the olfactory epithelium that do not show staining with the RALDH 1 antiserum. Scale bar represents 20 um.
CRBP I is associated with cells expressing RALDH 1 and RALDH 2
Expression of cellular retinol binding protein type I (CRBP I) was reported to be in the membranous coverings of postnatal mice fila olfactoria (Gustafson et al., 1999). We questioned whether CRBP I showed a similar expression pattern in postnatal rats and, if so, whether it colocalized with RALDH 1 or RALDH 2.
Colocalization of CRBP I with RALDH 1 was observed in olfactory ensheathing cell processes on the periphery of fila olfactoria in the lamina propria underlying the olfactory epithelium (Figure 7 A-7 C). Diffuse CRBP I labeling was observed along the apical processes of sustentacular cells (S-labeled arrows, Figure 7D). Punctate CRBP I labeling was observed along sustentacular cell processes, primarily in the central aspect of the olfactory epithelium (arrowheads, Figure 7A, 7C, and 7D), in fila olfactoria (FO-labeled arrows, Figure 7A-7C), and in close association with Bowman's gland cells (BG-labeled arrows, Figure 7D). CRBP I expression was also observed in Bowman's gland ducts in the olfactory epithelium (BGD-labeled arrows, Figure 7A and 7C) and in cell processes / basement membrane-type structures throughout the lamina propria (Figure 7A, 7C, 7D and Figure Eight, below).
Figure 7. CRBP I labeling follows RALDH 1 labeling in postnatal rat olfactory sensory tissue.

A-C. Frozen section double labeled with RALDH 1 (green) antiserum and CRBP I (red) antibody. Confocal image of the tissue section showing: A. CRBP 1-CY3 signal only. B. RALDH 1-FITC signal only. C. merged RALDH 1-CRBP I signals. D. Image showing merged RALDH 1-CRBP I signals in a different region of the same tissue section. RALDH 1 and CRBP I show colocalization (yellow) in an ensheathing cell process (ECP-labeled arrows) located on the periphery of a nerve fiber bundle in the lamina propria (LP, plates A-C). Elsewhere, the labeling patterns of CRBP I and RALDH 1 overlap, but do not exhibit colocalization as defined by a yellow pseudocolored signal. For example, note the diffuse CRBP I staining associated with the apical and basal processes of RALDH 1-labeled sustentacular cells (S-labeled arrows in plate D) and the punctuate CRBP I staining in the central area of the olfactory epithelium (OE, arrowheads in plates C and D) that also appears to be associated with sustentacular cell processes. Additionally, punctate CRBP I staining is associated with RALDH 1-labeled fila olfactoria (FO) and Bowman's Gland cells (BG-labeled arrows) in the LP (plates C and D). CRBP I also labels Bowman's gland duct cells in the OE (BGD-labeled arrows in plates A and C) and cell processes / basement membrane-type structures throughout the LP that are not labeled by RALDH 1 (plates A and C). Scale bars represent 20 μm.
The staining patterns of CRBP 1 and RALDH 2 followed very similar (Figure 8 A and 8B), and closely apposed, trajectories in the lamina propria (Figure 8C and 8D). Co-expression of CRBP I and RALDH 2 was observed in cell processes surrounding fila olfactoria (arrows, Figure 8D), as well as in cell processes / basement membrane-type structures along the basal lamina (BL-labeled arrows, Figure 8D). In other areas, the proteins exhibited non-overlapping patterns of expression (Figure 8C-8D).
Effect of Vitamin A Status on the expression of RALDH 1 and 2
Expression of RALDH 1 protein was altered in the olfactory epithelium and underlying lamina propria of vitamin A-deficient (VAD) postnatal rats (Figure 9), as indicated by relatively higher staining intensities of RALDH1-DAB conjugates in VAD olfactory region tissue sections as compared with tissue from age-matched vitamin A-sufficient (VAS) control tissue (Figure 9A-D). Dense aggregates of RALDH-1 stained Bowman's glands were observed in some regions of olfactory region sections where there were few or no RALDH 1-stained sustentacular (sus) cells or olfactory ensheathing cell processes (ecp) and, conversely, RALDH 1 expression was highly elevated in sustentacular cells and ensheathing cell processes in areas where Bowman's gland expression was negligible (compare areas designated by bg-labeled arrows with areas designated by sus-ecp-labeled arrows, Figure 9E and 9F). The variable pattern of RALDH 1 expression across olfactory region sections appeared to be accentuated in VAD tissue as compared with age-matched, VAS control tissue, particularly in areas of relatively high Bowman's gland staining by RALDH 1 (Figure 9E and 9F). We observed similar results in repeated experiments using tissues from three different VAD and three different VAS animals using two different methods of detection (DAB, Figure 9, and FITC, data not shown).
Vitamin A deficiency had no apparent effect on the relative intensities or expression patterns of RALDH 2-FITC conjugates on olfactory tissue sections from the same animals used to evaluate effects of vitamin A status on RALDH 1 (Figure 10).
Figure 10. RALDH 2 expression is not affected by vitamin A deficiency in the postnatal rat olfactory organ.

Olfactory region tissue sections were prepared from VAD and VAS rats, as described in Figure 9, and reacted with RALDH 2 antiserum. Signal detection was with the ABC-TSA system, as described in Materials and Methods, using FITC as the fluorophore. Shown are confocal images of VAD (A and C) and VAS (tissue sections) shown at different powers of magnification. Scale bars are 50 μm in A and B; 20 μm in C and D. Similar signal intensities and labeling patterns of fibrous structures in lamina propria are seen throughout the olfactory organ of VAD and age-matched, control animals.
