Abstract
Estrogen receptor alpha (ERα) mediates estrogen (E2) actions in the brain and is critical for normal reproductive function and behavior. In the classical pathway, ERα binds to estrogen response elements (EREs) to regulate gene transcription. ERα can also participate in several non-classical pathways, including ERE-independent gene transcription via protein-protein interactions with transcription factors and rapid, non-genotropic pathways. To distinguish between ERE-dependent and ERE-independent mechanisms of E2 action in vivo, we have created ERα null mice that possess an ER knock-in mutation (E207A/G208A; “AA”), in which the mutant ERα cannot bind to DNA but retains activity in ERE-independent pathways (ERα−/AA mice). Understanding the molecular mechanisms of ERα action will be helpful in developing pharmacological therapies that differentiate between ERE-dependent and –independent processes. This review focuses on how the ERα−/AA model has contributed to our knowledge of ERα signaling mechanisms in estrogen regulation of the reproductive axis and sexual behavior.
Keywords: estrogen receptor alpha, estrogen response element, non-classical signaling, negative feedback, sexual behavior
The biological effects of estrogens are mediated through at least two distinct nuclear receptors, ERα and ERβ, which belong to the nuclear hormone receptor superfamily (Mangelsdorf et al., 1995). In the classical pathway of estrogen action, E2 binds to ER, inducing conformational changes within the receptor that promote dimerization and interaction with coactivator and corepressor molecules. The ligand-receptor complex binds with high affinity to specific estrogen response elements (EREs) in the regulatory regions of target genes to either activate or repress gene expression (Glass, 1994; McKenna et al., 1999; Smith and O'Malley, 2004; Tsai and O'Malley, 1994). However, not all estrogen-responsive genes contain EREs or ERE-like sequences (O'Lone et al., 2004). Thus, while ER has traditionally been thought of as a nuclear, ligand-dependent transcription factor, the molecular mechanisms of E2 action are more complex. It is now recognized that E2 actions are mediated by at least three other “non-classical” ER pathways: (1) ligand-independent ER signaling, in which gene activation occurs through second-messenger pathways that alter intracellular kinase and phosphatase activity, resulting in altered phosphorylation of ER (Weigel and Zhang, 1998); (2) rapid, non-genotropic effects through a membrane-associated ER, which are discussed in detail elsewhere in this issue (Kelly MJ, pg XX); and (3) genotropic, ERE-independent signaling, in which ER regulates genes independent of direct DNA binding via protein-protein interaction with other transcription factors, such as c-Fos/c-Jun B (AP-1), Sp1, and NF-κB (Gaub et al., 1990; Jakacka et al., 2002; Kushner et al., 2000; Ray et al., 1994; Safe, 2001; Webb et al., 1995) (Fig.1).
Figure 1. ER Signaling.
ERα signaling pathways include: (1) genotropic ERE-dependent (“classical”), in which liganded ER dimerizes on an ERE; (2) genotropic ERE-independent (“tethered”), in which liganded ER interacts with other transcription factors bound to their response elements; (3) membrane initiated, in which a membrane associated ER acts through kinases to phosphorylate other transcription factors and eNOS; (4) ligand independent, in which ER is activated by phosphorylation. Pathways 2–4 are collectively referred to as “non-classical”.
ER knockout mice have provided invaluable evidence for the biological functions of ERα and ERβ. However, the exact cellular and molecular mechanisms of E2 action are only recently being uncovered. This review describes the non-classical estrogen receptor knock-in mouse model and highlights how it has contributed to our knowledge of the relative contributions of classical and non-classical ERα signaling in vivo.
