Abstract
Na+ transport across epithelia is mediated in part by the epithelial Na+ channel ENaC. Previous work indicates that Na+ is an important regulator of ENaC, providing a negative feedback mechanism to maintain Na+ homeostasis. ENaC is synthesized as an inactive precursor, which is activated by proteolytic cleavage of the extracellular domains of the α and γ subunits. Here we found that Na+ regulates ENaC in part by altering proteolytic activation of the channel. When the Na+ concentration was low, we found that the majority of ENaC at the cell surface was in the cleaved/active state. As Na+ increased, there was a dose-dependent decrease in ENaC cleavage and, hence, ENaC activity. This Na+ effect was dependent on Na+ permeation; cleavage was increased by the ENaC blocker amiloride and by a mutation that decreases ENaC activity (αH69A) and was reduced by a mutation that activates ENaC (βS520K). Moreover, the Na+ ionophore monensin reversed the effect of the inactivating mutation (αH69A) on ENaC cleavage, suggesting that intracellular Na+ regulates cleavage. Na+ did not alter activity of Nedd4-2, an E3 ubiquitin ligase that modulates ENaC cleavage, but Na+ reduced ENaC cleavage by exogenous trypsin. Our findings support a model in which intracellular Na+ regulates cleavage by altering accessibility of ENaC cleavage sites to proteases and provide a molecular explanation for the earlier observation that intracellular Na+ inhibits Na+ transport via ENaC (Na+ feedback inhibition).
Transport of Na+ across epithelia is critical to maintain Na+ homeostasis. Defects in Na+ transport cause inherited forms of hypertension (e.g. Liddle syndrome) and hypotension (pseudohypoaldosteronism type 1) (1). In the distal nephron of the kidney, lung, colon, and sweat duct, transport is mediated by the epithelial Na+ channel ENaC, a heterotrimer composed of homologous α, β, and γ subunits (2–5). ENaC is located at the apical membrane, where it functions as a conduit for Na+ to enter the cell (reviewed in Refs. 6 and 7). Coupled with Na+ exit at the basolateral membrane via the Na+-K+-ATPase, ENaC provides a pathway for Na+ reabsorption across these tissues.
Although Na+ is the permeant ion for this pathway, Na+ also regulates its own transport through negative feedback mechanisms (8–16). Under conditions of Na+/volume excess, the Na+ concentration in the distal nephron is high (>50 mm) (17), which inhibits ENaC in order to minimize Na+ reabsorption. Conversely, under conditions of Na+/volume depletion, the Na+ concentration is low (∼1 mm) (17), which activates ENaC to maximize Na+ reabsorption. By countering changes in Na+ delivery to the distal nephron, this pathway functions to maintain Na+ homeostasis. Previous work indicates that both extracellular Na+ (“Na+ self-inhibition” (10, 11, 18)) and intracellular Na+ (“Na+ feedback inhibition” (12–16)) regulate ENaC activity. However, an understanding of the underlying mechanisms has remained elusive.
In this work, we tested the hypothesis that Na+ regulates ENaC gating in part by altering the proteolytic cleavage state of the channel. Recent studies indicate that proteases including furin (19), channel-activating protease 1 (20), and neutrophil elastase (21) cleave the extracellular domains of α- and γENaC, converting channels from a quiescent state to an active Na+-conducting state. One pool of channels is cleaved and activated in the Golgi apparatus before trafficking to the cell surface (22). A second pool reaches the cell surface in the uncleaved inactive state, where it can be proteolytically cleaved and activated by proteases at the cell surface (22–24). Using biochemical and electrophysiological approaches, we asked whether Na+ alters proteolytic cleavage of ENaC.
EXPERIMENTAL PROCEDURES
cDNA Constructs—Human α-, β-, and γENaC (4, 5), and Nedd4-2 (25) in pMT3 were cloned as described previously. Mutations were generated by site-directed mutagenesis (QuikChange, Stratagene): αH69A, αY644A, αCl mut (R175A, R177A, R178A, R181A, R201A, R204A), βS520K, βY620A, and γY627A. α-FLAG and γ-FLAG were generated by insertion of a FLAG epitope (DYKDDDDK) at the C terminus (24).