DISCUSSION
The results of studies reported here indicate that RALDH 1 and RALDH 2 exhibit high levels of expression in different non-neuronal cell types throughout the postnatal rat olfactory organ and are found in close association with proteins that bind and transport retinol/retinaldehyde substrates and retinoic acid products utilized or produced by RALDH-catalyzed reactions. RALDH 1, but not RALDH 2, expression is altered in olfactory tissue in response to vitamin A deficiency. The RALDH isozymes and the retinoid binding proteins exhibit spatio-temporal changes in their patterns of distribution within postnatal olfactory epithelium and underlying lamina propria. The redundancy in RALDH isozyme expression, the variable patterns of RALDH protein expression within the tissue, and the differential responses of RALDH 1 and 2 protein levels to tissue retinoid status suggests that a complex system, based on dynamic changes in the location and expression levels of RALDHs and retinoid binding proteins within postnatal olfactory tissue microenvironments, may be regulating retinoic acid availability in response to physiological demands, including changes in the developmental and maturational states of neurons in the olfactory epithelium and axons in the underlying lamina propria.
mRNAs encoding RALDH 1, 2, and 3 were all expressed in postnatal rat olfactory tissue
A qualitative estimate of the relative amounts of mRNA encoding the three RALDH transcripts was made by comparing transcript accumulation in RT-PCR reaction mixes that contained equivalent amounts of starting cDNA and primers shown to be capable of optimal amplification of each transcript. By this analysis, RALDH 1 mRNA was most abundant, RALDH 2 and RALDH 3 mRNA levels were 77% and 17%, respectively, of those of RALDH 1.
ALDH-PB mRNA was not detected in olfactory tissue by RT-PCR analysis
The rats used for the studies reported here were not induced with phenobarbital. Phenobarbital is a substrate for ALDH-PB, and treatment of responsive rats with this drug induces both ALDH-PB mRNA and protein expression. As a consequence of this induction, liver ALDH-PB enzyme activities are elevated to levels that can be as high as 10-fold greater than those observed in untreated rats (Tank et al., 1986; Dunn et al., 1989). ALDH-PB mRNA has been shown to be weakly expressed in liver from untreated rats and to be absent from the heart, brain, spleen, muscle, kidney and testis of these animals (Kathmann et al., 2000).
We observed relatively high levels of RALDH 1 mRNA in postnatal olfactory tissue from uninduced rats
In contrast, ALDH-PB mRNA was not detected in this tissue under the conditions used for these experiments, suggesting that ALDH-PB transcripts are either not present, or are expressed at negligible levels in the postnatal rat olfactory organ. Positive control reactions indicated the specificity of the PCR primers since ALDH-PB DNA was amplified from rat genomic DNA. Thus, although the RALDH 1 antiserum recognizes both purified, recombinant RALDH 1 and purified, recombinant ALDH-PB proteins (Kathmann et al., 2000), we interpret the strong, positive reactivity of the RALDH 1 antiserum with olfactory tissue to be attributable to reactivity with RALDH protein. This interpretation is supported by in situ hybridization studies carried out by others in postnatal mouse olfactory tissue sections (Niederreither et al., 2002a), showing an RALDH 1 mRNA labeling pattern of olfactory epithelium and underlying lamina propria that is similar to the labeling pattern we observe in rat olfactory tissue sections reacted with the RALDH 1 antiserum.
RALDH 1 immunoreactivity was localized to cells with morphologies characteristic of sustentacular cells, olfactory ensheathing cells, and Bowman's Gland cells
Colocalization with phenotypic markers for these cells (SUS 4, sustentacular cells and Bowman's Glands; and GFAP, olfactory ensheathing cells) confirmed the morphological idenitification of RALDH 1 expression sites. The low affinity nerve growth factor receptor/p75, which has also been used as a phenotypic marker for olfactory ensheathing cells in the peripheral olfactory system (Gong et al., 1994; Au and Roskams, 2003), did not colocalize with RALDH 1. Our results indicate that p75 showed positive immunoreactivity with processes traversing and encompassing nerve fiber bundles in the lamina propria of postnatal rats, consistent with the expected location of olfactory ensheathing cells; however, p75 labeling did not colocalize with GFAP. Whereas labeling of olfactory ensheathing cells with GFAP and RALDH 1 was primarily in processes associated with the internal aspects of fila olfactoria (Figure 5A and 5B), labeling with p75 was primarily restricted to the outer boundary of nerve fiber bundles in both single-labeled specimens (Figure 5D), and double-labeled tissue sections (Figure 5E). These results suggest there may be two distinct types of olfactory ensheathing cells in the lamina propria underlying the olfactory epithelium of postnatal rats, or, alternatively, that these markers are expressed in different regions of the same cells or in cells that are in different stages of development. Evidence for the existence of more than one type or sub-type of olfactory ensheathing cell in the rodent olfactory organ is supported by in vitro and in vivo studies reported from other laboratories (Pixley, 1992; Au and Roskams, 2003).
RALDH 1 did not colocalize with RALDH 2, indicating that RALDH 1 and RALDH 2 are expressed by different non-neuronal cell types in postnatal olfactory tissue
Consistent with its lack of colocalization with RALDH 1, RALDH 2 did not colocalize with GFAP. The labeling pattern of RALDH 2 was very similar to that of p75, but actual overlap of RALDH 2 and p75 signal was not observed. RALDH 2 and p75 were both present in fibrous structures closely associated with the external aspect of nerve fiber bundles and in fibrous structures along the basal lamina. Expression of RALDH 2 and p75 was intermittent throughout the lamina propria, and it appeared as though expression of one antigen within a particular cellular or tissue site precluded expression of the other. The significance of the lack of p75-RALDH 2 colocalization in what appear to be morphologically-similar structures is not known, but may indicate differences in the differentiation, metabolic, or functional state(s) of the cellular sites expressing the two proteins.
Based on morphologic considerations, one site of RALDH 2 expression in the peripheral olfactory tract may be GFAP− olfactory ensheathing cell processes that are located peripheral to RALDH 1+/GFAP+ olfactory ensheathing cell processes
Alternatively, RALDH 2 may be expressed by olfactory fibroblast cells, whose processes are known to envelop the outer layer of nerve fiber bundles (Field et al., 2003). Higher levels of microscopic resolution will be needed to resolve this issue and to firmly identify the cellular expression sites of RALDH 2 in the olfactory organ.