The non-classical estrogen receptor knock-in mouse
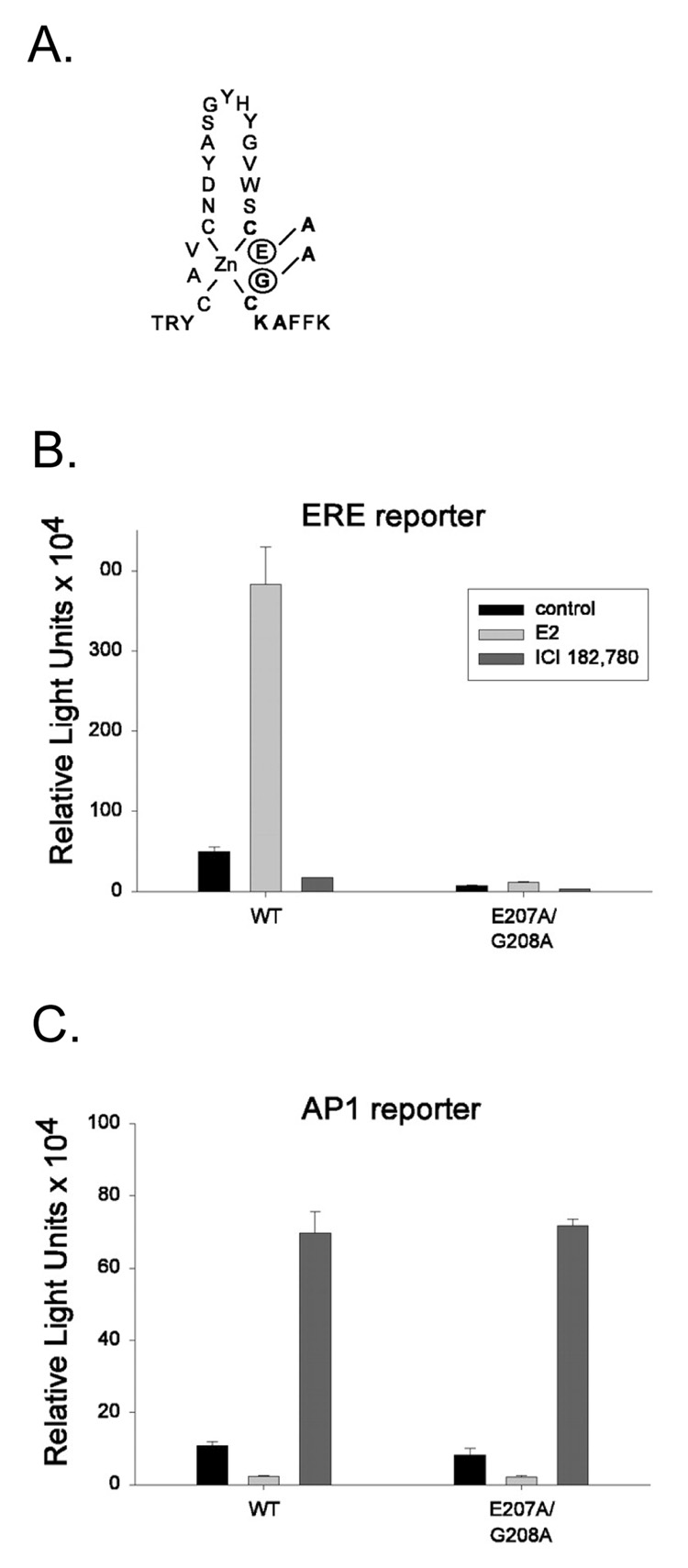
To better define non-classical signaling mechanisms of ERα action, Jakacka and colleagues generated selective DNA binding mutations in the mouse ERα and observed their effects in vitro (Jakacka et al., 2001). One such mutation within the first zinc finger of the DNA binding domain, E207A/G208A (“AA”), completely eliminated ERE binding and activation of ERE-containing reporter genes, but retained full transcriptional activity of reporter genes containing AP1 response elements and interacted with Jun when tested in mammalian cell two-hybrid assays (Fig. 2). Of note, the AA mutant ERα protein has normal structure, equal expression and activity compared to the wild-type protein, and does not exert dominant-negative effects in vitro (Jakacka et al., 2002). These findings demonstrated that ERα-DNA binding is not necessary for activity in the non-classical AP1 pathway.
Figure 2. The non-classical ERα knock-in mutation.
(A) Schematic illustration of the introduced double mutation (E at position 207 to A and G at position 208 to A) in the first zinc finger of the DNA-binding domain of mouse ERα. (B,C) Transfection studies were performed in ER-deficient TSA cells, using either ERE (B) or AP1 (C) reporters. Cells were transfected with WT ERα or the E207A/G208A mutant. Cells were treated with ethanol vehicle, 1 nM E2, or 100 nM ICI 182,780, and luciferase activity was measured. The E207A/G208A mutant was incapable of activating transcription through EREs but retained the full capacity to activate transcription through AP1. (Jakacka et al., 2002)
This E207A/G208A mutation was introduced into the mouse ERα by targeted insertion (knock-in) in order to distinguish between classical and non-classical ERα actions in vivo (Jakacka et al., 2002). Initial characterization of the resulting ERα+/AA mice revealed that females are acyclic and display reduced serum progesterone, anovulation, uterine defects, and inhibited mammary gland development. These results are surprising given that ERα+/− females are fertile (Dupont et al., 2000; Lubahn et al., 1993), and therefore suggest a putative antagonism of the wild-type allele by the AA mutation, or an imbalance of the relative activities of classical and non-classical pathways. Nonetheless, the phenotype of these heterozygous ERα+/AA mice demonstrates the physiological importance of non-classical ERα signaling in the development and function of the female reproductive system.