Biochemistry—Human embryonic kidney (HEK)2 293T cells were transfected with α-, β-, and γENaC (α-or γENaC contained FLAG epitope, as indicated in the figure legends) using Lipofectamine 2000 (Invitrogen). The cells were maintained in Dulbecco's modified Eagle's medium containing 10 μm amiloride. Two days after transfection, the medium was replaced with a solution containing 0–135 mm Na+ (135 mm NaCl/NMDG Cl, 5 mm KCl, 1.2 mm MgCl2, 1.2 mm CaCl2, 5 mm HEPES, pH 7.4), and the cells were incubated for 0–60 min at 37 °C, as indicated in the figure legends. In some studies, amiloride (10 μm) or monensin (10 μm) was present in this solution. In Fig. 6A, the cells were then incubated in 135 mm Na+ solution with 5 μg/ml trypsin for 5 min. The cells were washed with 4 °C phosphate-buffered saline (PBS) containing 1 mm CaCl2 and 1 mm MgCl2 (PBS-Ca/Mg), and then surface proteins were biotinylated with 0.5 mg/ml sulfo-N-hydroxysuccinimide-biotin (Pierce) for 30 min at 4 °C. Excess biotin was quenched with 100 mm glycine in PBS-Ca/Mg for 20 min at 4 °C. The cells were lysed in 1% Nonidet P-40, 150 mm NaCl, 50 mm Tris, pH 7.4, and protease inhibitors (Sigma) at 4 °C, and then centrifuged at 14,000 rpm for 10 min to remove insoluble material. Biotinylated proteins were isolated with neutravidin-agarose beads (Pierce) for 16 h at 4 °C. Following extensive washing, biotinylated proteins were eluted with SDS sample buffer (4% SDS, 100 mm dithiothreitol, 20% glycerol, and 100 mm Tris-Cl, pH 6.8) and separated by SDS-PAGE. Following transfer to a nitrocellulose membrane and blocking in 5% bovine serum albumin, biotinylated ENaC was detected by Western blot using anti-FLAG M2-peroxidase-conjugated antibody (1:2000, Sigma) and enhanced chemiluminescence (ECL Plus; Amersham Biosciences). The full-length and proteolytically cleaved ENaC bands were quantitated by densitometry (ImageJ). As a control for specificity, we did not detect biotinylation of Nedd4-2 or serum and glucocorticoid-regulated kinase, two cytoplasmic proteins.
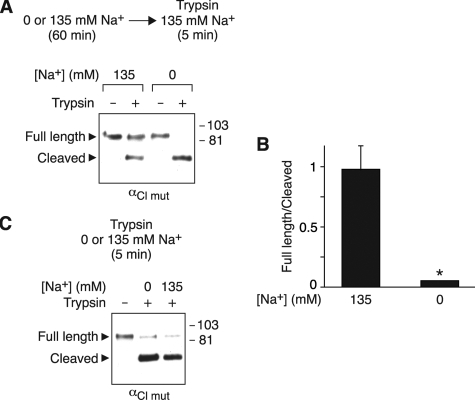
FIGURE 6.
Na+ alters ENaC cleavage by trypsin. A and B, HEK 293T cells expressing αCl mut-FLAG, β, and γ were incubated for 60 min in 0 or 135 mm Na+ solution and then treated with 5 μg/ml trypsin for 5 min in 135 mm Na+ solution. α-FLAG at the cell surface was detected by biotinylation and immunoblot (A), and the ratio of full-length/cleaved bands for the trypsin-treated groups was quantitated by densitometry (B) (mean ± S.E., n = 3; *, p < 0.02). C, HEK 293T cells expressing αCl mut-FLAG, β, and γ were treated with 5 μg/ml trypsin for 5 min in 0 or 135 mm Na+ solution. α-FLAG at the cell surface was detected by biotinylation and immunoblot.
Electrophysiology—Fischer rat thyroid (FRT) cells were grown on permeable filter supports and maintained in F-12 Coon's medium (Sigma) with 5% fetal calf serum (Sigma), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C. One day after seeding, cells were cotransfected with α-, β-, and γENaC using TFX50 (Promega), as described previously (25). H441 cells (American Type Culture Collection) were grown on permeable filter supports in RPMI with 8.5% fetal calf serum, 20 mm l-glutamine, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium, 100 nm dexamethasone, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C.
Two days after transfection (FRT) or 5 days after seeding (H441), the cells were preincubated for 45 min in Ringer solution containing 135 mm NaCl or 135 mm NMDG-Cl (0 mm Na+), 5 mm KCl, 1.2 mm CaCl2, 1.2 mm MgCl2, 5 mm HEPES (pH 7.4) at 37 °C. The filters were then transferred to modified Ussing chambers (Warner Instrument Corp.) with the apical and basolateral membranes bathed in 135 mm Na+ Ringer at 37 °C. Amiloride-sensitive short circuit current was determined as the current difference with and without amiloride (10 μm) in the apical bathing solution. To activate uncleaved ENaC channels, trypsin (2 μg/ml) was added to the apical solution for 5 min. The amiloride-sensitive short circuit current was measured before and after treatment with trypsin.
