Abstract
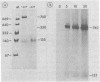
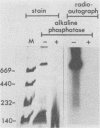
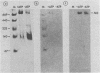
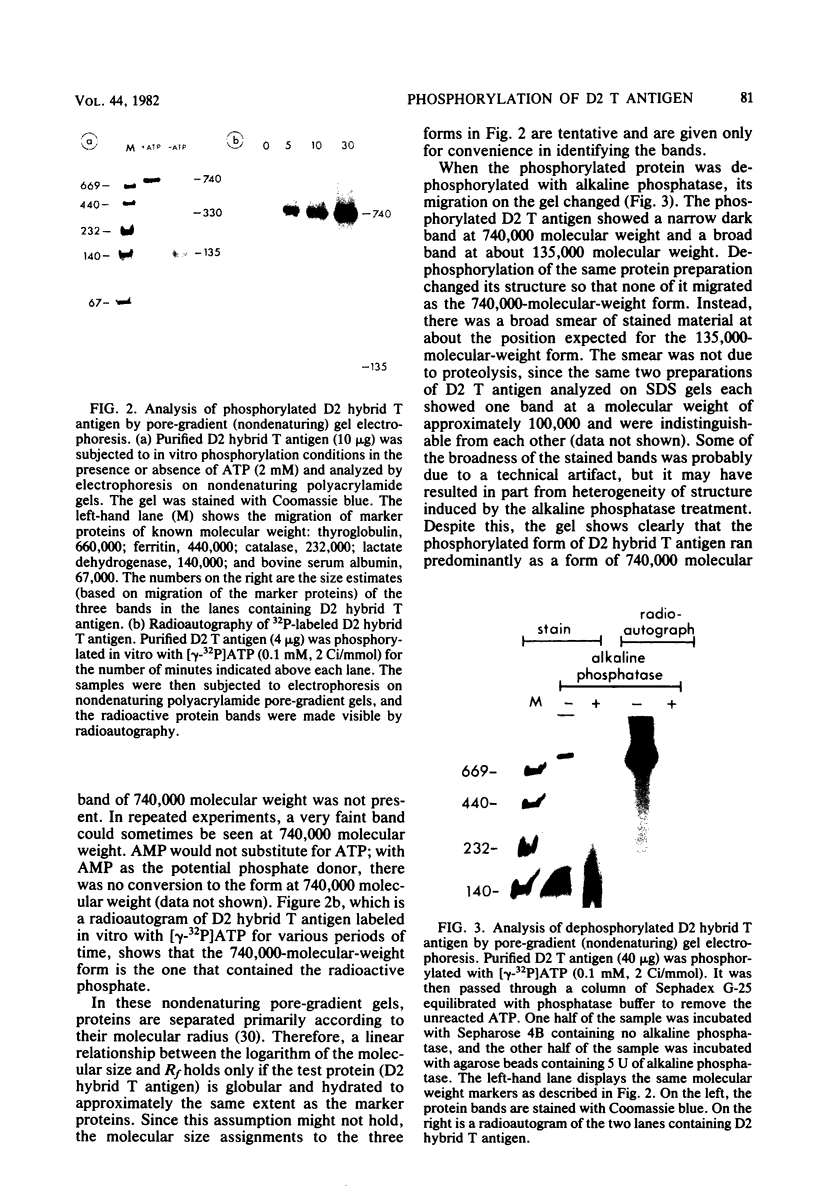
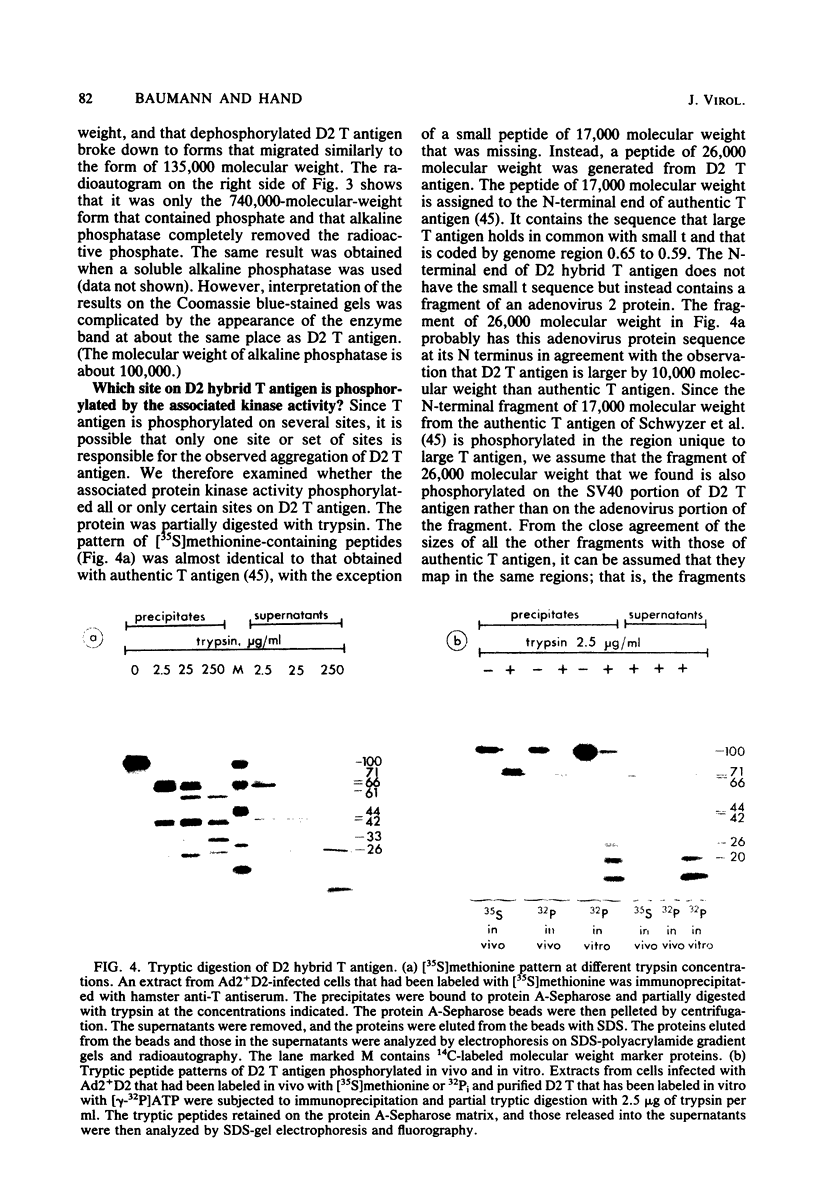
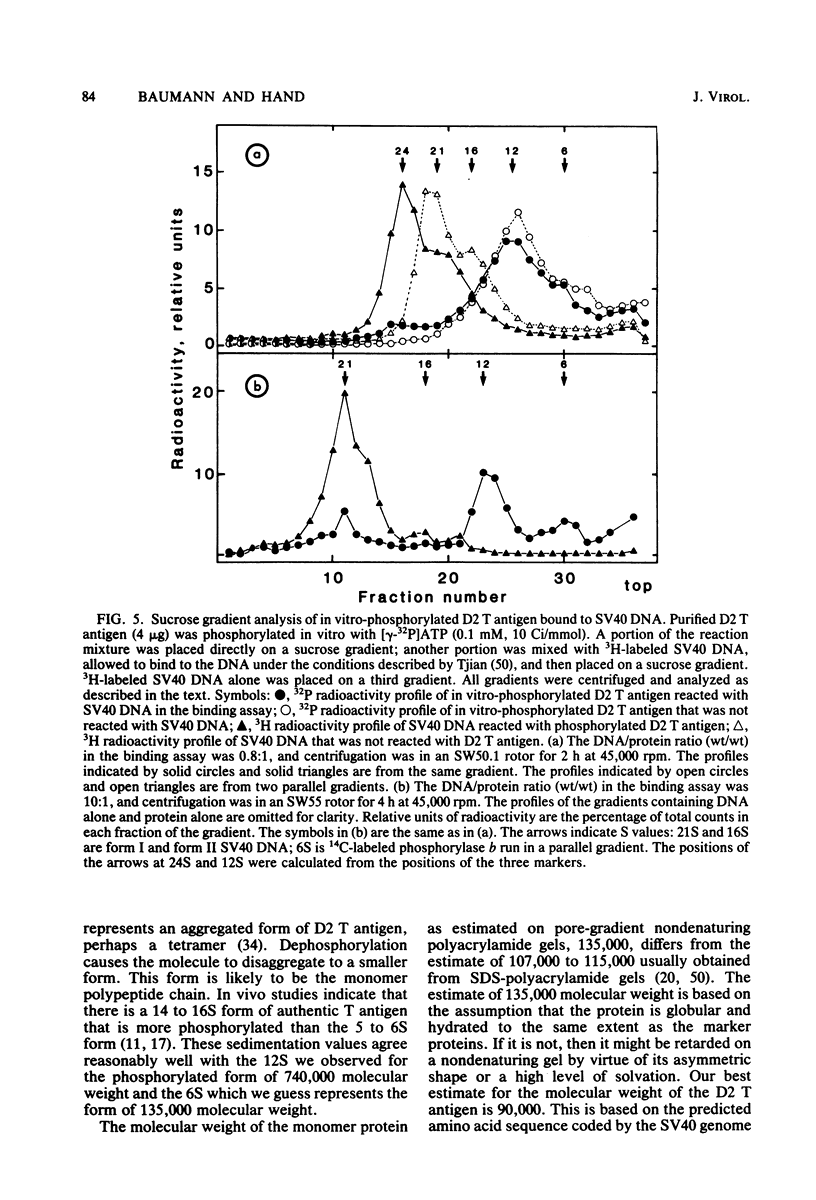
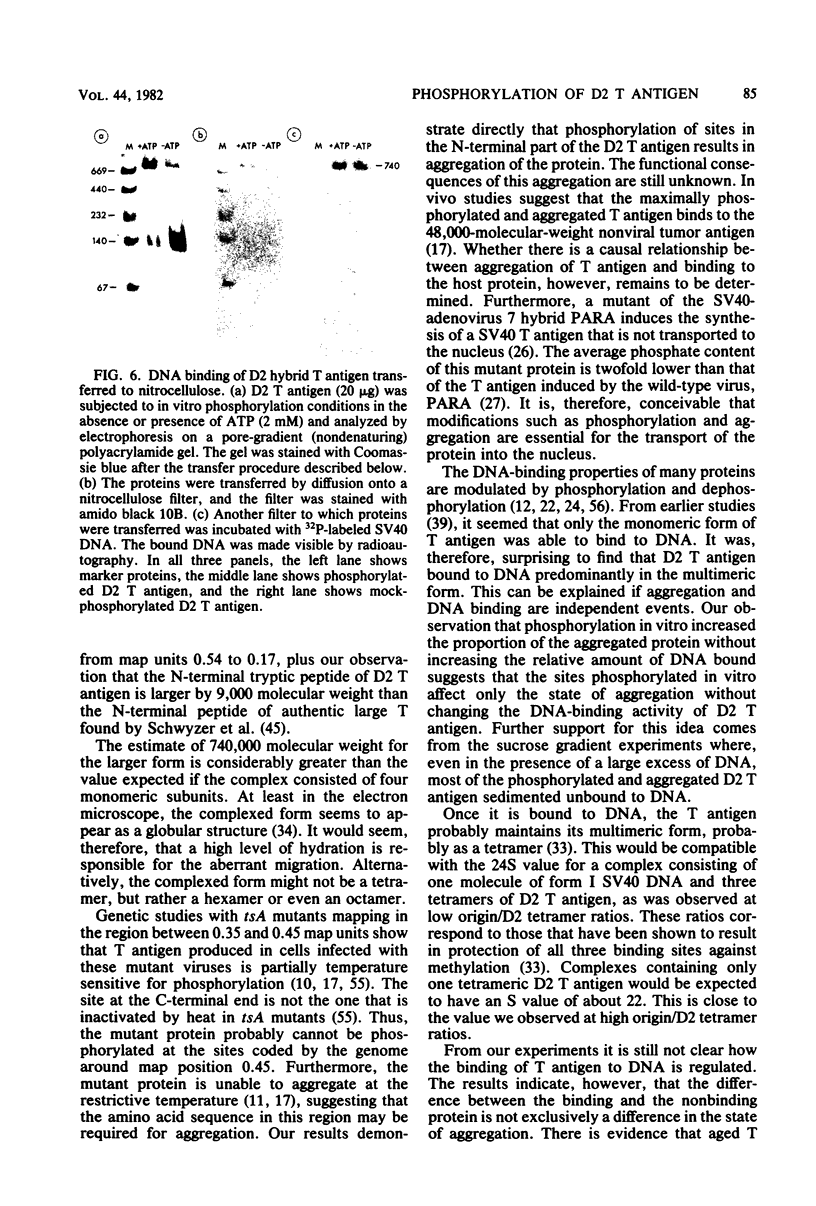
D2 hybrid T antigen is a protein closely related to simian virus 40 large T antigen and is synthesized in large quantities in cells infected with Ad2+D2, an adenovirus-simian virus 40 hybrid. We have analyzed the effects of phosphorylation on the structure and DNA binding of this protein. On nondenaturing pore-gradient gels, the purified protein migrated with an apparent molecular weight of 135,000, with a minor band at 330,000 molecular weight. In vitro phosphorylation catalyzed by the protein kinase activity associated with the protein resulted in a structural change so that most of it migrated with an apparent molecular weight of 740,000. Treatment of the phosphorylated form of the protein with alkaline phosphatase (which removed 95% of the phosphate) caused the disappearance of the 740,000-molecular-weight form and reappearance of the smaller forms. Partial tryptic digestion showed that D2 T antigen has two major regions of phosphorylation, only one of which was phosphorylated in vitro. The region phosphorylated in vitro was responsible for the aggregation of D2 T antigen and was tentatively assigned to the N-terminal part of the protein. As shown by protein blotting onto nitrocellulose filters, it was mainly the form of 740,000 molecular weight that bound to simian virus 40 DNA. However, sucrose gradient analyses showed that only a fraction of the in vitro-phosphorylated protein bound to DNA, suggesting that aggregation alone is not sufficient for binding.
