Abstract
Previous work has demonstrated that the character of mouse cortical interneuron subtypes can be directly related to their embryonic temporal and spatial origins. The relationship between embryonic origin and the character of mature interneurons is likely reflected by the developmental expression of genes that direct cell fate. However, a thorough understanding of the early genetic events that specify subtype identity has been hampered by the perinatal lethality resulting from the loss of genes implicated in the determination of cortical interneurons. Here we employ a conditional loss-of-function approach to demonstrate that the transcription factor Nkx2-1 is required for the proper specification of specific interneuron subtypes. Removal of this gene at distinct neurogenic timepoints results in a switch in the subtypes of neurons observed at more mature ages. Our strategy reveals a causal link between the embryonic genetic specification by Nkx2-1 in progenitors and the functional attributes of their neuronal progeny in the mature nervous system.
Introduction
Genetic studies have indicated that in response to extrinsic signals (predominantly Shh and FGF8) the subpallium gives rise to a wide diversity of GABAergic neurons –both striatal projection and cortical interneurons (Gutin et al., 2006; Kohtz et al., 1998; Rallu et al., 2002; Shimamura and Rubenstein, 1997; Storm et al., 2006). These populations arise primarily from the three ventrally situated ganglionic eminences: the lateral, medial and caudal ganglionic eminences (the LGE, MGE, and CGE respectively). Transplantation and genetic in vivo fate mapping (Butt et al., 2005; Miyoshi et al., 2007; Nery et al., 2002; Wichterle et al., 2001) have indicated that a wide variety of telencephalic structures receive distinct populations of neurons from each of these eminences. While the range of neurons arising from each eminence is quite diverse, the MGE and CGE together give rise to the majority, if not all, cortical interneurons.
Beginning with the classic studies of Ramon y Cajal, a large body of work from numerous groups has indicated that cortical interneurons comprise a diverse array of subtypes defined on the basis of a number of immunohistochemical, morphological and electrophysiological criteria (Gonchar and Burkhalter, 1997; Kawaguchi and Kubota, 1997; Markram et al., 2004; Somogyi et al., 1998). Recent in vitro and in vivo studies have indicated that the MGE and CGE give rise to distinct non-overlapping populations of cortical interneurons (Butt et al., 2005; Xu et al., 2004). The MGE produces the majority of cortical interneurons and these contribute to all cortical layers. By contrast, CGE-derived cortical interneurons primarily reside in superficial cortical layers. Current analysis from numerous labs demonstrates that the parvalbumin (PV)- and somatostatin (SST)- expressing subtypes are MGE-derived, and the vasointestinal polypeptide (VIP) and calretinin (CR) expressing populations are predominantly generated within the CGE (Butt et al., 2005; Cobos et al., 2006; Nery et al., 2003; Xu et al., 2004). Consistent with these immunohistochemical findings, morphological and physiological analyses suggest that the PV-expressing fast-spiking (FS) basket and chandelier interneurons and SST-expressing intrinsic burst spiking (IB) and non-fast spiking (NFS) Martinotti neurons arise from the MGE (Butt et al., 2005; Miyoshi et al., 2007). By contrast, the VIP/CR-expressing adapting firing interneurons appear to be CGE-derived (Butt et al., 2005).
What then is the molecular mechanism utilized within these two eminences to give rise to distinct populations of cortical interneurons? At present Nkx2-1 is the only known transcription factor that precisely delineates MGE from CGE progenitors. It is expressed by all progenitors within the MGE and is entirely excluded from the CGE (Butt et al., 2005; Sussel et al., 1999). Moreover, Nkx genes and their homologs are known regulators of neuronal cell fate. Both the ventral nervous system defective (vnd) gene in Drosophila and the Nkx2-2, Nkx6-1 and Nkx6-2 genes in the mouse spinal cord act to direct progenitors to adopt specific ventral neural identities, while repressing the fates of neighboring regions (Briscoe et al., 1999; Chu et al., 1998; McDonald et al., 1998; Sander et al., 2000; Weiss et al., 1998). Consistent with this, previous loss of function analysis has indicated that in Nkx2-1 null mutants, the MGE takes on the molecular character of the LGE (Sussel et al., 1999). Unfortunately, as Nkx2-1−/− mice die at birth the impact of this gene on the generation of the interneuron diversity observed in the mature cortex (Markram et al., 2004; Miyoshi et al., 2007) is unclear.