RESULTS
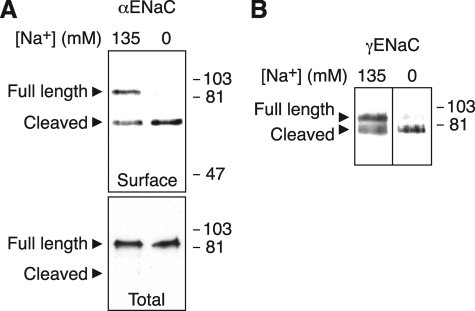
Na+ Decreases ENaC Cleavage—To test the hypothesis that Na+ alters proteolytic cleavage of ENaC, we incubated HEK 293T cells expressing α-, β-, and γENaC (α or γ subunit contained a C-terminal FLAG) in extracellular solutions containing either 135 or 0 mm Na+. Following biotinylation of the cells, we detected ENaC subunits at the cell surface by isolation with immobilized NeutrAvidin followed by immunoblot (anti-FLAG). When the cells were incubated for 1 h in 135 mm Na+, two forms of αENaC were present at the cell surface (Fig. 1A); the 90-kDa band corresponds to full-length αENaC, and the 65-kDa band is the C-terminal fragment of the proteolytically cleaved subunit. These data are consistent with previous work (22, 24). Under these conditions, more full-length αENaC was present at the cell surface than cleaved αENaC (Fig. 1A and quantitated in Fig. 2B). Incubation of the cells in 0 mm Na+ solution (Na+ replaced with NMDG) dramatically increased cleavage of αENaC, converting the majority of αENaC to the cleaved form (Fig. 1A and quantitated in Fig. 2B). Similar results were obtained when Na+ was replaced with Cs+ or mannitol (not shown). Na+ had no effect on αENaC in the total cellular pool (Fig. 1A, bottom panel), which mainly reflects immature full-length ENaC in the biosynthetic pathway. Na+ also reduced proteolytic cleavage of γENaC at the cell surface; a larger proportion of γENaC was cleaved in cells incubated in 0 mm Na+ than in 135 mm Na+ (Fig. 1B).
FIGURE 1.
Na+ inhibits ENaC cleavage. HEK 293T cells were transfected with the following ENaC subunits (5.33 μg each): α-FLAG, β, and γ (A) or α, β, and γ-FLAG (B). 48 h after transfection, cells were incubated in 135 or 0 mm Na+ solution for 60 min. Cell surface ENaC was biotinylated, isolated with NeutrAvidin beads, and detected by immunoblot (anti-FLAG). Total αENaC was detected by immunoprecipitation (anti-FLAG) of cell lysate followed by immunoblot (anti-FLAG). Full-length and proteolytically cleaved C-terminal fragments are indicated by arrowheads.
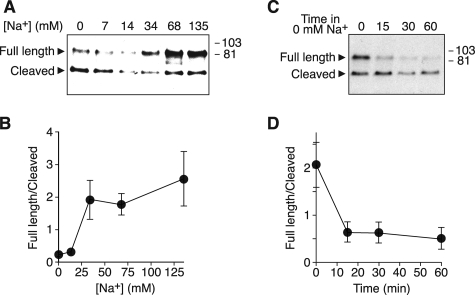
FIGURE 2.
Dose-response relationship and time course. A and B, HEK 293T cells expressing α-FLAG and β- and γENaC were incubated for 60 min in solution containing Na+ concentrations from 0 to 135 mm. α-FLAG at the cell surface was detected by biotinylation and immunoblot (A). B, full-length and cleaved bands were quantitated by densitometry, and the ratio (full-length/cleaved) was plotted versus Na+ concentration (mean ± S.E., n = 4). C and D, HEK 293T cells expressing α-FLAG and β- and γENaC were incubated for 60 min in 135 mm Na+ solution and then shifted to 0 mm Na+ solution for times between 0 and 60 min. α-FLAG at the cell surface was detected by biotinylation and immunoblot (C). D, full-length and cleaved bands were quantitated by densitometry, and the ratio (full-length/cleaved) was plotted versus time in 0 mm Na+ solution (mean ± S.E., n = 3).
In Fig. 2, A and B, we varied the Na+ concentration to determine a dose-response relationship. As extracellular Na+ increased from 0 to 34 mm, the ratio of full-length to cleaved αENaC increased. At Na+ concentrations above 34 mm, there was no significant additional change in the ratio. Interestingly, previous work indicates that Na+ permeation through ENaC saturates in a similar range (10).
To determine the time course over which Na+ removal alters cleavage, we incubated cells in 135 mm Na+ solution and then shifted them to 0 mm Na+ for times between 0 and 60 min. After 15 min in 0 mm Na+, ENaC cleavage increased, resulting in a decrease in the ratio of full-length to cleaved channels (Fig. 2, C and D). This change in ratio was maximal at 15 min; there was no further increase in cleavage at 30 or 60 min.