The RALDH 3 antiserum used in these studies did not reveal a unique labeling pattern for RALDH 3 in postnatal rat olfactory tissue
The RALDH 3 antiserum has been shown to exhibit robust and specific labeling in other tissues; for example, it has been shown to be highly reactive with a subpopulation of periglomerular cells in mouse olfactory bulb that do not exhibit labeling with antibodies directed against RALDH 1 (Wagner et al., 2002). The relatively high expression levels of RALDH 1 and 2 mRNA in rat olfactory tissue and low levels of RALDH 3 mRNA, as observed in studies reported here using RT-PCR analysis, are consistent with results of Niederreither et al. (2002a) who showed relatively high levels of RALDH 1 mRNA expression, moderate levels of RALDH 2 mRNA expression, and relatively low levels of RALDH 3 mRNA expression in postnatal day 22 mouse olfactory region sections, by in situ hybridization analysis. Together, these results suggest that RALDH 3 protein levels are likely to be low in sensory regions of the postnatal rodent olfactory organ. Notably, the labeling patterns produced by the RALDH 1 and 2 riboprobes in the Niederreither et al (2002a) study are similar to those produced by the RALDH 1 and 2 antisera reported here, with RALDH 1 mRNA expression being evident in the olfactory epithelium and underlying lamina propria, and RALDH 2 mRNA expression restricted to the lamina propria. The RALDH 3 riboprobes appear to primarily label cells located along the basal lamina of the olfactory epithelium (Niederreither et al, 2002a). Double labeling experiments using the RALDH 1 and RALDH 3 antisera showed complete overlap of the RALDH 1 and RALDH 3 signals (data not shown). Colocalization of these signals is most likely a reflection of cross-reactivity of the RALDH 3 antiserum with RALDH 1 protein, rather than an indication that both isozymes are expressed by the same cell. Failure to find a unique site of RALDH 3 protein expression in sensory regions of the postnatal olfactory organ is likely due to the very low abundance of RALDH 3 protein in this tissue.
At present, the most conservative interpretation of available biochemical data regarding the catalytic properties of RALDH 1, 2, and 3 is that they are each capable of catalyzing the synthesis of retinoic acid in in vivo microenvironments that provide substrate access (Table 1A)
It is currently not possible to assess their relative efficiencies in vivo because a rigorous definition of the physiologic conditions encountered by the isozymes has not been determined. It has been suggested that RALDH 2 displays greater catalytic efficiency for all-trans retinaldehyde than RALDH I (Gagnon et al., 2002) and may serve a more important role in facilitating retinoic acid synthesis in vivo. There are two problems with these interpretations. First, the measured Kms (0.6−11.6 uM) and catalytic efficiencies (0.32−49) for these isozymes fall within the same ranges (Table 1A). The wide variability in reported values is likely due to differences in experimental conditions and assay methods. Second, the assays used to generate these data were not designed to provide physiologically relevant evidence regarding potential differences in isozyme activities in vivo. For example, the optimal pH and temperature of catalysis for RALDH 1 and 2 are outside of the predicted physiologic range, and the reported in vitro assays were carried out at 24−25°C (Gagnon et al., 2002; Montplaisir et al., 2002; Gagnon et al., 2003), and, in some cases, a pH of 8.5 (Wang et al., 1996; Penzes et al., 1997; Kathmann et al., 2000). The assessment of relative physiologic functionality is further complicated by a need to take into consideration potential effect(s) that factors in an isozyme's microenvironment have on its actual in vivo activity. As an example, knowledge of local magnesium concentrations is required, since it has been demonstrated that MgCl2 inhibits RALDH 1 and enhances RALDH 2 activity (Kathmann et al., 2000; Gagnon et al., 2002; Gagnon et al., 2003).
The observation that multiple RALDH isozymes are expressed in the peripheral olfactory system is not unique to this postnatal tissue
Multiple RALDH isozymes have also been characterized in eye, liver, lung, skin, and reproductive tissues in postnatal animals (Mey et al., 1997; Zhai et al., 2001; Fan et al., 2003; Everts et al., 2004; Li et al., 2004). The physiologic relevance of the noted redundancy in RALDH isozyme expression in late stage mammalian embryos and postnatal animals is not understood. The expression of multiple functional RALDHs may provide a means of fine-tuning the availability of retinoic acid to cells during advanced stages of development when organisms are composed of more complex and specialized tissue and organ systems. Increased regulation may be required to ensure that adequate amounts of this essential growth (and possibly, maintenance) factor are available, particularly under conditions of vitamin A deficiency or changing retinoid status, and/or to prevent the accumulation of toxic levels of retinaldehyde or retinoic acid during late stages of fetal development and throughout adult life.
Regardless of the reason for RALDH redundancy, the presence of RALDH 1, RALDH 2, or RALDH 3 indicates the potential for local synthesis of retinoic acid exists in the cell where the isozyme is expressed
Thus, sustentacular cells, Bowman's gland cells, olfactory ensheathing cells, and other, unidentified stromal cells and/or processes are potential sites for retinoic acid synthesis in the postnatal olfactory epithelium and underlying lamina propria. CRBP I is a cytosolic protein that binds retinol and retinaldehyde. CRABP II is a cytosolic protein that binds retinoic acid. The association of CRBP I signal with RALDH 1 signal in sustentacular cell processes and Bowman's gland cells, and the colocalization of CRBP I with RALDH 2 in the stroma suggest that the retinoic acid precursors, retinol and/or retinaldehyde, are present at these tissue sites. Colocalization of CRABP II with RALDH 1 in sustentacular cells and Bowman's gland cells indicates that retinoic acid is synthesized in these cells, since this protein has been shown to track retinoic acid synthesis in other tissue systems (Zheng et al., 1999).
