Abstract
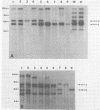
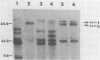
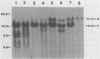
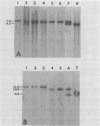
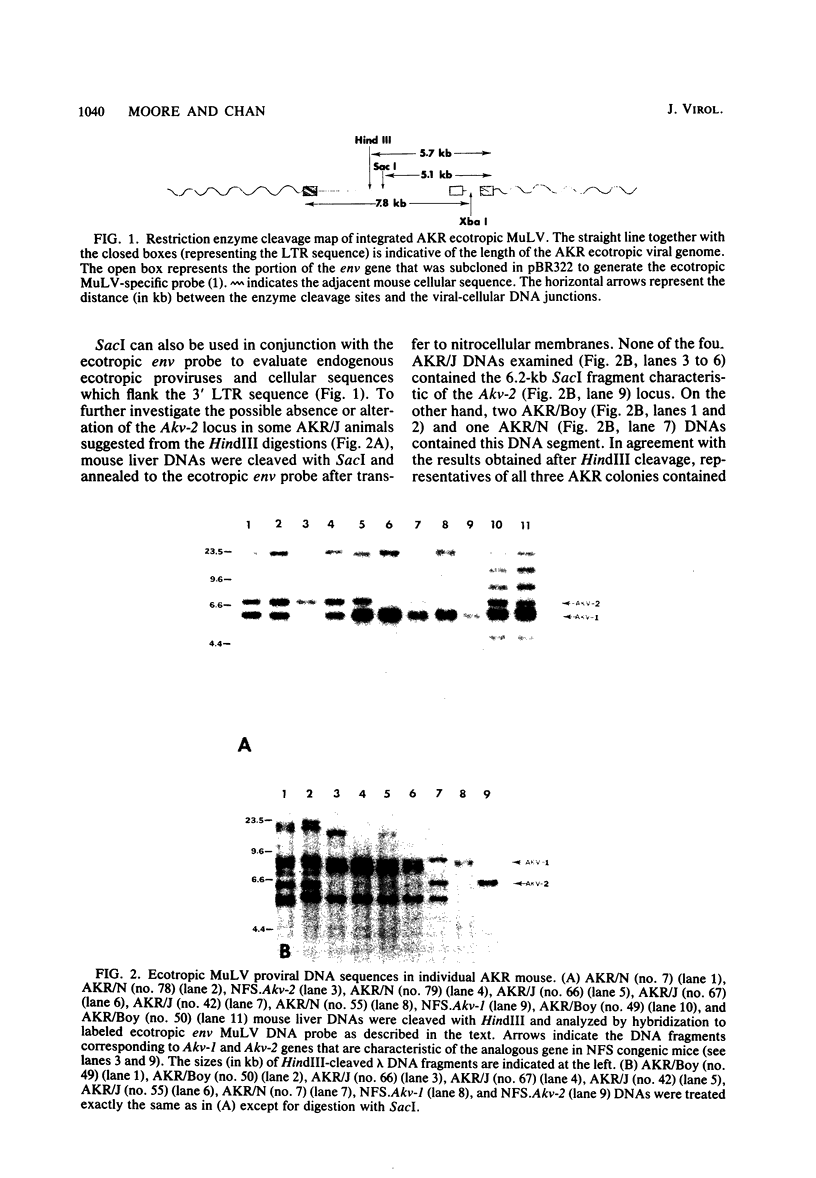
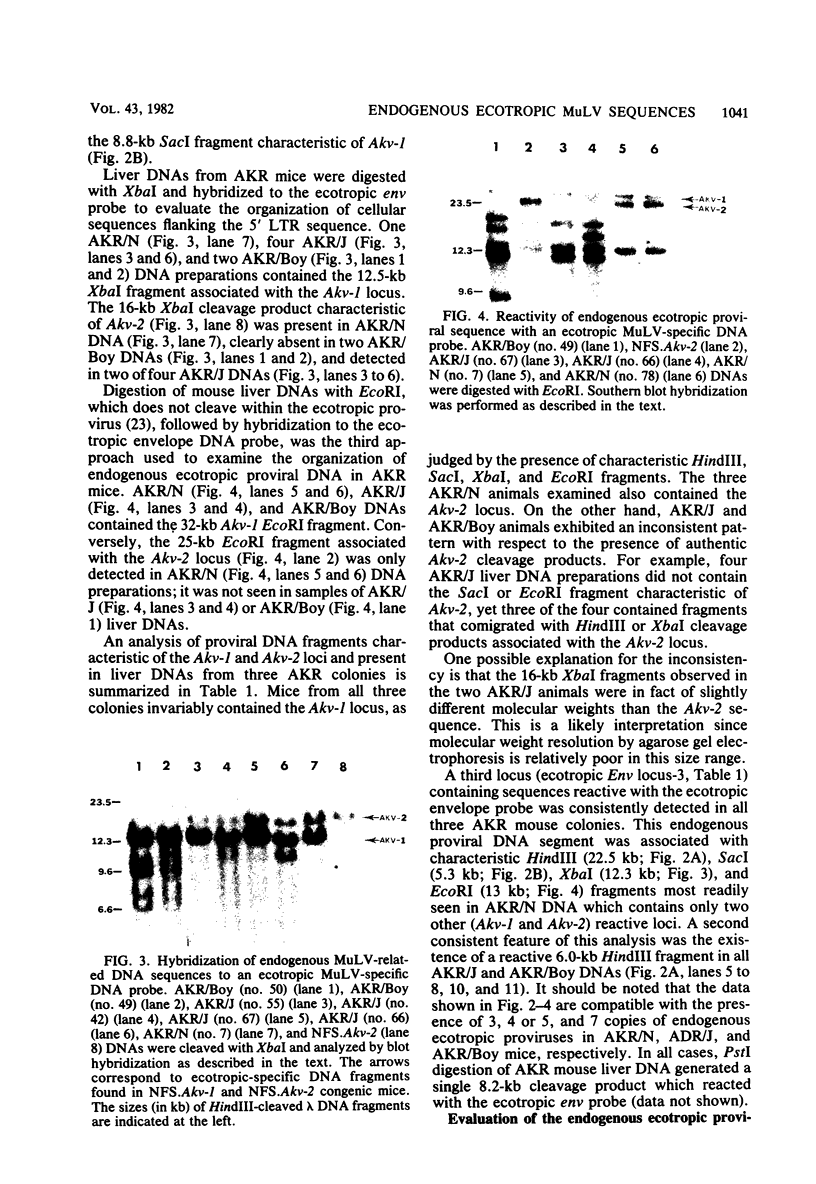
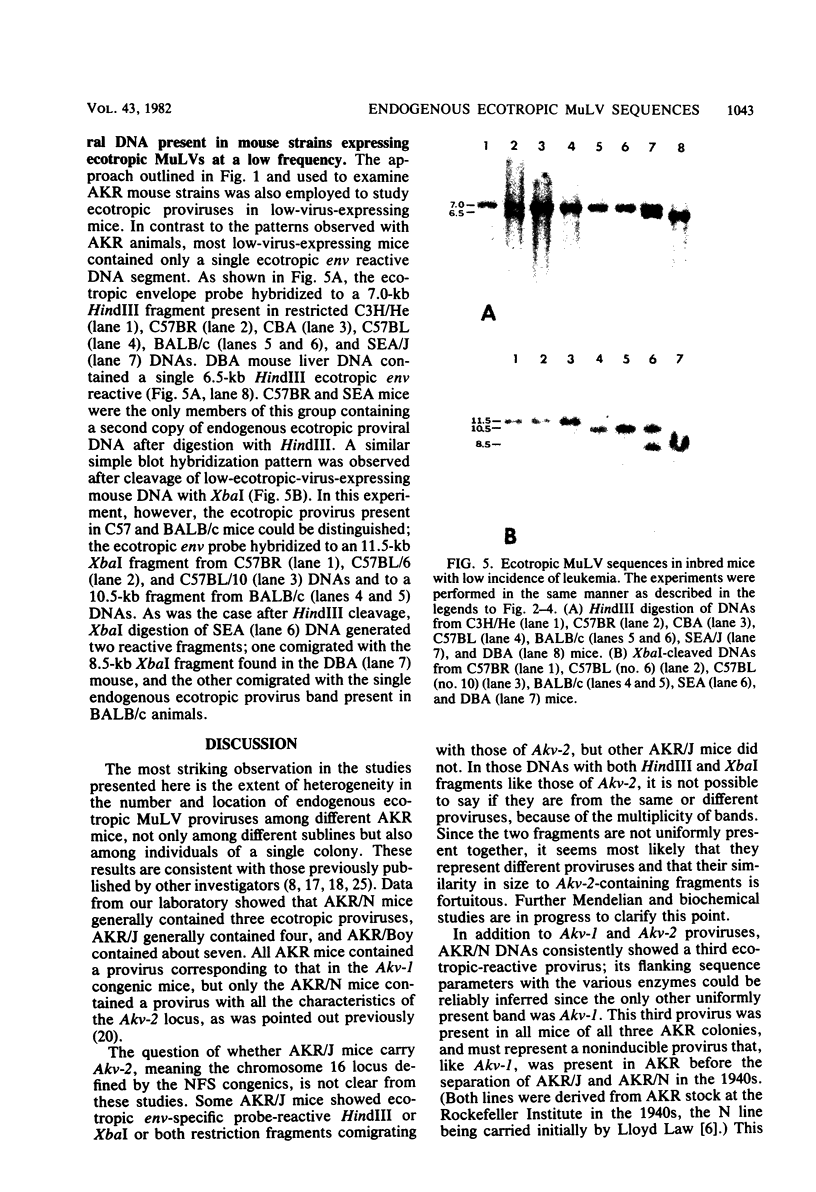
The arrangement of endogenous ecotropic retroviruses in selected high- and low-ecotropic-virus-producing mouse strains was examined by Southern blot hybridization analysis, using an ecotropic retrovirus-specific DNA probe. High-ecotropic-virus-producing mouse strains of the AKR family displayed heterogeneity with respect to the number of copies and the sites of insertion of endogenous ecotropic specific DNA. This diversity was seen even among individuals of the same AKR subline. Contrastingly, individuals within the same low-ecotropic-retrovirus-producing mouse strain showed no evidence of variability in their endogenous ecotropic proviral sequences. These results favored the hypothesis that germ line proviral reinsertion was responsible for the proviral sequence heterogeneity observed in high-ecotropic-virus-producing mouse strains.
Full text
PDF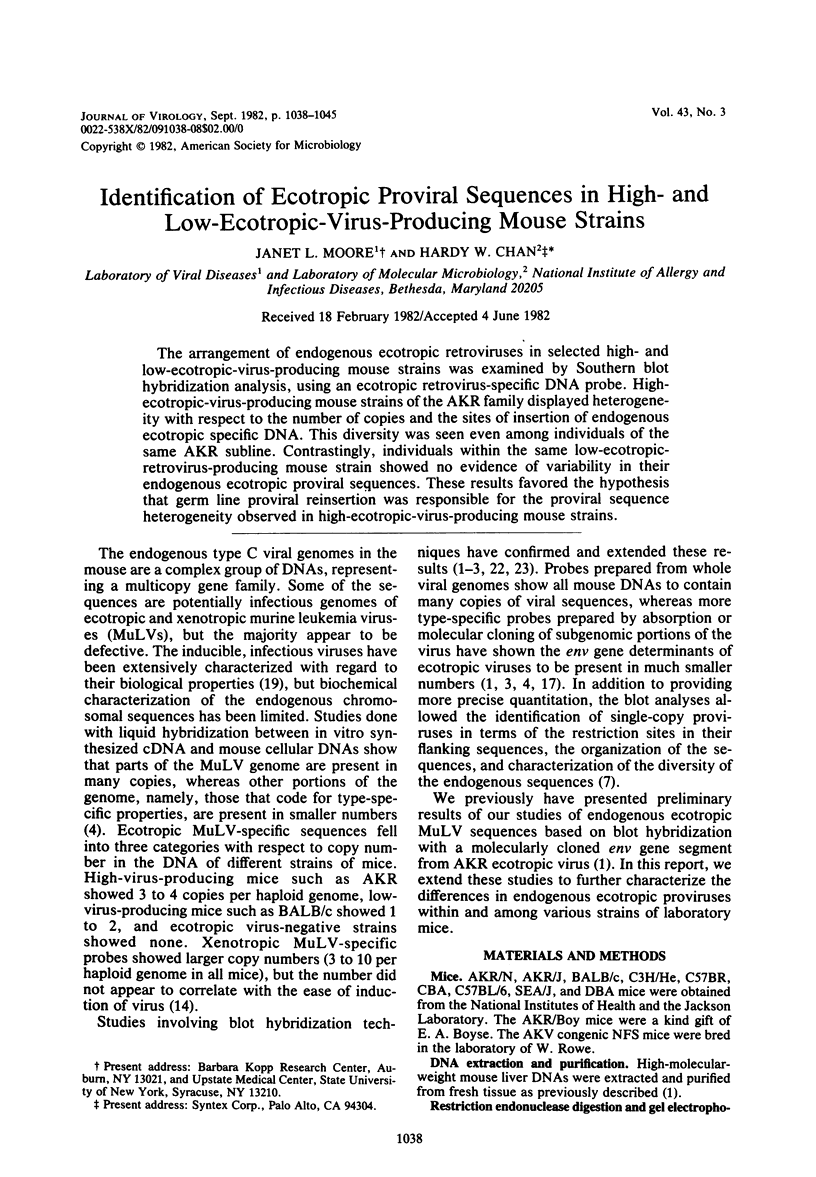