Full text
PDF

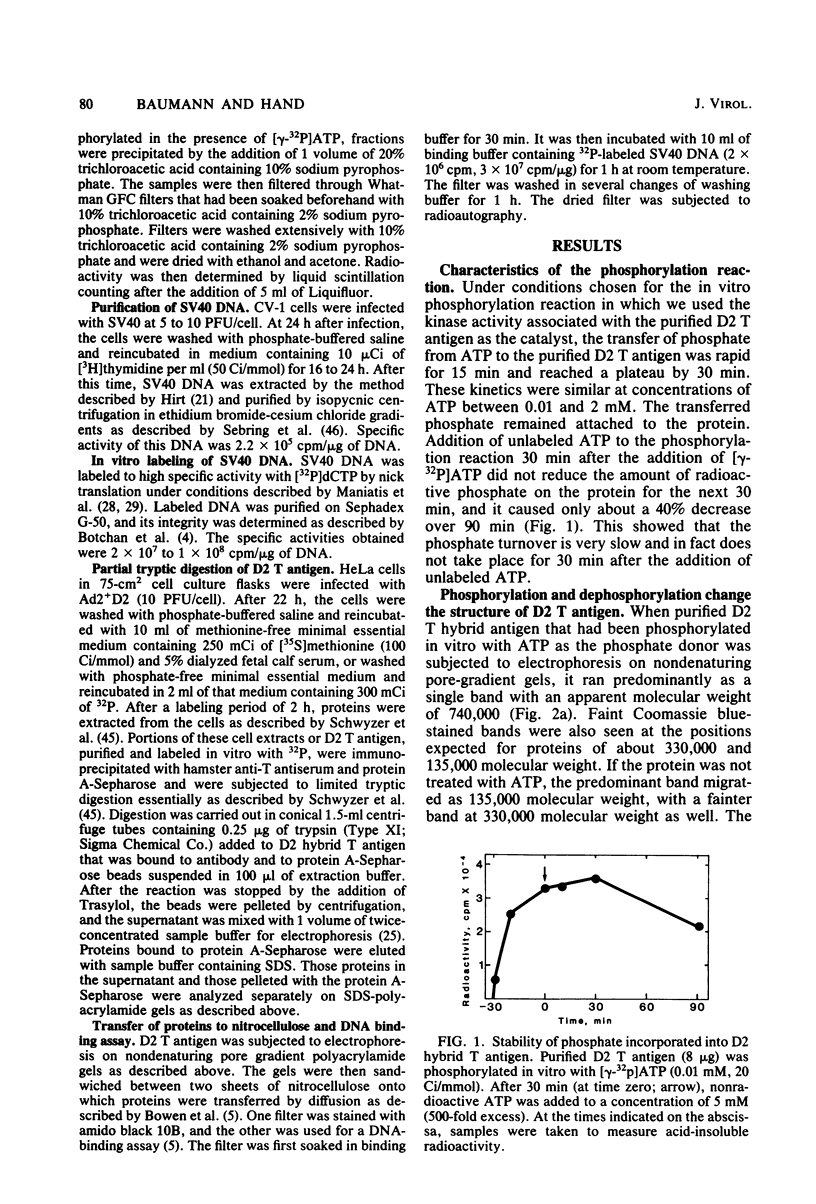



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann E. A., Hand R. Protein kinase activity associated with the D2 hybrid protein related to simian virus 40 T antigen: some characteristics of the reaction products. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3688–3692. doi: 10.1073/pnas.76.8.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. B., Hager L., Dulbecco R. Simian virus 40 T antigen binds to DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3754–3757. doi: 10.1073/pnas.71.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alisa R. M., Gershey E. L. Simian virus 40 T antigen binds to host cell chromosomes. Nature. 1978 Jul 13;274(5667):164–166. doi: 10.1038/274164a0. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Edwards C. A., Khoury G., Martin R. G. Phosphorylation of T-antigen and control T-antigen expression in cells transformed by wild-type and tsA mutants of simian virus 40. J Virol. 1979 Feb;29(2):753–762. doi: 10.1128/jvi.29.2.753-762.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Nowak B., Burger C. Detection and characterization of multiple forms of simian virus 40 large T antigen. J Virol. 1981 Jan;37(1):92–102. doi: 10.1128/jvi.37.1.92-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasy T. M., Inoue A., Johnson E. M., Allfrey V. G. Phosphorlyation of H1 and H5 histones by cyclic AMP-dependent protein kinase reduces DNA binding. Biochim Biophys Acta. 1979 Sep 27;564(2):322–334. doi: 10.1016/0005-2787(79)90229-6. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Giacherio D., Hager L. P. A poly(dT)-stimulated ATPase activity associated with simian virus 40 large T antigen. J Biol Chem. 1979 Sep 10;254(17):8113–8116. [PubMed] [Google Scholar]
- Goldman N., Brown M., Khoury G. Modification of SV40 T antigen by poly ADP-ribosylation. Cell. 1981 May;24(2):567–572. doi: 10.1016/0092-8674(81)90347-0. [DOI] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Complex of simian virus 40 large tumor antigen and 48,000-dalton host tumor antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):105–109. doi: 10.1073/pnas.78.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Simian virus 40 large T antigen isoelectric focuses as multiple species with varying phosphate content. Virology. 1979 Dec;99(2):413–416. doi: 10.1016/0042-6822(79)90020-5. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Spangler G., Livingston D. M. Protein kinase activity associated with simian virus 40 T antigen. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2610–2614. doi: 10.1073/pnas.76.6.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R. Functions of T antigens of SV40 and polyomavirus. Biochim Biophys Acta. 1981 Aug 31;651(1):1–24. doi: 10.1016/0304-419x(81)90002-0. [DOI] [PubMed] [Google Scholar]
- Hassell J. A., Lukanidin E., Fey G., Sambrook J. The structure and expression of two defective adenovirus 2/simian virus 40 hybrids. J Mol Biol. 1978 Apr 5;120(2):209–247. doi: 10.1016/0022-2836(78)90065-7. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Stern H. The effect of dephosphorylation on the properties of a helix-destabilizing protein from meiotic cells and its partial reversal by a protein kinase. Eur J Biochem. 1979 Mar 15;95(1):31–38. doi: 10.1111/j.1432-1033.1979.tb12936.x. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Kuehn G. D., Affolter H. U., Atmar V. J., Seebeck T., Gubler U., Braun R. Polyamine-mediated phosphorylation of a nucleolar protein from Physarum polycephalum that stimulates rRNA synthesis. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2541–2545. doi: 10.1073/pnas.76.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Biochemical characterization of nuclear and cytoplasmic forms of SV40 tumor antigens encoded by parental and transport-detective mutant SV40-adenovirus 7 hybrid viruses. Virology. 1980 Sep;105(2):314–327. doi: 10.1016/0042-6822(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Inhibition of nuclear migration of wild-type SV40 tumor antigen by a transport-defective mutant of SV40-adenovirus 7 hybrid virus. Virology. 1980 Sep;105(2):303–313. doi: 10.1016/0042-6822(80)90032-x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Mechali M., Abadiedebat J., de Recondo A. M. Eukaryotic DNA polymerase alpha. Structural analysis of the enzyme from regenerating rat liver. J Biol Chem. 1980 Mar 10;255(5):2114–2122. [PubMed] [Google Scholar]
- Montenarh M., Henning R. Simian virus 40 T-antigen phosphorylation is variable. FEBS Lett. 1980 May 19;114(1):107–110. doi: 10.1016/0014-5793(80)80870-2. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Rio D. C., Robbins A. K., Tjian R. SV40 gene expression is modulated by the cooperative binding of T antigen to DNA. Cell. 1981 Aug;25(2):373–384. doi: 10.1016/0092-8674(81)90056-8. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Williams R. C., Tjian R. Oligomeric structure of a simian virus 40 T antigen in free form and bound to DNA. J Mol Biol. 1981 Jun 5;148(4):347–353. doi: 10.1016/0022-2836(81)90180-7. [DOI] [PubMed] [Google Scholar]
- Oren M., Winocour E., Prives C. Differential affinities of simian virus 40 large tumor antigen for DNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):220–224. doi: 10.1073/pnas.77.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. SV40: T antigen, the A function and transformation. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):267–276. doi: 10.1101/sqb.1974.039.01.035. [DOI] [PubMed] [Google Scholar]
- Paucha E., Mellor A., Harvey R., Smith A. E., Hewick R. M., Waterfield M. D. Large and small tumor antigens from simian virus 40 have identical amino termini mapping at 0.65 map units. Proc Natl Acad Sci U S A. 1978 May;75(5):2165–2169. doi: 10.1073/pnas.75.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., McLaughlin B. C., Oxford J. S. Simian virus 40-induced T and tumor antigens. J Virol. 1969 Nov;4(5):574–579. doi: 10.1128/jvi.4.5.574-579.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Beck Y., Gidoni D., Oren M., Shure H. DNA binding and sedimentation properties of SV40 T antigens synthesized in vivo and in vitro. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):123–130. doi: 10.1101/sqb.1980.044.01.014. [DOI] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rio D., Robbins A., Myers R., Tjian R. Regulation of simian virus 40 early transcription in vitro by a purified tumor antigen. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5706–5710. doi: 10.1073/pnas.77.10.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Scheidtmann K. H., Kaiser A., Carbone A., Walter G. Phosphorylation of threonine in the proline-rich carboxy-terminal region of simian virus 40 large T antigen. J Virol. 1981 Apr;38(1):59–69. doi: 10.1128/jvi.38.1.59-69.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer M., Weil R., Frank G., Zuber H. Amino acid sequence analysis of fragments generated by partial proteolysis from large simian virus 40 tumor antigen. J Biol Chem. 1980 Jun 25;255(12):5627–5634. [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Robbins A. Enzymatic activities associated with a purified simian virus 40 T antigen-related protein. Proc Natl Acad Sci U S A. 1979 Feb;76(2):610–614. doi: 10.1073/pnas.76.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Van Roy F., Fransen L., Fiers W. Phosphorylation patterns of tumour antigens in cells lytically infected or transformed by simian virus 40. J Virol. 1981 Oct;40(1):28–44. doi: 10.1128/jvi.40.1.28-44.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Flory P. J., Jr Phosphorylation of SV40 large T antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):165–169. doi: 10.1101/sqb.1980.044.01.019. [DOI] [PubMed] [Google Scholar]
- Wilcox K. W., Kohn A., Sklyanskaya E., Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol. 1980 Jan;33(1):167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]