Unfortunately, the resulting infertility of ERα+/AA females precludes the generation of ERαAA/AA homozygous mutants. However, ERα+/AA males appear to have normal fertility (Jakacka et al., 2002) and can therefore be used to generate ERα−/AA compound heterozygotes when bred with ERα+/− females. Introducing the AA knock-in mutation on the ERαKO background effectively eliminates all ERE-dependent signaling, therefore a rescue of ERα−/− phenotypes by the AA mutation suggests a physiological role for non-classical mechanisms. Thus, these ERα−/AA mice provide a unique opportunity to examine isolated ERE-independent signaling. As such, they have been successfully used to identify a physiological role for non-classical ERα signaling in uterus (O'Brien et al., 2006) and bone (Syed et al., 2005).
Homeostatic feedback actions of estrogen in the female reproductive axis
Throughout most of the ovulatory cycle, estrogens exert suppressive effects on GnRH and LH secretion. However, as estrogen levels rise from the growing ovarian follicle, their effects become stimulatory, evoking a preovulatory GnRH surge and subsequent LH surge, which triggers ovulation. While it is known that estrogen feedback appears to be primarily mediated by ERα (Couse et al., 2003; Wintermantel et al., 2006), the underlying signaling mechanisms that contribute to estrogen positive and negative feedback remain unclear. To address this question, we have used the ERα−/AA mouse model to examine the relative contributions of ERE-dependent and –independent ERα signaling pathways in conveying estrogen feedback in vivo (Glidewell-Kenney et al., 2007).
Negative feedback on LH
ERα−/− females are infertile and display elevated serum LH compared to wild-type counterparts (Couse et al., 1999; Couse and Korach, 1999; Glidewell-Kenney et al., 2007). The non-classical knock-in mutation reduces serum LH levels in intact ERα−/AA females compared to ERα−/− females (Fig. 3), thus reducing hyperstimulation of the ovary, and consequently reduces the presence of hemorrhagic cysts (Glidewell-Kenney et al., 2007). The reduction in LH also rescues steroidogenesis, as evidenced by a complete restoration of serum E2 in ERα−/AA females. ERα−/AA mice display a normal elevation of LH in response to ovariectomy, and estrogen replacement in ovariectomized (OVX) ERα−/AA females is sufficient to reduce serum LH to intact levels, suggesting that ERE-independent mechanisms mediate, at least in part, the negative feedback actions of estrogen on LH (Fig. 3). However, as LH remains elevated in intact and E2-replaced OVX ERα−/AA females compared to ERα+/+ controls, it remains a possibility that classical, ERE-dependent mechanisms contribute to some aspects of negative feedback.
Figure 3. Estrogen feedback in the female.
ERE-independent ERα signaling is sufficient to convey estrogen negative but not positive feedback. Serum LH from intact, OVX, OVX/estrogen-replaced females killed in the morning for negative feedback and afternoon for positive feedback (n=5–16). Serum LH is significantly elevated in OVX ERα+/+ females compared to intact ERα+/+ females (a), and significantly elevated in OVX ERα−/AA females compared to intact ERα−/AA females (b). The post-OVX rise in LH was reduced by E2 treatment (negative feedback) in ERα+/+ (c) and ERα−/AA females (d). Estrogen treatment (positive feedback) significantly increased serum LH in ERα+/+ females (e), but not in ERα−/− or ERα−/AA females. (Glidewell-Kenney et al., 2007)
In the ERα−/AA model the mutant ERα may mediate estrogen negative feedback through genotropic, ERE-independent mechanisms. Several genes have been implicated in mediating estrogen suppression of GnRH/LH secretion, but whether they are regulated by classical or non-classical mechanisms remain to be determined. For example, Kiss1 expressing neurons in the arcuate nucleus (ARC) of the hypothalamus relay negative feedback effects of E2 on GnRH secretion; Kiss1 expression in the ARC is inhibited by E2 via ERα, presumably reducing kisspeptin production and its stimulatory effects on GnRH release (Popa et al., 2008). Recent evidence has identified three ERE half-sites within the human Kiss1 promoter, but Kiss1 expression can also be regulated indirectly through ERα-Sp protein complexes at GC-rich motifs in the Kiss1 promoter in vitro (Li et al., 2007). Whether the non-classical ERα mutant in ERα−/AA female mice is capable of mediating E2 inhibition of Kiss1 remains to be determined.