In this manuscript we utilize a conditionally null Nkx2-1 (Nkx2-1c) (Kusakabe et al., 2006) allele to investigate the consequences of removal of Nkx2-1 gene function on the specification of cortical interneuron subtypes. We have previously determined the temporal origins of cortical interneurons arising from the MGE using an inducible genetic fate mapping strategy using the Olig2 CreERTM driver line (Miyoshi et al., 2007). By performing a similar analysis in the context of the conditional Nkx2-1 allele, we were able to study how the removal of Nkx2-1 affects the neuronal subtypes that developmentally arise from the MGE. An attractive aspect of this analysis is that our strategy allowed us to simultaneously remove Nkx2-1 gene function, while genetically marking the MGE precursors with a fluorescent EGFP reporter, so that their fate can be followed. We observed that both early and late removal of Nkx2-1 dramatically alters the fate of MGE-derived precursors in a temporally specific manner. At both time points examined we noted that cortical interneurons are re-specified, such that they adopt the character of CGE-derived neurons (Butt et al., 2005). Specifically we observed that VIP/CR, adapting firing interneurons and late spiking neurogliaform interneurons are generated at the expense of PV-expressing, fast spiking basket neurons and SST-expressing, non-fast spiking Martinotti populations. In addition, removal of Nkx2-1 gene function at E10.5 resulted in a substantial reduction in cortical interneuron numbers and a concomitant increase in the production of striatal medium spiny neurons (MSN). We conclude that Nkx2-1 function is required throughout neurogenesis both for the production of MGE-derived cortical interneuron subtypes and the suppression of surrounding cell fate programs.
Results
Genetic fate mapping of MGE-derived neurons and conditional removal of Nkx2-1 gene function
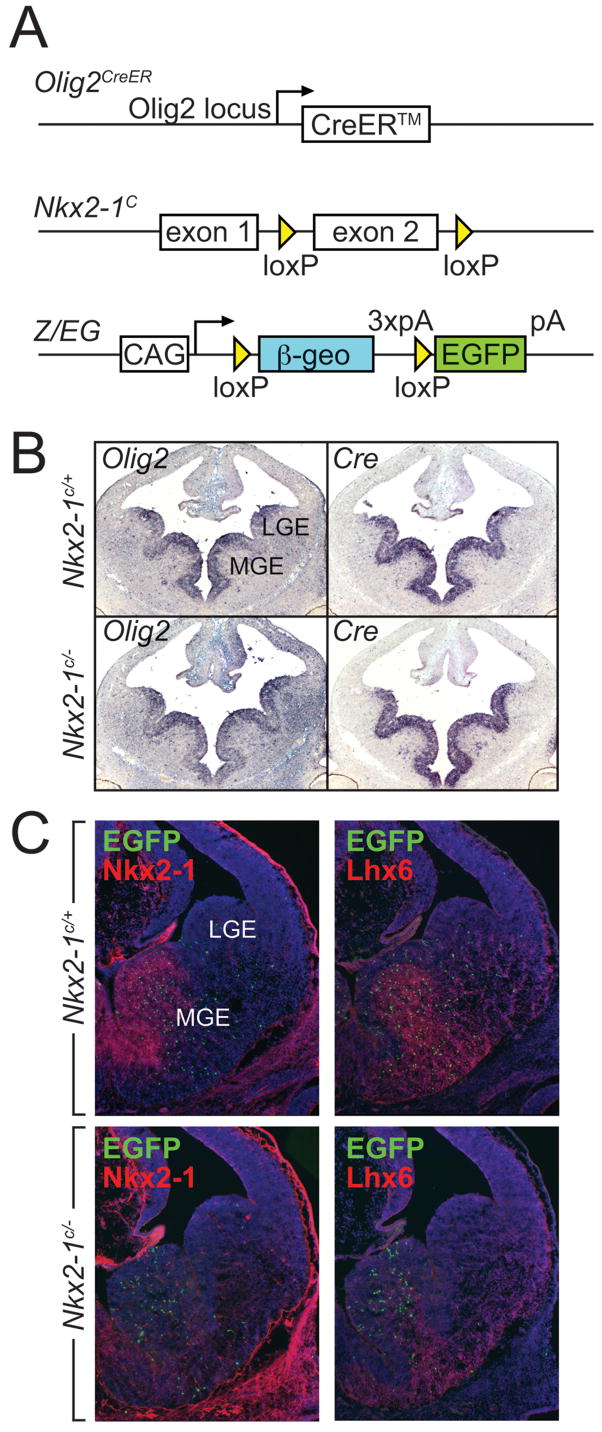
To examine how the fate of MGE-derived progenitors is affected by the loss of Nkx2-1 gene function, we used a genetic fate mapping strategy together with an Nkx2-1 loxP-flanked conditional loss of function allele (Fig. 1a)) (Kusakabe et al., 2006). Our fate mapping approach employed a tamoxifen-inducible form of the Cre recombinase (Feil et al., 1997) under the control of