The localization of RALDH isozymes to non-neuronal cells in the postnatal rat olfactory organ suggests these cells are potential sources of retinoic acid for processes involved in different aspects of olfactory neuron development and turnover
Sustentacular cells and Bowman's Gland cells are well-positioned to provide retinoic acid as a local or diffusible signal to affect neuron progenitor proliferation and differentiation in the olfactory epithelium; olfactory ensheathing cells are well positioned to promote local, retinoic acid-induced effects on neurite outgrowth and targeting to glomeruli in the olfactory bulb, and these sites are also positioned to affect neuron death (apoptosis) and axon turnover. Retinoic acid has been shown to affect cell proliferation, differentiation, neurite outgrowth, and apoptosis in other experimental models (Schultz and Larsson, 2004; Coelho et al., 2005). This interpretation of the significance of our results is supported by our previous findings that retinoid status influences basal cell proliferation and mature olfactory receptor neuron number in the postnatal olfactory epithelium (Asson-Batres et al., 2003b), work demonstrating the tissue regenerating potential of retinoic acid (reviewed in Maden and Hind, 2003), and studies characterizing the influence of glia on neurite outgrowth and targeting in the developing olfactory system (Kafitz and Greer, 1999; Li et al., 2005) and (reviewed in Tolbert et al., 2004).
Significance and Observation
Retinoic acid is a known morphogen, growth factor, and teratogen. As a morphogen, it affects development of organ systems during embryogenesis. As a growth factor, it has been shown to inhibit cell proliferation and promote differentiation of stem cells in cell culture systems. As a teratogen, it has been shown to cause dysmorphogenesis. A deficiency in or an excess of retinoic acid is known to cause pathologies and even death in mammals. Thus, it is not surprising that this lipophilic molecule is under tight regulation. Regulation of retinoic acid availability appears to be present at many levels, including spatio-temporal changes in the distribution of enzymes involved in its synthesis and proteins involved in its transport, use, and/or degradation. In this study, we show that RALDH 1 and RALDH 2 exhibit differential patterns of expression within the olfactory region, and that the distribution patterns of these enzymes are dynamic.
When we first began these studies, we were skeptical that the variable patterns of apparent RALDH 1 or 2 concentration and/or distribution within the postnatal olfactory region might be due to technical problems. However, after repeating the experiments with numerous tissue preparations, including frozen sections and paraffin-embedded sections, multiple immunohistochemistry protocols, including colorimetric detection, indirect immunofluorescence, and the ABC-TSA method of detection described in Materials and Methods, using tissue dissected from at least 12 animals, we conclude that our results are not due to technical artifacts, but are a representation of dynamic patterns of expression by these two proteins in the olfactory region of postnatal rats under conditions of vitamin A sufficiency and vitamin A deficiency.
Norlin et al (2002), also reported seeing a variable pattern of expression of RALDH 2 mRNA in postnatal mouse olfactory tissue sections; however, these investigators interpreted the relatively low to high staining intensities produced by the RALDH 2 riboprobe along the dorso-medial to ventro-lateral axis of the olfactory turbinate region as an indication of a gradient of RALDH 2 expression. Further, they showed evidence that the gradient corresponded with zones of expression of specific olfactory receptor neurons, and suggested that graded availability of retinoic acid may influence the development of particular neuron subsets within the postnatal olfactory system. Our results differ from those of Norlin et al (2002) in several ways. Although we see variation in RALDH 2 signal intensity within and between animals, the variability does not follow a gradient (Figure 3). Also, we see expression of RALDH 2 protein in the dorsal meatus region of the olfactory region, whereas, Norlin et al (2002), did not (see Figure 4A, Norlin et al, 2002). The differences in our findings may be due to the difference in species (rat as opposed to mouse) and/or molecule under study (protein as opposed to RNA) in the two studies.
Our results support a more general role for retinoic acid in influencing olfactory receptor neuron development. Just as basal cell proliferation is observed to occur in patches throughout the postnatal olfactory epithelium (Weiler and Farbman, 1997; Asson-Batres et al, 2003b), it is our view that retinoic acid production, utilization, and degradation, may also occur in dispersed areas. In this model, we propose that as neurons die and turnover, different areas of the olfactory region will become active, first in expanding the population of neuron precursor cells in the affected region (via basal cell proliferation), and then, in activating processes that inhibit continued proliferation and that promote the differentiation and maturation of functional olfactory receptor neurons. Our data and the results of many in vitro studies support the notion that retinoic acid inhibits cell proliferation and promotes differentiation. Thus, we anticipate that retinoic acid may be regulated such that it would be absent when cell proliferation was required to expand the population of available precursor cells, and present, when needed to promote early differentiation events along the neuron development pathway, and/or to promote neurite outgrowth to complete the maturation process. A variable pattern of RALDH 1 and/or RALDH 2 expression could provide one means of regulating retinoic acid availability for these processes. Further work is needed to investigate this possibility.
Table 1B.
Comparison of in vitro kinetic constants for RALDHS using other aldehydes as substrates.
| Enzyme |
Substrate |
Km (μM) |
Vmax/Km |
References |
|---|---|---|---|---|
| Rat RALDH 1 |
Acetaldehyde | 41 | ------- | (Labrecque et al., 1993; Kathmann et al., 2000) |
| Benzaldehyde | 0.12 | ------- | ||
| Propanal |
5.5 |
------- |
|
|
| Rat RALDH 2 |
Acetaldehyde | 645 | 0.2 | (Wang et al., 1996) |
| Benzaldehyde | 305 | 0.7 | ||
| Propanal | 61 | 2.4 | ||
| Hexanal | 28 | 23 | ||
| Octanal | 5 | 152 | ||
| Decanal |
3 |
214 |
|
|
| Mouse RALDH 2 |
Acetaldehyde | 1400 | 0.46 | (Bordelon et al., 2004) |
| Hexanal | 5 | 164 | ||
| Octanal | 10 | 108 | ||
| Decanal |
1 |
960 |
|
|
| Chick RALDH 3 | Acetaldehyde | 3200 | ------- | (Grun et al., 2000) |
| Benzaldehyde | 4.3 | 5.3 | ||
| Octanal | 0.24 | 679 |
OTHER ACKNOWLEDGEMENTS
We thank Gareth Davis for technical assistance in preparation of tissue sections; Marilyn Getchell for evaluating immunohistochemical results and for assistance with identification of morphologic features in the olfactory organ; the Vanderbilt Cell Imaging Shared Resource Facility and Sam Wells for assistance with interpretation of the RALDH 1-CRABP II colocalization results; Gary Olson for use of his laboratory's cryostat; and Vanderbilt Histopathology and Vanderbilt Immunohistochemistry Core Laboratories for cutting some tissue sections and for batch automated staining of tissue sections for evaluation of Vitamin A status on RALDH 1.