The rescue of negative feedback in ERα−/AA females may also reflect rapid, non-genotropic actions of E2 originating at the plasma membrane, as the knock-in mutation is specific to the DNA-binding domain and leaves the membrane-localization domain intact (Jakacka et al., 2001). Evidence from ovariectomized ewes (Nett et al., 1984), monkeys (Pau et al., 1990), and guinea pigs (Condon et al., 1988) demonstrates that E2 can rapidly decrease LH secretion, indicating non-genotropic mechanisms of E2 action. Furthermore, the fact that conjugated forms of E2 can rapidly prevent GnRH-induced LH secretion in ovine pituitary cells suggests that E2’s suppressive effects on LH release are mediated by a plasma membrane-associated E2-binding protein (Arreguin-Arevalo and Nett, 2006). It is therefore possible that ERE-independent estrogen negative feedback is mediated by genotropic mechanisms, non-genotropic mechanisms, or both.
Positive feedback on LH
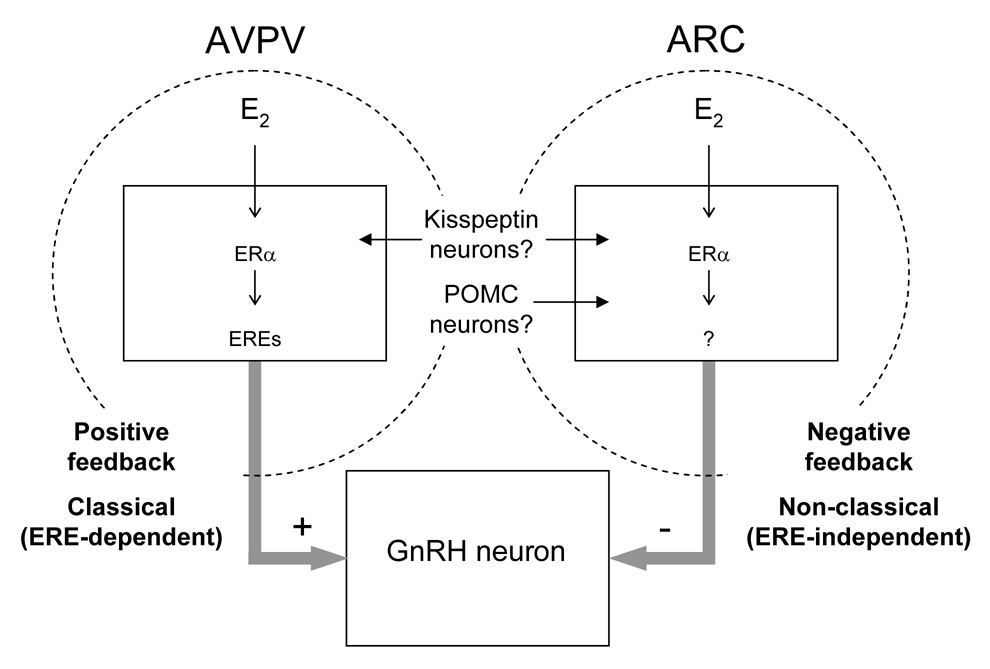
Positive feedback actions of estrogen on GnRH neurons, which drive the preovulatory LH surge, are mediated by ERα-expressing neuronal afferents within the GnRH neuronal network (Wintermantel et al., 2006). Similar to ERα−/− females, ERα−/AA females do not exhibit an LH surge, spontaneous ovulation, or estrous cyclicity, indicating that they do not respond to the positive feedback actions of E2 (Fig. 3) (Glidewell-Kenney et al., 2007). These results suggest that while non-classical ERα signaling mechanisms are sufficient to restore E2’s negative feedback effects on LH, they are not sufficient to mediate E2’s stimulatory actions on the LH surge (Fig. 4).
Figure 4. Proposed model for classical and non-classical ERα signaling in estrogen positive and negative feedback.
Negative feedback actions of estrogen on GnRH/LH secretion are mediated in part by non-classical (ERE-independent) ERα signaling mechanisms. In contrast, positive feedback actions of estrogen on GnRH/LH surges are mediated by classical (ERE-dependent) ERα signaling mechanisms. Whether these classical and non-classical mechanisms occur in specific cell types that mediate estrogen feedback (e.g. kisspeptin neurons, POMC neurons) remain to be determined.