the endogenous Olig2 locus (Olig2 CreER; Takebayashi et al., 2002) (Fig 1A,B). By using this Cre-driver line in the context of a compound Z/EG (a Cre-responsive flurorescent reporter, (Novak et al., 2000);Nkx2-1c/−background, we were able to genetically label MGE-derived precursors (as previously described in Miyoshi et al., 2007), while simultaneously removing Nkx2-1 gene function from this MGE progenitor population (E10.5, Fig. 1C; E9.5 data not shown). Use of this experimental strategy had a number of benefits. First, it allowed us to conditionally remove Nkx2-1 at specific time points during neurogenesis. Second, we were able to overcome the perinatal lethality observed in the null allele, as Cre under the control of the Olig2 locus is not expressed within the developing lung and thyroid primordium. Finally, as Z/EG recombination is restricted to temporally distinct cohorts of MGE-derived precursors, we could readily assess the cell autonomous effects of gene removal on cellular phenotype.
Figure 1. Temporally regulated genetic fate mapping and Nkx2-1 loss-of-function in Olig2CreER precursors.
(A) The genetic strategy for examining Nkx2-1 conditional loss-of-function. while simultaneously fate mapping of MGE-derived precursors This is achieved by combineing the Olig2CreER driver with a floxed Nkx2-1 (Nkx2-1C) allele for conditional loss-of-function and the Z/EG reporter line for genetic fate mapping purposes. Expression from the Z/EG reporter line is directed from the CAG, Hybrid promoter and contains a Polyadenylation signal (pA). (B) The Olig2 locus directs the expression of the inducible form of the Cre recombinase, CreER, in Nkx2-1C/+ control and Nkx2-1C/−conditionally mutant embryos in a pattern identical to the endogenous Olig2 transcript. (C) Administration of tamoxifen (4mg) at E10.5 and concomitant activation of Cre activity results in the complete loss of Nkx2-1 from the MGE at E12.5 in Nkx2-1C/−conditionally mutant embryos and in the permanent labeling with EGFP of MGE-derived cells as they exit the ventricular zone of Nkx2-1C/+ control and Nkx2-1C/− conditionally mutant embryos. Lhx6, whose expression is directly activated by Nkx2-1 and provides a convenient postmitotic marker for MGE-derived interneuron populations, is downregulated as a consequence of the loss of Nkx2-1.