Contract grant sponsor: NIH: contract grant numbers: S-06-GM-08092 and 5 K02 DC00180−020
LITERATURE CITED
- Ahmad O. Master's Thesis. Tennessee State University; 2001. Immunolocalization of the cellular retinoic acid binding proteins types I and II and retinoic acid receptor beta in the olfactory mucosa of the mature male Long Evans rat. [Google Scholar]
- Anchan RM, Drake DP, Haines CF, Gerwe EA, LaMantia AS. Disruption of local retinoid-mediated gene expression accompanies abnormal development in the mammalian olfactory pathway. J Comp Neurol. 1997;379(2):171–84. [published erratum appears in J Comp Neurol 1997 Jul 28;384(2):321]
- Asson-Batres MA, Ahmad O, Smith WB. Expression of the cellular retinoic acid binding proteins, type II and type I, in mature rat olfactory epithelium. Cell Tissue Res. 2003a;312(1):9–19. doi: 10.1007/s00441-003-0709-1. [DOI] [PubMed] [Google Scholar]
- Asson-Batres MA, Zeng MS, Savchenko V, Aderoju A, McKanna J. Vitamin A deficiency leads to increased cell proliferation in olfactory epithelium of mature rats. J Neurobiol. 2003b;54(4):539–54. doi: 10.1002/neu.10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au E, Roskams AJ. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia. 2003;41(3):224–36. doi: 10.1002/glia.10160. [DOI] [PubMed] [Google Scholar]
- Berggren K, McCaffery P, Drager U, Forehand CJ. Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme, RALDH-2. Dev Biol. 1999;210(2):288–304. doi: 10.1006/dbio.1999.9286. [DOI] [PubMed] [Google Scholar]
- Bhat PV, Labrecque J, Boutin JM, Lacroix A, Yoshida A. Cloning of a cDNA encoding rat aldehyde dehydrogenase with high activity for retinal oxidation. Gene. 1995;166(2):303–6. doi: 10.1016/0378-1119(96)81752-5. [DOI] [PubMed] [Google Scholar]
- Bordelon T, Montegudo SK, Pakhomova S, Oldham ML, Newcomer ME. A disorder to order transition accompanies catalysis in retinaldehyde dehydrogenase type II. J Biol Chem. 2004;279(41):43085–91. doi: 10.1074/jbc.M406139200. [DOI] [PubMed] [Google Scholar]
- Bucco RA, Zheng WL, Wardlaw SA, Davis JT, Sierra-Rivera E, Osteen KG, Melner MH, Kakkad BP, Ong DE. Regulation and localization of cellular retinol-binding protein, retinol-binding protein, cellular retinoic acid-binding protein (CRABP), and CRABP II in the uterus of the pseudopregnant rat. Endocrinology. 1996;137(7):3111–22. doi: 10.1210/endo.137.7.8770937. [DOI] [PubMed] [Google Scholar]
- Buiakova OI, Baker H, Scott JW, Farbman A, Kream R, Grillo M, Franzen L, Richman M, Davis LM, Abbondanzo S, Stewart CL, Margolis FL. Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proc Natl Acad Sci U S A. 1996;93(18):9858–63. doi: 10.1073/pnas.93.18.9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CE, Parsons LM, Hosang M, Shooter EM. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984;259(11):6882–9. [PubMed] [Google Scholar]
- Chun TY, Carman JA, Hayes CE. Retinoid repletion of vitamin A-deficient mice restores IgG responses. J Nutr. 1992;122(5):1062–9. doi: 10.1093/jn/122.5.1062. [DOI] [PubMed] [Google Scholar]
- Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr. 2002;22:347–81. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- Coelho SM, Vaisman M, Carvalho DP. Tumour re-differentiation effect of retinoic acid: a novel therapeutic approach for advanced thyroid cancer. Curr Pharm Des. 2005;11(19):2525–31. doi: 10.2174/1381612054367490. [DOI] [PubMed] [Google Scholar]
- Duester G, Mic FA, Molotkov A. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem Biol Interact. 2003;143−144:201–10. doi: 10.1016/s0009-2797(02)00204-1. [DOI] [PubMed] [Google Scholar]
- Dunn TJ, Koleske AJ, Lindahl R, Pitot HC. Phenobarbital-inducible aldehyde dehydrogenase in the rat. cDNA sequence and regulation of the mRNA by phenobarbital in responsive rats. J Biol Chem. 1989;264(22):13057–65. [PubMed] [Google Scholar]
- Elder JT, Astrom A, Pettersson U, Tavakkol A, Krust A, Kastner P, Chambon P, Voorhees JJ. Retinoic acid receptors and binding proteins in human skin. J Invest Dermatol. 1992;98(6 Suppl):36S–41S. doi: 10.1111/1523-1747.ep12462180. [DOI] [PubMed] [Google Scholar]
- Everts HB, King LE, Jr., Sundberg JP, Ong DE. Hair cycle-specific immunolocalization of retinoic acid synthesizing enzymes Aldh1a2 and Aldh1a3 indicate complex regulation. J Invest Dermatol. 2004;123(2):258–63. doi: 10.1111/j.0022-202X.2004.23223.x. [DOI] [PubMed] [Google Scholar]
- Everts HB, Sundberg JP, Ong DE. Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp Cell Res. 2005;308(2):309–19. doi: 10.1016/j.yexcr.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Fan X, Molotkov A, Manabe S, Donmoyer CM, Deltour L, Foglio MH, Cuenca AE, Blaner WS, Lipton SA, Duester G. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol Cell Biol. 2003;23(13):4637–48. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbman AI. Cell Biology of Olfactory Epithelium. In: Finger TE, Silver WL, Restrepo D, editors. The Neurobiology of Taste and Smell. Wiley-Liss; New York: 2000. pp. 131–58. [Google Scholar]
- Field P, Li Y, Raisman G. Ensheathment of the olfactory nerves in the adult rat. J Neurocytol. 2003;32(3):317–24. doi: 10.1023/B:NEUR.0000010089.37032.48. [DOI] [PubMed] [Google Scholar]