Several lines of evidence support the idea that genotropic mechanisms underlie estrogen positive feedback and that rapid, non-genotropic actions of estrogen are not sufficient for GnRH neuron activation and LH surges. For example, prolonged estrogen exposure is required for positive feedback and maximum pituitary responsiveness to GnRH in the rat (Legan et al., 1975), mouse (Bronson, 1981), monkey (Xia et al., 1992), and ewe (Evans et al., 1997), suggesting that slower, genotropic actions are involved in this process. Interestingly, GnRH surges can occur even after estrogen withdrawal, suggesting that the neuronal network supporting the surge process is sufficiently activated by previous E2 exposure and does not require short-term E2 action (Evans et al., 1997). Furthermore, acute treatments of E2 are not sufficient to induce LH surges in the ewe (Arreguin-Arevalo and Nett, 2006). Our findings extend this idea of genotropic-dependent LH surges to include a specific requirement for ERα-DNA binding, and therefore suggest that downstream genes involved in estrogen regulation of GnRH/LH surges likely contain EREs. The progesterone receptor (PR) gene is one downstream candidate known to be necessary for E2-induction of the LH surge (Chappell and Levine, 2000; Chappell et al., 1999; Levine, 1997), and is indeed regulated by estrogen through classical, ERE-dependent ERα signaling (Kraus et al., 1994). Accordingly, immunohistochemical analyses from our laboratory demonstrate that estrogen-induced PR expression is reduced in the anteroventral periventricular (AVPV) nucleus of the hypothalamus, a region critical for LH surges, in both ERα−/AA and ERα−/− females (our unpublished observations). Thus, the lack of PR induction in these animals likely contributes, at least in part, to the lack of E2 positive feedback. Together these findings demonstrate that estrogen positive feedback on LH relies on classical, ERE-dependent signaling pathways.
It is well known that estrogen positive feedback is a sexually dimorphic neuroendocrine process, as LH surges do not occur in males. Previous studies have clearly shown that estrogen signaling through ERα (and ERβ) in the perinatal period mediates the sexual differentiation of the AVPV, a region critical for estrogen positive feedback (Bodo et al., 2006; Simerly et al., 1997). In the AVPV, female mice have more tyrosine hydroxylase immunoreactive (TH-ir) neurons than males and ERαKO males display a feminized AVPV. Immunohistochemical analyses from our laboratory demonstrate that the number of TH-ir neurons in the medial AVPV is significantly greater in both ERα−/− and ERα−/AA males compared to ERα+/+ counterparts, indicating that the mutant ERα is not sufficient to defeminize the AVPV (Fig. 5). Thus, sexual differentiation of this region, and consequently estrogen positive feedback and LH surges, appears to require classical, ERE-dependent ERα signaling mechanisms.
Figure 5. Non-classical ERα signaling mechanisms are not sufficient to mediate estrogen’s defeminizing effects on the sexually dimorphic AVPV.
Immunohistochemical analysis revealed that brains from both ERα−/− and ERα−/AA males have significantly more TH-ir neurons in the medial AVPV than wild-type male counterparts. *p<0.05, compared to ERα+/+ males.
Testicular function and testosterone production in the male
The importance of ERα signaling in male fertility is demonstrated by descriptions of profound testicular dysfunction in ERαKO mice. Although the reproductive tract develops normally during the prenatal period, ERαKO males display atrophy of the testes and seminiferous tubules, tubule dysmorphogenesis, and reduced sperm counts (Eddy et al., 1996). We have used the ERα−/AA mouse model to investigate the relative contributions of classical and non-classical mechanisms to these phenotypes. The knock-in mutation completely restores sperm counts and sperm motility in ERα−/AA males, suggesting that ERE-independent signaling is sufficient for normal sperm production (our unpublished observations). Interestingly, while the majority of ERα−/− males exhibit severe testis pathology, tubules in ERα−/AA males appear normal or with only mild dysmorphogenesis at young ages. However, as ERα−/AA age, their testis phenotype becomes moderate to severe, suggesting that the rescue is transient (our unpublished observations). These findings suggest that while ERE-independent mechanisms contribute to some aspects of testis physiology and function, ERE-dependent mechanisms are required for long-term maintenance of male fertility.