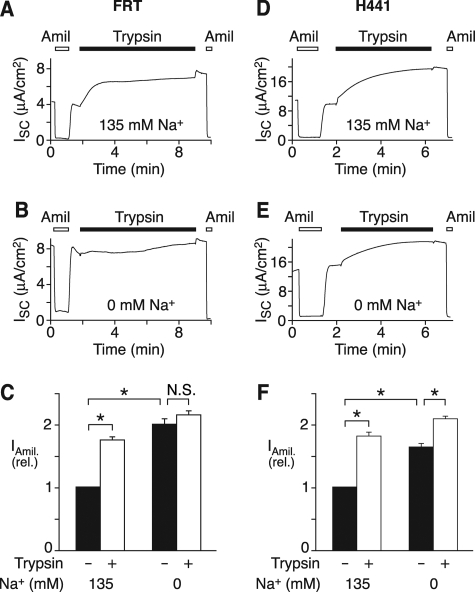
Na+ Alters ENaC Cleavage in Epithelia—As an independent assay to test the effect of Na+ on ENaC cleavage, we expressed ENaC in FRT epithelia. After preincubation in 135 mm Na+ (Fig. 3A) or 0 mm Na+ (Fig. 3B) solution for 45 min, the cells were mounted in an Ussing chamber with 135 mm Na+ solution (apical and basolateral), and short circuit current was immediately recorded. Basal amiloride-sensitive current reflects channels that are proteolytically cleaved and activated by endogenous proteases. We found that the initial amiloride-sensitive current was larger in cells preincubated in 0 mm Na+ than in cells preincubated in 135 mm Na+ (Fig. 3, A and B, quantitated in Fig. 3C). This finding is consistent with the notion that Na+ inhibits ENaC cleavage/activation. If Na+ reduces cleavage by endogenous proteases, then it should increase the pool of channels at the cell surface that are available to be cleaved by exogenous trypsin (19, 24). To test this prediction, we incubated the apical surface of epithelia with trypsin and quantitated the increase in amiloride-sensitive current. When cells were preincubated in 135 mm Na+, trypsin increased amiloride-sensitive current 75% (Fig. 3, A and C). In contrast, trypsin minimally increased current (8%) when cells were incubated in 0 mm Na+ (Fig. 3, B and C), indicating that a larger proportion of channels had been cleaved by endogenous proteases. Together, the biochemical and functional data suggest that Na+ inhibits ENaC cleavage by endogenous proteases.
FIGURE 3.
Na+ alters ENaC cleavage in epithelia. A–C, FRT epithelia transfected with α-, β-, and γENaC (0.33 μg each) were preincubated in 135 mm Na+ (A) or 0 mm Na+ (B) solution for 45 min, then transferred to an Ussing chamber with 135 mm Na+ solution bathing the apical and basolateral membranes. Representative short circuit current recordings are shown (A and B). Trypsin (2 μg/ml, black bars) and amiloride (10 μm, white bars) were added to apical bathing solution, as indicated. C, quantitation of amiloride-sensitive current before and after exposure to trypsin in cells preincubated in 0 or 135 mm Na+ solution, as indicated (mean ± S.E., n = 19; *, p < 0.001). N.S., not significant. D–F, H441 epithelia were preincubated in 135 mm Na+ (A) or 0 mm Na+ (B) solution for 45 min and then transferred to an Ussing chamber with 135 mm Na+ solution bathing apical and basolateral membranes. Representative short circuit current recordings are shown (D and E). Trypsin and amiloride were added to apical bathing solution, as indicated. F, quantitation of amiloride-sensitive current before and after exposure to trypsin in cells preincubated in 0 or 135 mm Na+ solution, as indicated (mean ± S.E., n = 13; *, p < 0.001).
To test if Na+ regulates ENaC cleavage in epithelia that express endogenous ENaC, we measured amiloride-sensitive current in H441 cells, a human lung carcinoma cell line (4). When the cells were preincubated in 135 mm Na+, trypsin increased amiloride-sensitive current 81% (Fig. 3, D and F). Preincubation in 0 mm Na+ increased basal amiloride-sensitive current and reduced ENaC activation by trypsin (28%) (Fig. 3, E and F). These results indicate that Na+ regulates ENaC cleavage not only in heterologous cells but also in Na+-transporting epithelia.
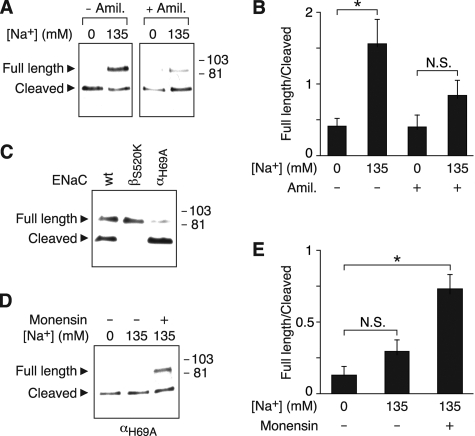
Requirement for Na+ Permeation—Fig. 2B indicates that there is a correlation between the dose-response relationship for the inhibition of ENaC cleavage by Na+ and that previously reported for Na+ permeation through ENaC (10); both saturate in a similar range. This suggested the possibility that permeation is required for extracellular Na+ to alter ENaC cleavage. To test this possibility, we used two strategies. First, we blocked ENaC with amiloride. In Fig. 4A, we incubated cells in 0 or 135 mm Na+ solution with or without amiloride. In the absence of amiloride, 135 mm Na+ reduced cleavage, increasing the ratio of full-length to cleaved αENaC (Fig. 4, A and B, similar to data in Fig. 1A). In contrast, in cells incubated with amiloride, Na+ did not have a significant effect on αENaC cleavage (Fig. 4, A and B). Thus, amiloride prevented extracellular Na+ from inhibiting ENaC cleavage.