Patterning alterations in conditional Nkx2-1 mutants
The Olig2CreER allele and Z/EG transgene were utilized for genetic fate mapping purposes in all experimental crosses- thus, our experimental group was Olig2CreER;Z/EG;Nkx2-1c/− mice, while our control group was Olig2CreER;Z/EG;Nkx2-1c/+ (which are heterozygous for Nkx2-1 after tamoxifen-induced recombination). Both groups of embryos were treated with tamoxifen at specific time-points between E9.5–E12.5. For clarity we will subsequently refer to these mice with reference to the timepoint of tamoxifen treatment (i.e. Nkx2-1E9.5LOF, Nkx2-1E10.5LOF, Nkx2-1E12.5LOF for the experimental (i.e. loss of function, LOF) groups and Nkx2-1E9.5Ctrl, Nkx2-1E10.5Ctrl or Nkx2-1E12.5Ctrl for the control (Ctrl) groups. To confirm the degree to which our strategy was effective in conditionally removing Nkx2-1 expression in E10.5 MGE progenitor populations, we conducted short-term immunological and in situ hybridization analyses of Nkx2-1 protein and its downstream transcriptional targets (Fig 1C, 2A, 2D). We observed that Cre-mediated recombination occurs more easily at the Nkx2-1 locus than at the Z/EG locus (see Fig. 1C). Thus, we assume that tamoxifen-induced recombination at E10.5 results in the loss of Nkx2-1 gene function in all cells that express EGFP. Additionally, Nkx2-1 target genes, encoding both the signaling molecule Sonic hedgehog (Shh) (Jeong et al., 2006) and the transcription factor Lhx6 (Du et al., 2008), showed reduced punctate levels of expression in the VZ and mantle. In both Nkx2-1E10.5LOF and Nkx2-1E12.5LOF mice, Lhx7, a gene related to Lhx6 and associated with the production of eminence-derived cholinergic neurons (Fragkouli et al., 2005; Marin et al., 2000; Zhao et al., 2003) was also strongly reduced in expression. Despite these changes, there was no discernible alteration in the gross morphology or size of the MGE in these animals compared to control littermates. This is in contrast to the pronounced reduction in the size of the MGE observed in Nkx2-1−/− animals (Sussel et al., 1999). Taken together, these observations suggest that Cre expression from the Olig2 locus effectively recombines the Nkx2-1 conditional allele, allowing us to observe the cell autonomous effects of Nkx2-1 in specifying cell identity.
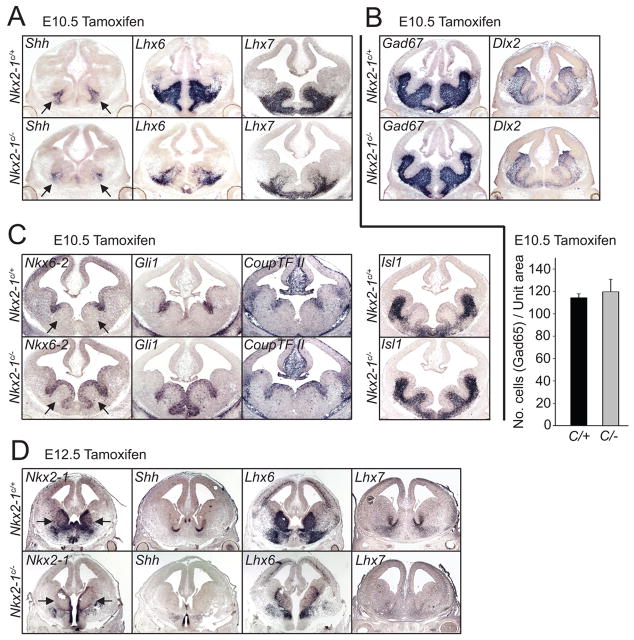
Figure 2. Conditional loss of function of Nkx2-1 at early (E10.5) and late (E12.5) time points is accompanied by changes in expression of genes involved in interneuron development.
The affects on gene expression of inducible loss of Nkx2-1 function at E10.5 (A–C) and E12.5 (D) were examined two days later using in situ hybridization of coronal sections of E12.5 (A–C) and E14.5 (D) embryos. (A) Shh, Lhx6 and Lhx7, whose expression is dependent on normal levels of Nkx2-1 in the MGE, are downregulated in Nkx2-1E10.5LOF mice. These are indicative of a reduction in the specification of the interneuron and cholinergic neuron lineages. (B) By contrast, Gad67 and Dlx2 are expressed at apparently normal levels within the subpallium, revealing that ventral GABAergic neuron development does not appear to be affected by the conditional loss of Nkx2-1 in Nkx2-1E10.5LOF mutant mice. The histogram below this photomicrograph demonstrates that the density of GAD65-positive neurons is not significantly decreased in the mutant population compared to the wild type controls. (C) The expression of Nkx6-2, Gli1 and Coup-TFII, which normally are normally expressed in both the ventricular zone of the sulcus separating the MGE and LGE, as well as portions of the CGE, are expanded throughout most the MGE ventricular zone in Nkx2-1E10.5LOF mice. Similarly, Islet1, which is normally confined to the SVZ and postmitotic regions of the LGE, expands such that it is also expressed through the SVZ of the MGE of in Nkx2-1E10.5LOF mutants. (D) Treatment of conditional Nkx2-1 mice with tamoxifen at E12.5 effectively removes Nkx2-1 expression by E14.5. Expression of Shh, Lhx6 and Lhx7 is also reduced in Nkx2-1E12.5LOF mutant mice suggesting that the loss of this gene at this age affects both the character of GABAergic and cholinergic interneuron lineages. Arrows in A, C and D indicate the position of the MGE. Error bars in the histogram of B represent SEM.