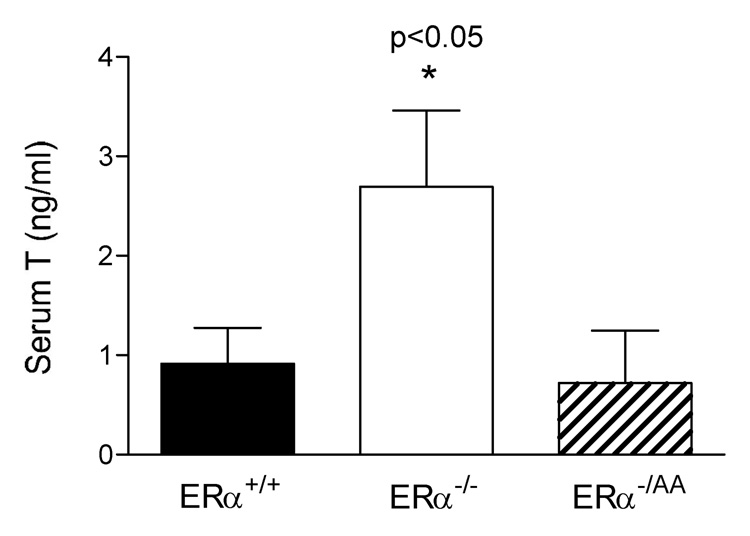
In addition to the abnormal morphological features of the testis, serum testosterone levels are significantly elevated in ERαKO males (Akingbemi et al., 2003; Delbes et al., 2005; Eddy et al., 1996; McDevitt et al., 2007; Wersinger et al., 1999). This elevation of testosterone appears to be independent of LH stimulation (Eddy et al., 1996; McDevitt et al., 2007; Wersinger et al., 1999) and more likely reflects increased androgen biosynthesis of individual Leydig cells (Akingbemi et al., 2003). We have demonstrated that serum T levels are completely restored in the ERα−/AA male (Fig. 6) (McDevitt et al., 2007), and hypothesize that ERE-independent ERα signaling mediates estrogen’s inhibitory actions on steroidogenesis. In support of this hypothesis, preliminary studies from our laboratory suggest that expression and activity of enzymes involved in T synthesis are elevated in ERα−/− testes but normal in ERα−/AA testes (our unpublished observations). Given the growing concern for the detrimental effects of environmental compounds with estrogenic activity, uncovering the mechanisms of E2’s actions will be particularly useful for generating new strategies to treat testicular development disorders and adult male infertility.
Figure 6. Serum T is rescued by the non-classical knock-in mutation.
Serum testosterone is significantly elevated in ERα−/− males (p<0.05) but not in ERα−/AA males (n=9–13). (McDevitt et al., 2007)
Estrogen regulation of sexual behavior
Male sexual behavior
It is well established that both organizational and activational effects of estrogen are critical for male sexual behavior (Meisel and Sachs, 1994; Scordalakes et al., 2002). Accordingly, copulation and other sexually motivated behaviors are severely impaired in ERαKO males (Eddy et al., 1996; McDevitt et al., 2007; Ogawa et al., 1997; Ogawa et al., 1998; Rissman et al., 1997; Wersinger and Rissman, 2000; Wersinger et al., 1997). We have utilized the ERα−/AA mouse model to investigate the relative contributions of classical and non-classical ERα signaling in male sexual behavior. In repeated tests, the majority of wild-type male mice mount and intromit, whereas sexual behavior is virtually absent in male ERα−/AA and ERα−/− mice (McDevitt et al., 2007). The few ERα−/AA and ERα−/− males that do engage in copulation display very few mounts and intromissions, and no ejaculation. As ERα−/AA males perform no better than ERα−/− counterparts, we conclude that one ERE-independent ERα mutant allele is not sufficient, and ERE-dependent ERα signaling is essential, for the development/and or maintenance of normal male sexual behavior.
Our findings are consistent with previous in vivo studies that clearly demonstrate the importance of the genotropic actions of steroids on male sexual behavior (Davidson, 1969; McGinnis and Kahn, 1997), and additionally suggest that the gene targets of E2 action likely contain EREs. Nonetheless, E2 also exerts rapid, non-genotropic effects on male sexual behavior. For example, E2 alters neuronal firing within minutes in male preoptic area slices (Silva and Boulant, 1986) and rapidly stimulates copulation in castrated rats (Cross and Roselli, 1999), castrated quail (Cornil et al., 2006), and aromatase knockout mice (Taziaux et al., 2007). However, E2-induced recovery of sexual behavior may rely on sub-threshold doses of or recent exposure to steroids, suggesting that slow, genotropic actions of T and/or E2 may prime the neural mechanisms that confer sensitivity to E2’s rapid actions. Indeed, evidence suggests that there may be integration of non-genotropic and genotropic pathways (Pedram et al., 2002; Vasudevan et al., 2001) and that E2 regulates male sexual behavior through a combination of mechanisms (Balthazart et al., 2004). It is also possible that the short-term actions of E2 exert minor, facilitating effects, such as increasing sensitivity to sensory cues, which are separate from the major genotropic stimulus that is required to initiate sexual behavior. Whether the rapid actions of E2 are specifically mediated by a membrane-associated ERα remains to be determined. The lack of sexual behavior in ERα−/AA males would suggest that any membrane-associated, non-genomic actions of E2 mediated by the mutant ERα are not sufficient to maintain normal masculine sexual behavior in the absence of ERE-dependent pathways. Unfortunately, as ERE-dependent signaling is absent in the ERα−/AA mouse model throughout development, we are unable to discern whether ERE-mediated ERα signaling is required for E2’s organizational effects, activational effects, or both. The creation of inducible knockout and knock-in mice would be a powerful tool for teasing apart the temporal effects of estrogen action on male sexual behavior.