- Gagnon I, Duester G, Bhat PV. Kinetic analysis of mouse retinal dehydrogenase type-2 (RALDH2) for retinal substrates. Biochim Biophys Acta. 2002;1596(1):156–62. doi: 10.1016/s0167-4838(02)00213-3. [DOI] [PubMed] [Google Scholar]
- Gagnon I, Duester G, Bhat PV. Enzymatic characterization of recombinant mouse retinal dehydrogenase type 1. Biochem Pharmacol. 2003;65(10):1685–90. doi: 10.1016/s0006-2952(03)00150-3. [DOI] [PubMed] [Google Scholar]
- Giguere V. Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr Rev. 1994;15(1):61–79. doi: 10.1210/edrv-15-1-61. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Schwob JE. Analysis of the globose basal cell compartment in rat olfactory epithelium using GBC-1, a new monoclonal antibody against globose basal cells. J Neurosci. 1996;16(12):4005–16. doi: 10.1523/JNEUROSCI.16-12-04005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Bailey MS, Pixley SK, Ennis M, Liu W, Shipley MT. Localization and regulation of low affinity nerve growth factor receptor expression in the rat olfactory system during development and regeneration. J Comp Neurol. 1994;344(3):336–48. doi: 10.1002/cne.903440303. [DOI] [PubMed] [Google Scholar]
- Graziadei GA, Graziadei PP. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979a;8(2):197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- Graziadei PP, Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979b;8(1):1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Grun F, Hirose Y, Kawauchi S, Ogura T, Umesono K. Aldehyde dehydrogenase 6, a cytosolic retinaldehyde dehydrogenase prominently expressed in sensory neuroepithelia during development. J Biol Chem. 2000;275(52):41210–8. doi: 10.1074/jbc.M007376200. [DOI] [PubMed] [Google Scholar]
- Gustafson AL, Eriksson U, Dencker L. CRBP I and CRABP I localisation during olfactory nerve development. Brain Res Dev Brain Res. 1999;114(1):121–6. doi: 10.1016/s0165-3806(99)00014-0. [DOI] [PubMed] [Google Scholar]
- Harada H, Miki R, Masushige S, Kato S. Gene expression of retinoic acid receptors, retinoid-X receptors, and cellular retinol-binding protein I in bone and its regulation by vitamin A. Endocrinology. 1995;136(12):5329–35. doi: 10.1210/endo.136.12.7588278. [DOI] [PubMed] [Google Scholar]
- Haselbeck RJ, Hoffmann I, Duester G. Distinct functions for Aldh1 and Raldh2 in the control of ligand production for embryonic retinoid signaling pathways. Dev Genet. 1999;25(4):353–64. doi: 10.1002/(SICI)1520-6408(1999)25:4<353::AID-DVG9>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind M, Corcoran J, Maden M. Alveolar proliferation, retinoid synthesizing enzymes, and endogenous retinoids in the postnatal mouse lung. Different roles for Aldh-1 and Raldh-2. Am J Respir Cell Mol Biol. 2002a;26(1):67–73. doi: 10.1165/ajrcmb.26.1.4575. [DOI] [PubMed] [Google Scholar]
- Hind M, Corcoran J, Maden M. Temporal/spatial expression of retinoid binding proteins and RAR isoforms in the postnatal lung. Am J Physiol Lung Cell Mol Physiol. 2002b;282(3):L468–L476. doi: 10.1152/ajplung.00196.2001. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Greer CA. Olfactory ensheathing cells promote neurite extension from embryonic olfactory receptor cells in vitro. Glia. 1999;25(2):99–110. [PubMed] [Google Scholar]
- Kathmann EC, Lipsky JJ. Cloning of a cDNA encoding a constitutively expressed rat liver cytosolic aldehyde dehydrogenase. Biochem Biophys Res Commun. 1997;236(2):527–31. doi: 10.1006/bbrc.1997.6998. [DOI] [PubMed] [Google Scholar]
- Kathmann EC, Naylor S, Lipsky JJ. Rat liver constitutive and phenobarbital-inducible cytosolic aldehyde dehydrogenases are highly homologous proteins that function as distinct isozymes. Biochemistry. 2000;39(36):11170–6. doi: 10.1021/bi001120m. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Beites CL, Crocker CE, Wu HH, Bonnin A, Murray R, Calof AL. Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Dev Neurosci. 2004;26(2−4):166–80. doi: 10.1159/000082135. [DOI] [PubMed] [Google Scholar]
- Kuppumbatti YS, Rexer B, Nakajo S, Nakaya K, Lopez R. CRBP suppresses breast cancer cell survival and anchorage-independent growth. Oncogene. 2001;20(50):7413–9. doi: 10.1038/sj.onc.1204749. [DOI] [PubMed] [Google Scholar]
- Labrecque J, Bhat PV, Lacroix A. Purification and partial characterization of a rat kidney aldehyde dehydrogenase that oxidizes retinal to retinoic acid. Biochem Cell Biol. 1993;71(1−2):85–9. doi: 10.1139/o93-013. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Martin PJ, Flajollet S, Dedieu S, Billaut X, Lefebvre B. Transcriptional activities of retinoic acid receptors. Vitam Horm. 2005;70:199–264. doi: 10.1016/S0083-6729(05)70007-8. [DOI] [PubMed] [Google Scholar]
- Li H, Wagner E, McCaffery P, Smith D, Andreadis A, Drager UC. A retinoic acid synthesizing enzyme in ventral retina and telencephalon of the embryonic mouse. Mech Dev. 2000;95(1−2):283–9. doi: 10.1016/s0925-4773(00)00352-x. [DOI] [PubMed] [Google Scholar]