A previous study reported that in Nkx2-1 null mice, markers normally restricted to the LGE expand into the MGE (Sussel et al., 1999). To evaluate how gene expression in the MGE is affected in Nkx2-1E10.5LOF mice, we examined whether alterations occur in the regional expression of a series of transcription factors within the subpallium. In comparison to Nkx2-1E10.5Ctrl littermates, mutant mice exhibited a shift in the expression of markers that are normally expressed in the CGE (Coup-TFII), as well as the ventricular zone of the sulcus region between the MGE and LGE (Nkx6-2, Gli1 and Coup-TFII). Expression of all three markers is strongly expanded in Nkx2-1E10.5LOF mice such that they extended ventromedially throughout the VZ of the MGE (Fig 2C). In particular the observed expansion of Gli1 expression seen in these mutants is surprising, as it occurs in the context of reduced Shh expression. Although the expression of Gli1 is known to be absolutely dependent on hedgehog-signaling (Bai et al., 2004), the relationship between temporal changes in hedgehog-signaling and Gli1 expression is complex (Harfe et al., 2004).
In addition and consistent to has been reported previously (Sussel et al., 1999), precursors leaving the MGE VZ of the Nkx2-1E10.5LOF mice up-regulated Islet1– a LIM homeodomain transcription factor normally associated with the LGE and the generation of striatal projection neurons (Stenman et al., 2003; Toresson et al., 2000; Wang and Liu, 2001). Importantly, the persistence of early pan-ventral markers (Dlx2, Gad67; Fig. 2B) after Nkx2-1 removal suggests that GABAergic neurogenesis was not grossly altered. It therefore appears that our strategy of using Olig2 CreER™ to remove Nkx2-1 in a precise temporal manner may not significantly alter the numbers of GABAergic neurons generated from the ventral eminences per se. It does however result in a shift in the expression of several ventral transcription factors that might in turn have consequences on neuronal subtype specification.
Early removal of Nkx2-1 results in seizures in juvenile mice
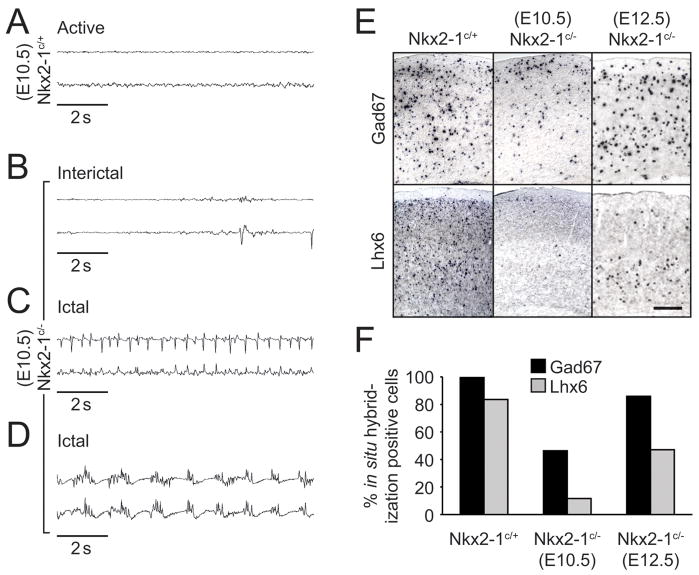
In contrast to Nkx2-1 null animals, conditionally mutant mice were viable postnatally irrespective of the time of tamoxifen administration (Nkx2-1E9.5LOF until P10, Nkx2-1E10.5LOF mice until P13–17 and Nkx2-1E12.5LOF at least until P30), probably due to sparing of the developing lung fields and thyroid (Kimura et al., 1996). Despite their prolonged viability, many of these mutant animals showed profound behavioral abnormalities as juveniles. Nkx2-1E9.5LOF (Supplementary Movie 1) and Nkx2-1E10.5LOF showed pronounced spontaneous seizures. Alterations in behavior were characterized by prolonged periods of quiescence, punctuated by violent tremors and an absence or reduction in voluntary movement. Electrocorticographic recordings were obtained using subcranial electrodes from freely moving control (Nkx2-1E10.5Cont) and mutant (Nkx2-1E10.5LOF) animals. Monitoring of Nkx2-1E10.5LOF juvenile animals revealed that visible seizures correlated with the occurrence of prolonged abnormal bursting activity within the cortex (Fig. 3B–D). Control Nkx2-1E10.5Ctrl