Female sexual behavior
In rodents, estrogen released during proestrous is necessary for eliciting reproductive behaviors. E2 also up-regulates progesterone receptors, thereby increasing sensitivity to the actions of progesterone. Together these steroids confer complete sexual responsiveness and permit a complex set of behaviors in the female. Because estrogen induces hypothalamic PR expression through ERα binding to EREs (Kraus et al., 1994), it is well established that classical signaling mechanisms contribute to the expression of female sexual activity. However, it is unknown whether non-classical mechanisms additionally play a role in reproductive behavior. ERαKO females do not display lordosis, reject male mice, and display reduced up-regulation of PR by E2 in the hypothalamus (Kudwa and Rissman, 2003; Ogawa et al., 1998; Shughrue et al., 1997). Preliminary studies from our laboratory have indicated that while ERα−/AA females are unreceptive to males and do not show lordosis behavior, they do display significantly less rejective behavior (e.g. kicking, fleeing, and rearing) and more proceptive behavior (e.g. approach of males, pausing for an attentive male) than ERα−/− counterparts (our unpublished observations). Interestingly, PR expression was induced by E2 in the ventral medial hypothalamus (VMH) of wild-type and ERα−/AA females but not in ERα−/− females. These findings are in contrast to the lack of E2-induced PRs in the AVPV (discussed above). As the VMH mediates proceptive behaviors in response to P, these findings together suggest that non-classical ERα signaling is sufficient to sustain gene expression and proceptive components of female sexual behavior in the absence of classical ERα-DNA binding. Furthermore, these results suggest that paracopulatory and copulatory behaviors may be mediated by different molecular mechanisms.
Conclusions
The ERα−/AA model clearly provides an exciting new opportunity for characterizing the classical and non-classical ERα signaling mechanisms in the brain and behavior. As estrogen regulation of physiology and behaviors requires fine-tuned control, it is perhaps not surprising that ERE-independent mechanisms are sufficient to mediate estrogen’s actions in some systems (e.g. negative feedback in the female) but not others (e.g. male sexual behavior). On-going studies in our laboratories are attempting to further distinguish between the contributions of genotropic and non-genotropic signaling in the ERα−/AA mouse. Understanding the distinct molecular mechanisms of ERα action in specific estrogen-target tissues and physiological systems will ultimately provide new possibilities for the development of pharmacological therapies that differentiate between ERE-dependent and –independent processes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology. 2003;144:84–93. doi: 10.1210/en.2002-220292. [DOI] [PubMed] [Google Scholar]
- Arreguin-Arevalo JA, Nett TM. A nongenomic action of estradiol as the mechanism underlying the acute suppression of secretion of luteinizing hormone in ovariectomized ewes. Biol Reprod. 2006;74:202–208. doi: 10.1095/biolreprod.105.044685. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Bodo C, Kudwa AE, Rissman EF. Both estrogen receptor-alpha and -beta are required for sexual differentiation of the anteroventral periventricular area in mice. Endocrinology. 2006;147:415–420. doi: 10.1210/en.2005-0834. [DOI] [PubMed] [Google Scholar]
- Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108:506–516. doi: 10.1210/endo-108-2-506. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141:1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Schneider JS, Kim P, Xu M, Lydon JP, O'Malley BW, Levine JE. Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology. 1999;140:3653–3658. doi: 10.1210/endo.140.8.6895. [DOI] [PubMed] [Google Scholar]
- Condon TP, Dykshoorn-Bosch MA, Kelly MJ. Episodic luteinizing-hormone release in the ovariectomized female guinea pig: rapid inhibition by estrogen. Biol Reprod. 1988;38:121–126. doi: 10.1095/biolreprod38.1.121. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;166:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Couse JF, Bunch DO, Lindzey J, Schomberg DW, Korach KS. Prevention of the polycystic ovarian phenotype and characterization of ovulatory capacity in the estrogen receptor-alpha knockout mouse. Endocrinology. 1999;140:5855–5865. doi: 10.1210/endo.140.12.7222. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- Cross E, Roselli CE. 17beta-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. Am J Physiol. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- Davidson JM. Effects of estrogen on the sexual behavior of male rats. Endocrinology. 1969;84:1365–1372. doi: 10.1210/endo-84-6-1365. [DOI] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R. Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor alpha. Endocrinology. 2005;146:2454–2461. doi: 10.1210/en.2004-1540. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ. Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology. 1997;138:5408–5414. doi: 10.1210/endo.138.12.5558. [DOI] [PubMed] [Google Scholar]
- Gaub MP, Bellard M, Scheuer I, Chambon P, Sassone-Corsi P. Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell. 1990;63:1267–1276. doi: 10.1016/0092-8674(90)90422-b. [DOI] [PubMed] [Google Scholar]