FIGURE 4.
Intracellular Na+ regulates ENaC cleavage. A and B, HEK 293T cells expressing α-FLAG and β- and γENaC were incubated for 60 min in 0 or 135 mm Na+ solution with or without 10 μm amiloride, as indicated. α-FLAG at the cell surface was detected by biotinylation and immunoblot (A), and the ratio of full-length/cleaved bands was quantitated by densitometry (B) (mean ± S.E., n = 4; *, p < 0.02). C, HEK 293T cells expressing α-FLAG (wild-type or H69A) or β-(wild-type or S520K) andγENaC were incubated for 60 min in 135 mm Na+ solution, and α-FLAG at the cell surface was detected by biotinylation and immunoblot. D and E, HEK 293T cells expressing α-FLAG and β- and γENaC were incubated for 30 min in 0 or 135 mm Na+ solution with or without 10 μm monensin, as indicated. α-FLAG at the cell surface was detected by biotinylation and immunoblot (D), and the ratio of full-length/cleaved bands was quantitated by densitometry (E) (mean ± S.E., n = 5; *, p < 0.01).
As a second strategy to investigate the role of Na+ permeation in regulating ENaC cleavage, we introduced mutations that alter ENaC gating. Introduction of a charged residue at the “Deg” position (βS520K) increases Na+ current by locking the channel in an open state (26). This mutation abolished ENaC cleavage (Fig. 4C). In contrast, cleavage was increased by an ENaC mutation that dramatically decreases its open-state probability (αH69A) (Fig. 4C) (27).
Together, the data indicate that there is an inverse relationship between ENaC current and proteolytic cleavage; interventions that reduced current (amiloride, αH69A) increased ENaC cleavage, whereas an intervention that increased current (βS520K) decreased cleavage. Because changes in ENaC current produce corresponding changes in the intracellular concentration of Na+ (13, 28), we hypothesized that intracellular Na+ regulates ENaC cleavage. In Fig. 4, D and E, we expressed ENaC containing the αH69A mutation to minimize Na+ entry through the channel. This mutation prevented Na+ from inhibiting αENaC cleavage (Fig. 4, D and E). To provide an alternative pathway for Na+ entry, we treated cells with a Na+ ionophore, monensin. In the presence of monensin, 135 mm Na+ decreased αENaC cleavage, as indicated by an increase in the ratio of full-length to cleaved αENaC (Fig. 4, D and E). These data suggest that an increase in intracellular Na+ is sufficient to regulate ENaC cleavage.
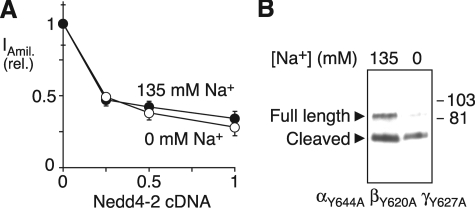
Na+ Does Not Alter Nedd4-2 Activity—In previous work, we found that ENaC cleavage was modulated by Nedd4-2, an E3 ubiquitin ligase that targets ENaC for degradation. Nedd4-2 decreased cleavage, whereas cleavage was increased by ENaC mutations that disrupt its Nedd4-2 binding (PY) motif (24). We therefore tested whether Na+ regulates ENaC cleavage by altering the activity of Nedd4-2. We expressed ENaC in FRT epithelia with increasing amounts of Nedd4-2 cDNA, preincubated the cells in 0 or 135 mm Na+ solution for 45 min, and then measured amiloride-sensitive Na+ currents (with cells bathed in 135 mm Na+). Under both conditions, Nedd4-2 produced an identical decrease in ENaC current (Fig. 5A). Likewise, mutation of the ENaC PY motifs did not prevent Na+ from inhibiting ENaC cleavage (Fig. 5B). Together the data indicate that Na+ does not regulate ENaC via changes in Nedd4-2 activity.
FIGURE 5.
Na+ does not alter Nedd4-2 activity. A, FRT epithelia expressing ENaC (0.17 μg of each subunit) with Nedd4-2 (0–0.5 μg, total DNA held constant with green fluorescent protein cDNA) were preincubated in 135 or 0 mm Na+ solution for 45 min. Plot shows amiloride-sensitive current (relative to 0 μg of Nedd4-2) versus quantity of Nedd4-2 cDNA (mean ± S.E., n = 6–8). B, HEK 293T cells expressing αY644A-FLAG and βY620A- and γY627AENaC were incubated for 60 min in 0 or 135 mm Na+ solution. α-FLAG at the cell surface was detected by biotinylation and immunoblot.