littermates never exhibited such activity but rather showed seemingly normal low amplitude desynchronized EEG activity (Fig. 3A). On occasion, activity in Nkx2-19.5LOF or Nkx2-1E10.5LOF animals resembled a myoclonic seizure with a phase of periodic interictal discharges (Fig. 3B) followed by large amplitude rhythmic spike (Fig. 3C) and spike-wave (Fig. 3D) seizure discharge that terminated after a period of 30 s or more. Nkx2-1E10.5LOF showed a reduced incidence of seizures when compared with Nkx2-19.5LOF animals. By contrast, Nkx2-1E12.5LOF animals never exhibited overtly abnormal behavior or synchronized bursting discharge in the EEG (Supplementary Movie 2).
Figure 3. Both the presence of myoclonic seizure activity and a reduction of GABAergic cortical inhibition are observed in Nkx2-1E10.5LOF mutant mice.
(A–D) Electroencephalographic examination of freely moving Nkx2-1E10.5Ctrl control (A) and Nkx2-1E10.5LOF mutant P15 mice reveals abnormal interictal discharges (B) during normal resting behavior. The initial period of a spontaneous seizure episode is characterized by the appearance of continuous single spike activity (C) As shown in this EEG trace recorded shortly after the onset of seizure, increases in frequency and amplitude occur within minutes and progress (D) into continuous spike and spike-wave rhythmic activity. This gradually returns to normal cortical activity at the end of the seizure episode. (E–F) In situ hybridization on brains from electroencephalographic monitored mice shows a correlation exists between the presence of seizure episodic behavior and the reduction in the number of GABAergic neurons in the cortex. This reduction is due mainly to a loss of MGE derived interneurons characterized by the expression of Lhx6. Nkx2-1E12.5LOF mutant mice display only a modest reduction of inhibitory profiles in the cortex when compared to Nkx2-1E10.5LOF mutants (F), insufficient to disrupt the normal cortical activity observed by a lack of seizure episodes in those mice.
Post-hoc marker and genetic fate mapping analysis of Nkx2-1E10.5LOF mice revealed that the occurrence of behavioral abnormalities closely correlated with a reduction in the numbers cortical GABAergic interneurons (Fig. 3F). In situ hybridization analysis of either Nkx2-1E9.5LOF (data not shown) or Nkx2-1E10.5LOF (Fig. 3E) animals exhibited a gross deficit in total Gad67 neurons (Fig. 3E,F), and more specifically MGE-derived cortical interneurons, as indicated by a pronounced reduction in cortical Lhx6 expression (Fig. 3E,F). We are uncertain as to the precise origin of the observed seizures. However, the decrease in interneuron numbers may cause the susceptibility of Nkx2-1E9.5LOF or Nkx2-1E10.5LOF mice to seizures, a phenotype also observed in other mutants that affect the number of GABAergic interneurons in the forebrain (Cobos et al., 2006; Powell et al., 2003). While removal of Nkx2-1 at or prior to E10.5 had a significant effect on the numbers of GABAergic interneurons present in the juvenile neocortex, removal of Nkx2-1 at E12.5 resulted in only a minor decrease in the total numbers of Gad67-positive cortical interneurons (Fig. 3F).