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan H. W., Bryan T., Moore J. L., Staal S. P., Rowe W. P., Martin M. A. Identification of ecotropic proviral sequences in inbred mouse strains with a cloned subgenomic DNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5779–5783. doi: 10.1073/pnas.77.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Qualitative and quantitative studies of AKR-type murine leukemia virus sequences in mouse DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1085–1101. doi: 10.1101/sqb.1974.039.01.124. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Germ-line MuLV reintegrations in AKR/J mice. Nature. 1982 Apr 29;296(5860):865–868. doi: 10.1038/296865a0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Joseph D. R. Serological and virological analysis of NIH (NIH X AKR) mice: evidence for three AKR murine leukemia virus loci. Virology. 1978 Jun 15;87(2):287–297. doi: 10.1016/0042-6822(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Rowe W. P., Boyse E. A., Stockert E., Sato H., Jacobs S. Relationship of infectious murine leukemia virus and virus-related antigens in genetic crosses between AKR and the Fv-1 compatible strain C57L. J Exp Med. 1976 Jan 1;143(1):32–46. doi: 10.1084/jem.143.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M. A., Chan H. W., Rowe W. P., Martin M. A. Molecular cloning of polyoma virus DNA in Escherichia coli: plasmid vector system. Science. 1979 Mar 2;203(4383):883–887. doi: 10.1126/science.217087. [DOI] [PubMed] [Google Scholar]
- Israel M. A., Vanderryn D. F., Meltzer M. L., Martin M. A. Characterization of polyoma viral DNA sequences in polyoma-induced hamster tumor cell lines. J Biol Chem. 1980 Apr 25;255(8):3798–3805. [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Chattopadhyay S. K., Teich N. M., Rowe W. P., Levine A. S. AKR murine leukemia virus genome: frequency of sequences in DNA of high-, low-, and non-virus-yielding mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3555–3559. doi: 10.1073/pnas.71.9.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint W., Quax W., van der Putten H., Berns A. Characterization of AKR murine leukemia virus sequences in AKR mouse substrains and structure of integrated recombinant genomes in tumor tissues. J Virol. 1981 Jul;39(1):1–10. doi: 10.1128/jvi.39.1.1-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint W., van der Putten H., Janssen F., Berns A. Mobility of endogenous ecotropic murine leukemia viral genomes within mouse chromosomal DNA and integration of a mink cell focus-forming virus-type recombinant provirus in the germ line. J Virol. 1982 Mar;41(3):901–908. doi: 10.1128/jvi.41.3.901-908.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Kozak C. A. Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4871–4874. doi: 10.1073/pnas.77.8.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P. Leukemia virus genomes in the chromosomal DNA of the mouse. Harvey Lect. 1978;71:173–192. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D., Bird S., Rowe W. P., Weinberg R. A. Identification of DNA fragments carrying ecotropic proviruses of AKR mice. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4554–4558. doi: 10.1073/pnas.76.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Origin of retroviruses from cellular moveable genetic elements. Cell. 1980 Oct;21(3):599–600. doi: 10.1016/0092-8674(80)90420-1. [DOI] [PubMed] [Google Scholar]
- Yoshimura F. K., Breda M. Lack of AKR ecotropic provirus amplification in AKR leukemic thymuses. J Virol. 1981 Sep;39(3):808–815. doi: 10.1128/jvi.39.3.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]