Na+ Alters ENaC Cleavage by Trypsin—We considered two alternative mechanisms by which Na+ might regulate cleavage. First, Na+ could alter the activity of one or more proteases that cleave ENaC. Second, Na+ could induce a conformational change in ENaC that alters accessibility of its cleavage sites. To begin to distinguish between these possibilities, we tested whether Na+ would alter ENaC cleavage during a brief exposure to trypsin. To eliminate cleavage by endogenous proteases (but not trypsin), we mutated residues inαENaC that contribute to its two furin cleavage sites (αCl mut) (19, 29). Following preincubation in 0 or 135 mm Na+ solution for 60 min, the cells were placed in 135 mm Na+ solution with or without trypsin for 5 min. We reasoned that if intracellular Na+ altered the activity of an endogenous protease, then it should not affect ENaC cleavage by trypsin, because trypsin was added to the extracellular solution and because the Na+ concentration was held constant (135 mm) during exposure to trypsin. Conversely, if Na+ altered accessibility of ENaC cleavage sites, it should alter cleavage by both endogenous proteases and by trypsin.
In the absence of trypsin, we observed a single band corresponding to full-length αENaC in cells incubated in both 135 and 0 mm Na+ (Fig. 6A), indicating that the mutations abolished cleavage by endogenous proteases. Trypsin generated a cleaved band similar in mass to that generated by endogenous proteases (65 kDa) (Fig. 6A). When cells were preincubated in 0 mm Na+, the majority of αENaC was cleaved by trypsin (Fig. 6, A and B). In contrast, a smaller fraction of αENaC was cleaved by trypsin in cells preincubated with 135 mm Na+ (Fig. 6A), resulting in an increase in the ratio of full-length/cleaved channels (Fig. 6B). Thus, Na+ inhibited ENaC cleavage by trypsin, similar to its effect on cleavage by endogenous proteases. However, Na+ did not alter the enzymatic activity of trypsin; when the Na+ concentration was varied only during the 5-min exposure to trypsin, there was no difference in ENaC cleavage (Fig. 6C).
DISCUSSION
Our current data indicate that Na+ regulates epithelial Na+ transport in part by altering the cleavage state of α- and γENaC, which in turn leads to changes in ENaC activity. In low Na+ medium, most of the channels were in the cleaved/active state, which maximized Na+ current. As the Na+ concentration increased, fewer channels were cleaved/active, resulting in decreased Na+ current. This reciprocal relationship between Na+ concentration and ENaC cleavage may function to counter changes in volume status in order to restore homeostasis.
Is cleavage regulated by extracellular Na+ or intracellular Na+? Although we varied extracellular Na+ in our studies, previous work indicates that Na+ permeation through ENaC produces an increase in the intracellular Na+ concentration (13, 28). Moreover, we found that the Na+ dose-response relationship for cleavage correlated closely to that previously reported for Na+ permeation through ENaC; the effect of Na+ on cleavage was maximal at 34 mm, a concentration at which Na+ permeation through ENaC saturates (10). This correlation suggests that Na+ permeation and cleavage might be mechanistically linked. Consistent with this concept, we found that Na+ entry was required in order for Na+ to regulate cleavage; amiloride (to block ENaC) and a mutation that decreases ENaC gating (αH69A) each blunted the effect of Na+ on ENaC cleavage. In addition, increased intracellular Na+ was sufficient to inhibit ENaC cleavage; an ENaC-activating mutation (βS520K) and a Na+ ionophore (monensin) each reduced cleavage. However, the ionophore data suggest that cleavage is not regulated by the movement of Na+ through ENaC per se but by the increase in intracellular Na+ that results from Na+ entry via ENaC. Thus, our data suggest that intracellular Na+ regulates ENaC cleavage.
How does our current data fit with previous work? It is clear from the literature that intracellular Na+ reduces ENaC current, a process known as Na+ feedback inhibition (reviewed in Ref. 18). In contrast to the rapid regulation of ENaC by extracellular Na+ (Na+ self-inhibition), feedback regulation occurs over a relatively slow time course (minutes). Moreover, a recent report found that intracellular Na+ inhibits ENaC through an effect on open probability (16). By linking intracellular Na+ to ENaC cleavage (and hence, gating), our data provide a molecular explanation that may in part explain the process of Na+ feedback inhibition. Interestingly, ENaC cleavage prevents regulation of the channel by extracellular Na+ (self-inhibition) (30). Thus, cleavage may provide a means to integrate the effects of intracellular and extracellular Na+ on ENaC activity.