- Li XH, Kakkad B, Ong DE. Estrogen directly induces expression of retinoic acid biosynthetic enzymes, compartmentalized between the epithelium and underlying stromal cells in rat uterus. Endocrinology. 2004;145(10):4756–62. doi: 10.1210/en.2004-0514. [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Olfactory ensheathing cells and olfactory nerve fibroblasts maintain continuous open channels for regrowth of olfactory nerve fibres. Glia. 2005 doi: 10.1002/glia.20241. [DOI] [PubMed] [Google Scholar]
- Liang X, Lu Y, Neubert TA, Resh MD. Mass spectrometric analysis of GAP-43/neuromodulin reveals the presence of a variety of fatty acylated species. J Biol Chem. 2002;277(36):33032–40. doi: 10.1074/jbc.M204607200. [DOI] [PubMed] [Google Scholar]
- Lin M, Zhang M, Abraham M, Smith SM, Napoli JL. Mouse retinal dehydrogenase 4 (RALDH4), molecular cloning, cellular expression, and activity in 9-cis-retinoic acid biosynthesis in intact cells. J Biol Chem. 2003;278(11):9856–61. doi: 10.1074/jbc.M211417200. [DOI] [PubMed] [Google Scholar]
- Luo T, Wagner E, Grun F, Drager UC. Retinoic acid signaling in the brain marks formation of optic projections, maturation of the dorsal telencephalon, and function of limbic sites. J Comp Neurol. 2004;470(3):297–316. doi: 10.1002/cne.20013. [DOI] [PubMed] [Google Scholar]
- Maden M, Hind M. Retinoic acid, a regeneration-inducing molecule. Dev Dyn. 2003;226(2):237–44. doi: 10.1002/dvdy.10222. [DOI] [PubMed] [Google Scholar]
- McGowan SE, Holmes AJ, Smith J. Retinoic acid reverses the airway hyperresponsiveness but not the parenchymal defect that is associated with vitamin A deficiency. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L437–L444. doi: 10.1152/ajplung.00158.2003. [DOI] [PubMed] [Google Scholar]
- Mey J, McCaffery P, Drager UC. Retinoic acid synthesis in the developing chick retina. J Neurosci. 1997;17(19):7441–9. doi: 10.1523/JNEUROSCI.17-19-07441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montplaisir V, Lan NC, Guimond J, Savineau C, Bhat PV, Mader S. Recombinant class I aldehyde dehydrogenases specific for all-trans- or 9-cis-retinal. J Biol Chem. 2002;277(20):17486–92. doi: 10.1074/jbc.M112445200. [DOI] [PubMed] [Google Scholar]
- Napoli JL, Posch KP, Fiorella PD, Boerman MH. Physiological occurrence, biosynthesis and metabolism of retinoic acid: evidence for roles of cellular retinol-binding protein (CRBP) and cellular retinoic acid-binding protein (CRABP) in the pathway of retinoic acid homeostasis. Biomed Pharmacother. 1991;45(4−5):131–43. doi: 10.1016/0753-3322(91)90101-x. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Fraulob V, Garnier JM, Chambon P, Dolle P. Differential expression of retinoic acid-synthesizing (RALDH) enzymes during fetal development and organ differentiation in the mouse. Mech Dev. 2002a;110(1−2):165–71. doi: 10.1016/s0925-4773(01)00561-5. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21(4):444–8. doi: 10.1038/7788. [see comments] [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Fraulob V, Chambon P, Dolle P. Retinaldehyde dehydrogenase 2 (RALDH2)- independent patterns of retinoic acid synthesis in the mouse embryo. Proc Natl Acad Sci U S A. 2002b;99(25):16111–6. doi: 10.1073/pnas.252626599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlin EM, Alenius M, Gussing F, Hagglund M, Vedin V, Bohm S. Evidence for gradients of gene expression correlating with zonal topography of the olfactory sensory map. Mol Cell Neurosci. 2001;18(3):283–95. doi: 10.1006/mcne.2001.1019. [DOI] [PubMed] [Google Scholar]
- Ong D, Newcomer M, Chytil F. Cellular retinoid-binding proteins. In: Sporn MB, Roberts ABGDS, editors. The Retinoids. 2d edition Raven; New York, N.Y.: 1994. pp. 283–317. [Google Scholar]
- Penzes P, Wang X, Sperkova Z, Napoli JL. Cloning of a rat cDNA encoding retinal dehydrogenase isozyme type I and its expression in E. coli. Gene. 1997;191(2):167–72. doi: 10.1016/s0378-1119(97)00054-1. [DOI] [PubMed] [Google Scholar]
- Pixley SK. The olfactory nerve contains two populations of glia, identified both in vivo and in vitro. Glia. 1992;5(4):269–84. doi: 10.1002/glia.440050405. [DOI] [PubMed] [Google Scholar]
- Posch KC, Burns RD, Napoli JL. Biosynthesis of all-trans-retinoic acid from retinal. Recognition of retinal bound to cellular retinol binding protein (type I) as substrate by a purified cytosolic dehydrogenase. J Biol Chem. 1992;267(27):19676–82. [PubMed] [Google Scholar]
- Rajan N, Blaner WS, Soprano DR, Suhara A, Goodman DS. Cellular retinol-binding protein messenger RNA levels in normal and retinoid-deficient rats. J Lipid Res. 1990;31(5):821–9. [PubMed] [Google Scholar]
- Rajan N, Kidd GL, Talmage DA, Blaner WS, Suhara A, Goodman DS. Cellular retinoic acid-binding protein messenger RNA: levels in rat tissues and localization in rat testis. J Lipid Res. 1991;32(7):1195–204. [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73(3):597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Schultz A, Larsson C. Ceramide influences neurite outgrowth and neuroblastoma cell apoptosis regulated by novel protein kinase C isoforms. J Neurochem. 2004;89(6):1427–35. doi: 10.1111/j.1471-4159.2004.02431.x. [DOI] [PubMed] [Google Scholar]
- Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269(1):33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- Sophos NA, Vasiliou V. Aldehyde dehydrogenase gene superfamily: the 2002 update. Chem Biol Interact. 2003;143−144:5–22. doi: 10.1016/s0009-2797(02)00163-1. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Shintani T, Sakuta H, Kato A, Ohkawara T, Osumi N, Noda M. Identification of RALDH-3, a novel retinaldehyde dehydrogenase, expressed in the ventral region of the retina. Mech Dev. 2000;98(1−2):37–50. doi: 10.1016/s0925-4773(00)00450-0. [DOI] [PubMed] [Google Scholar]
- Tank AW, Deitrich RA, Weiner H. Effects of induction of rat liver cytosolic aldehyde dehydrogenase on the oxidation of biogenic aldehydes. Biochem Pharmacol. 1986;35(24):4563–9. doi: 10.1016/0006-2952(86)90779-3. [DOI] [PubMed] [Google Scholar]
- Tisay KT, Key B. The extracellular matrix modulates olfactory neurite outgrowth on ensheathing cells. J Neurosci. 1999;19(22):9890–9. doi: 10.1523/JNEUROSCI.19-22-09890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert LP, Oland LA, Tucker ES, Gibson NJ, Higgins MR, Lipscomb BW. Bidirectional influences between neurons and glial cells in the developing olfactory system. Prog Neurobiol. 2004;73(2):73–105. doi: 10.1016/j.pneurobio.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Vassar R, Ngai J, Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993;74(2):309–18. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Verhaagen J, Oestreicher AB, Grillo M, Khew-Goodall YS, Gispen WH, Margolis FL. Neuroplasticity in the olfactory system: differential effects of central and peripheral lesions of the primary olfactory pathway on the expression of B-50/GAP43 and the olfactory marker protein. J Neurosci Res. 1990;26(1):31–44. doi: 10.1002/jnr.490260105. [DOI] [PubMed] [Google Scholar]
- Wagner E, Luo T, Drager UC. Retinoic acid synthesis in the postnatal mouse brain marks distinct developmental stages and functional systems. Cereb Cortex. 2002;12(12):1244–53. doi: 10.1093/cercor/12.12.1244. [DOI] [PubMed] [Google Scholar]
- Wang X, Penzes P, Napoli JL. Cloning of a cDNA encoding an aldehyde dehydrogenase and its expression in Escherichia coli. Recognition of retinal as substrate. J Biol Chem. 1996;271(27):16288–93. doi: 10.1074/jbc.271.27.16288. [DOI] [PubMed] [Google Scholar]
- Weiler E, Farbman AI. Proliferation in the rat olfactory epithelium: age-dependent changes. J Neurosci. 1997;17(10):3610–22. doi: 10.1523/JNEUROSCI.17-10-03610.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides J, Hall M, Anchan R, LaMantia AS. Retinoid signaling distinguishes a subpopulation of olfactory receptor neurons in the developing and adult mouse. J Comp Neurol. 1998;394(4):445–61. [PubMed] [Google Scholar]
- Wilkinson M. Purification of RNA. In: Brown TAEd., editor. Essential Molecular Biology: A Practical Approach. IRL Press; Oxford: 1991. pp. 69–87. [Google Scholar]
- Yee KK, Rawson NE. Retinoic acid enhances the rate of olfactory recovery after olfactory nerve transection. Brain Res Dev Brain Res. 2000;124(1−2):129–32. doi: 10.1016/s0165-3806(00)00108-5. [DOI] [PubMed] [Google Scholar]
- Yee KK, Rawson NE. Immunolocalization of retinoic acid receptors in the mammalian olfactory system and the effects of olfactory denervation on receptor distribution. Neuroscience. 2005;131(3):733–43. doi: 10.1016/j.neuroscience.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Eriksson U. Cellular retinol-binding protein type I is prominently and differentially expressed in the sensory epithelium of the rat cochlea and vestibular organs. J Comp Neurol. 1994;349(4):596–602. doi: 10.1002/cne.903490407. [DOI] [PubMed] [Google Scholar]
- Young JT. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol. 1981;1(4):309–12. doi: 10.1016/s0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Lindqvist E, de Urquiza AM, Tomac A, Eriksson U, Perlmann T, Olson L. Role of retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. Eur J Neurosci. 1999;11(2):407–16. doi: 10.1046/j.1460-9568.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Sperkova Z, Napoli JL. Cellular expression of retinal dehydrogenase types 1 and 2: effects of vitamin A status on testis mRNA. J Cell Physiol. 2001;186(2):220–32. doi: 10.1002/1097-4652(200102)186:2<220::AID-JCP1018>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ross AC. Retinoic acid repletion restores the number of leukocytes and their subsets and stimulates natural cytotoxicity in vitamin A-deficient rats. J Nutr. 1995;125(8):2064–73. doi: 10.1093/jn/125.8.2064. [DOI] [PubMed] [Google Scholar]
- Zheng WL, Bucco RA, Sierra-Rivera E, Osteen KG, Melner MH, Ong DE. Synthesis of retinoic acid by rat ovarian cells that express cellular retinoic acid-binding protein-II. Biol Reprod. 1999;60(1):110–4. doi: 10.1095/biolreprod60.1.110. [DOI] [PubMed] [Google Scholar]
- Zheng WL, Ong DE. Spatial and temporal patterns of expression of cellular retinol-binding protein and cellular retinoic acid-binding proteins in rat uterus during early pregnancy. Biol Reprod. 1998;58(4):963–70. doi: 10.1095/biolreprod58.4.963. [DOI] [PubMed] [Google Scholar]