- Glass CK. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev. 1994;15:391–407. doi: 10.1210/edrv-15-3-391. [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci U S A. 2007;104:8173–8177. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol. 2002;16:2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276:13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- Kraus WL, Montano MM, Katzenellenbogen BS. Identification of multiple, widely spaced estrogen-responsive regions in the rat progesterone receptor gene. Mol Endocrinol. 1994;8:952–969. doi: 10.1210/mend.8.8.7997237. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF. Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol. 2003;15:978–983. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96:50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod. 1997;56:293–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- Li D, Mitchell D, Luo J, Yi Z, Cho SG, Guo J, Li X, Ning G, Wu X, Liu M. Estrogen regulates KiSS1 gene expression through estrogen receptor alpha and SP protein complexes. Endocrinology. 2007;148:4821–4828. doi: 10.1210/en.2007-0154. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE. Estrogen response element-independent estrogen receptor (ER)-alpha signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERalpha knockout mice. Endocrinology. 2007;148:5288–5294. doi: 10.1210/en.2007-0673. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Kahn DF. Inhibition of male sexual behavior by intracranial implants of the protein synthesis inhibitor anisomycin into the medial preoptic area of the rat. Horm Behav. 1997;31:15–23. doi: 10.1006/hbeh.1997.1367. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 3–105. [Google Scholar]
- Nett TM, Crowder ME, Wise ME. Role of estradiol in inducing an ovulatory-like surge of luteinizing hormone in sheep. Biol Reprod. 1984;30:1208–1215. doi: 10.1095/biolreprod30.5.1208. [DOI] [PubMed] [Google Scholar]
- O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281:26683–26692. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- O'Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci U S A. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Modifications of testosterone-dependent behaviors by estrogen receptor-alpha gene disruption in male mice. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- Pau KY, Gliessman PM, Hess DL, Ronnekleiv OK, Levine JE, Spies HG. Acute administration of estrogen suppresses LH secretion without altering GnRH release in ovariectomized rhesus macaques. Brain Res. 1990;517:229–235. doi: 10.1016/0006-8993(90)91031-b. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Hughes CC, Levin ER. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J Biol Chem. 2002;277:50768–50775. doi: 10.1074/jbc.M210106200. [DOI] [PubMed] [Google Scholar]
- Popa SM, Clifton DK, Steiner RA. The Role of Kisspeptins and GPR54 in the Neuroendocrine Regulation of Reproduction. Annu Rev Physiol. 2008;70:213–238. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- Ray A, Prefontaine KE, Ray P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem. 1994;269:12940–12946. [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav. 1997;31:232–243. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- Safe S. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm. 2001;62:231–252. doi: 10.1016/s0083-6729(01)62006-5. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Imwalle DB, Rissman EF. Oestrogen's masculine side: mediation of mating in male mice. Reproduction. 2002;124:331–338. doi: 10.1530/rep.0.1240331. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lubahn DB, Negro-Vilar A, Korach KS, Merchenthaler I. Responses in the brain of estrogen receptor alpha-disrupted mice. Proc Natl Acad Sci U S A. 1997;94:1108–1112. doi: 10.1073/pnas.94.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NL, Boulant JA. Effects of testosterone, estradiol, and temperature on neurons in preoptic tissue slices. Am J Physiol. 1986;250:R625–R632. doi: 10.1152/ajpregu.1986.250.4.R625. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci U S A. 1997;94:14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, O'Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- Syed FA, Modder UI, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S. Skeletal effects of estrogen are mediated by opposing actions of classical and nonclassical estrogen receptor pathways. J Bone Miner Res. 2005;20:1992–2001. doi: 10.1359/JBMR.050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27:6563–6572. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc Natl Acad Sci U S A. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- Weigel NL, Zhang Y. Ligand-independent activation of steroid hormone receptors. J Mol Med. 1998;76:469–479. doi: 10.1007/s001090050241. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Haisenleder DJ, Lubahn DB, Rissman EF. Steroid feedback on gonadotropin release and pituitary gonadotropin subunit mRNA in mice lacking a functional estrogen receptor alpha. Endocrine. 1999;11:137–143. doi: 10.1385/ENDO:11:2:137. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Rissman EF. Dopamine activates masculine sexual behavior independent of the estrogen receptor alpha. J Neurosci. 2000;20:4248–4254. doi: 10.1523/JNEUROSCI.20-11-04248.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Van Vugt D, Alston EJ, Luckhaus J, Ferin M. A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology. 1992;131:2812–2820. doi: 10.1210/endo.131.6.1446619. [DOI] [PubMed] [Google Scholar]