What is the mechanism by which intracellular Na+ inhibits ENaC cleavage? Our data suggest a model in which Na+ inhibits cleavage by reducing accessibility of proteases to the ENaC cleavage sites, which are located in the extracellular domains of α- and γENaC (19, 31). We speculate that this occurs through a conformational change in the extracellular domains. This could result from direct binding of Na+ to one or more ENaC subunit. Such a mechanism has precedent in the literature. For example, binding of Na+ to thrombin induces a conformational change that increases enzyme activity (32). Alternatively, Na+ could induce a conformational change indirectly though binding to an accessory protein or by activating a signaling cascade. Consistent with this last possibility, Abriel and Horisberger (15) found that internal perfusion of Xenopus oocytes removed a factor required for Na+ feedback inhibition. Although a variety of molecules have been implicated (e.g. Go guanine nucleotide-binding regulatory proteins, Ca2+, ATP, pH, and AKAP15), the data in the literature are conflicting (12, 15, 33–35).
It seems less likely that Na+ could alter accessibility through a change in ENaC localization within the cell. For example, if Na+ increased ENaC endocytosis, it would reduce exposure time to proteases at the cell surface and, hence, reduce cleavage. However, three observations argue against such a mechanism. First, Na+ did not reduce ENaC surface expression (e.g. Fig. 2A, compare 135 and 0 mm Na+), arguing that it does not affect ENaC trafficking. Second, Na+ did not alter ENaC inhibition by Nedd4-2, an important regulator of ENaC endocytosis. Third, mutation of ENaC PY motifs (which bind to Nedd4-2) did not prevent Na+ from inhibiting ENaC cleavage. These findings contrast with previous observations in oocytes and salivary duct cells that implicated a role for Nedd4 and ENaC PY motifs in the inhibition of ENaC by intracellular Na+ (13, 36). Thus, it seems likely that intracellular Na+ regulates ENaC activity by more than one mechanism.
We found that Na+ reduced ENaC cleavage both in heterologous cells and in epithelia that express endogenous ENaC. This has two implications. First, it suggests that the mechanism by which Na+ regulates cleavage is direct (e.g. Na+ binding directly to the channel) or that the cellular machinery required is conserved in cells that do not normally express ENaC. Second, it suggests that Na+ may have a physiologically relevant role in regulating ENaC cleavage, because it was observed in Na+-transporting epithelia. In fact, we may have underestimated the degree to which Na+ inhibited cleavage in epithelia, because our data in Fig. 6 indicate that Na+ reduces ENaC cleavage by trypsin.
In conclusion, we found that intracellular Na+ reduces the proteolytic cleavage and activation of ENaC. These results provide a mechanism by which intracellular Na+ may regulate ENaC gating, an underlying component of Na+ feedback inhibition. In earlier work, we found that Nedd4-2 also alters ENaC cleavage (24). Together these data suggest that cleavage is a critical convergence point for the regulation of epithelial Na+ transport. Defects in this regulation may disrupt Na+ homeostasis and contribute to the pathogenesis of hypertension and other diseases of Na+ transport.
Acknowledgments
We thank Diane Olson and Kaela Kramer for assistance and acknowledge the University of Iowa DNA Core Facility for reagents and DNA sequencing.
This work was supported, in whole or in part, by the National Institutes of Health. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HEK, human embryonic kidney; NMDG, N-methyl-d-glutamic acid; PBS, phosphate-buffered saline; FRT, Fischer rat thyroid; E3, ubiquitin-protein isopeptide ligase.
References
- 1.Lifton, R. P. (1996) Science 272 676–680 [DOI] [PubMed] [Google Scholar]
- 2.Canessa, C. M., Horisberger, J. D., and Rossier, B. C. (1993) Nature 361 467–470 [DOI] [PubMed] [Google Scholar]
- 3.Canessa, C. M., Schild, L., Buell, G., Thorens, B., Gautschi, I., Horisberger, J. D., and Rossier, B. C. (1994) Nature 367 463–467 [DOI] [PubMed] [Google Scholar]
- 4.McDonald, F. J., Snyder, P. M., McCray, P. B., Jr., and Welsh, M. J. (1994) Am. J. Physiol. 266 L728–L734 [DOI] [PubMed] [Google Scholar]
- 5.McDonald, F. J., Price, M. P., Snyder, P. M., and Welsh, M. J. (1995) Am. J. Physiol. 268 C1157–C1163 [DOI] [PubMed] [Google Scholar]
- 6.Schild, L. (2004) Rev. Physiol. Biochem. Pharmacol. 151 93–107 [DOI] [PubMed] [Google Scholar]
- 7.Snyder, P. M. (2005) Endocrinology 146 5079–5085 [DOI] [PubMed] [Google Scholar]
- 8.Macrobbie, E. A., and Ussing, H. H. (1961) Acta Physiol. Scand. 53 348–365 [DOI] [PubMed] [Google Scholar]
- 9.Frindt, G., Silver, R. B., Windhager, E. E., and Palmer, L. G. (1993) Am. J. Physiol. 264 F565–F574 [DOI] [PubMed] [Google Scholar]
- 10.Chraibi, A., and Horisberger, J. D. (2002) J. Gen. Physiol. 120 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng, S., Bruns, J. B., and Kleyman, T. R. (2004) J. Biol. Chem. 279 9743–9749 [DOI] [PubMed] [Google Scholar]
- 12.Komwatana, P., Dinudom, A., Young, J. A., and Cook, D. I. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 8107–8111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellenberger, S., Gautschi, I., Rossier, B. C., and Schild, L. (1998) J. Clin. Invest. 101 2741–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awayda, M. S. (1999) Am. J. Physiol. 277 C216–C224 [DOI] [PubMed] [Google Scholar]
- 15.Abriel, H., and Horisberger, J. D. (1999) J. Physiol. (Lond.) 516 31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anantharam, A., Tian, Y., and Palmer, L. G. (2006) J. Physiol. (Lond.) 574 333–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose, B. D. (1984) Clinical Physiology of Acid-Base and Electrolyte Disorders, pp. 125–126, McGraw-Hill, New York
- 18.Garty, H., and Palmer, L. G. (1997) Physiol. Rev. 77 359–396 [DOI] [PubMed] [Google Scholar]
- 19.Hughey, R. P., Bruns, J. B., Kinlough, C. L., Harkleroad, K. L., Tong, Q., Carattino, M. D., Johnson, J. P., Stockand, J. D., and Kleyman, T. R. (2004) J. Biol. Chem. 279 18111–18114 [DOI] [PubMed] [Google Scholar]
- 20.Vallet, V., Chraibi, A., Gaeggeler, H. P., Horisberger, J. D., and Rossier, B. C. (1997) Nature 389 607–610 [DOI] [PubMed] [Google Scholar]
- 21.Caldwell, R. A., Boucher, R. C., and Stutts, M. J. (2005) Am. J. Physiol. 288 L813–L819 [DOI] [PubMed] [Google Scholar]
- 22.Hughey, R. P., Bruns, J. B., Kinlough, C. L., and Kleyman, T. R. (2004) J. Biol. Chem. 279 48491–48494 [DOI] [PubMed] [Google Scholar]
- 23.Caldwell, R. A., Boucher, R. C., and Stutts, M. J. (2004) Am. J. Physiol. 286 C190–C194 [DOI] [PubMed] [Google Scholar]
- 24.Knight, K. K., Olson, D. R., Zhou, R., and Snyder, P. M. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 2805–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snyder, P. M., Olson, D. R., and Thomas, B. C. (2002) J. Biol. Chem. 277 5–8 [DOI] [PubMed] [Google Scholar]
- 26.Snyder, P. M., Bucher, D. B., and Olson, D. R. (2000) J. Gen. Physiol. 116 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grunder, S., Jaeger, N. F., Gautschi, I., Schild, L., and Rossier, B. C. (1999) Pfluegers Arch. 438 709–715 [DOI] [PubMed] [Google Scholar]
- 28.Palmer, L. G., and Speez, N. (1986) Am. J. Physiol. 250 F273–F281 [DOI] [PubMed] [Google Scholar]
- 29.Kabra, R., Knight, K. K., Zhou, R., and Snyder, P. M. (2008) J. Biol. Chem. 283 6033–6039 [DOI] [PubMed] [Google Scholar]
- 30.Sheng, S., Carattino, M. D., Bruns, J. B., Hughey, R. P., and Kleyman, T. R. (2006) Am. J. Physiol. 290 F1488–F1496 [DOI] [PubMed] [Google Scholar]
- 31.Bruns, J. B., Carattino, M. D., Sheng, S., Maarouf, A. B., Weisz, O. A., Pilewski, J. M., Hughey, R. P., and Kleyman, T. R. (2007) J. Biol. Chem. 282 6153–6160 [DOI] [PubMed] [Google Scholar]
- 32.Pineda, A. O., Carrell, C. J., Bush, L. A., Prasad, S., Caccia, S., Chen, Z. W., Mathews, F. S., and Di Cera, E. (2004) J. Biol. Chem. 279 31842–31853 [DOI] [PubMed] [Google Scholar]
- 33.Silver, R. B., Frindt, G., Windhager, E. E., and Palmer, L. G. (1993) Am. J. Physiol. 264 F557–F564 [DOI] [PubMed] [Google Scholar]
- 34.Harvey, B. J., Thomas, S. R., and Ehrenfeld, J. (1988) J. Gen. Physiol. 92 767–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bengrine, A., Li, J., and Awayda, M. S. (2007) FASEB J. 21 1189–1201 [DOI] [PubMed] [Google Scholar]
- 36.Dinudom, A., Harvey, K. F., Komwatana, P., Young, J. A., Kumar, S., and Cook, D. I. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 7169–7173 [DOI] [PMC free article] [PubMed] [Google Scholar]