Conditional loss of Nkx2-1 alters cortical interneuron diversity
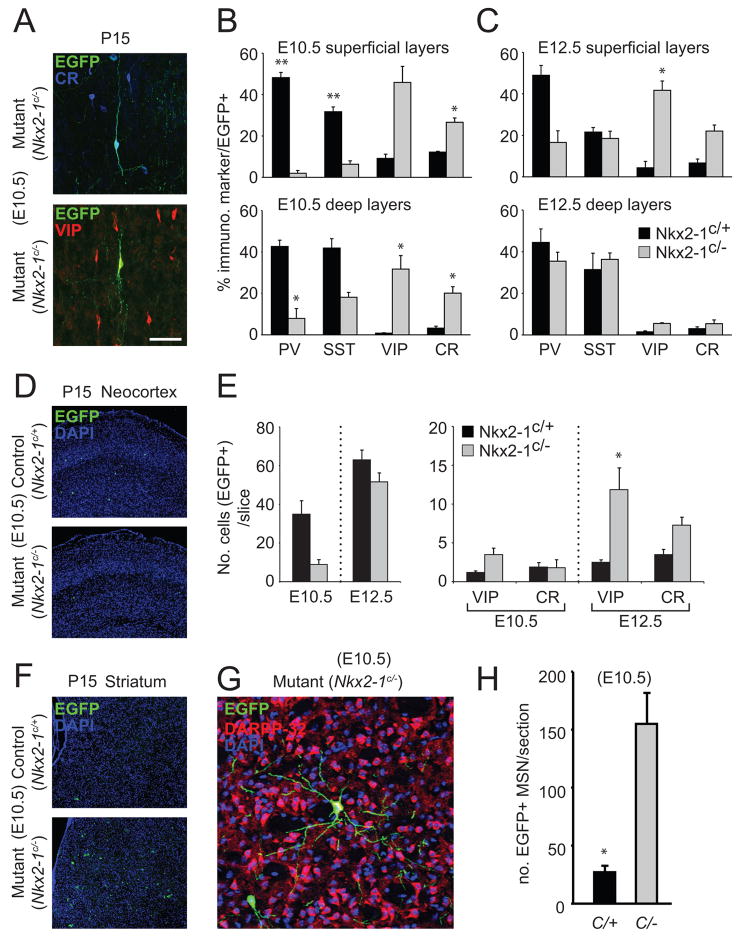
To evaluate the effect of Nkx2-1 removal on cortical interneuron diversity, we performed immunohistochemistry for known markers of interneuron subtypes in both control (Nkx2-1E10.5Ctrl and Nkx2-1E12.5Cont) and conditional null (Nkx2-1E10.5LOF and Nkx2-1E12.5LOF) animals with tamoxifen administration at E10.5 and E12.5. Consistent with their MGE origin (Miyoshi et al., 2007), EGFP-expressing cells in Nkx2-1E10.5Ctrl and Nkx2-1E12.5Ctrl mice predominantly co-labeled with either parvalbumin (PV) or somatostatin (SST) (Fig. 4B,C). By contrast, in Nkx2-1E10.5LOF and Nkx2-1E12.5LOF mice we observed numerous EGFP+ interneurons expressing calretinin (CR) and vasointestinal peptide (VIP), which resembled CGE-derived neurons (Fig. 4A) (Butt et al., 2005; Xu et al., 2004)). Analysis of the overall EGFP populations revealed a profound shift toward CR and VIP-expressing subtypes across all layers in Nkx2-1E10.5LOF animals (Fig. 4B). However, in Nkx2-1E12.5LOF animals the change was restricted to the more superficial (I–III) layers, while deeper (IV-VI) layers were less affected (Fig. 4C). In order to ascertain that this shift was due to an absolute increase in numbers and not a distortion of the small percentage of CR+ and VIP+ interneurons found in control animals, we counted the total number of EGFP+, CR+ and VIP+ interneurons per slice in all experimental conditions (Fig. 4E). First, it was evident that in control mice the overall number of EGFP interneurons in both wildtype and mutant animals is increased at E12.5 compared to E10.5 – at least partially reflecting the fact that E12.5 is near the peak in interneuron neurogenesis (fig 4E). Second, similar to the result obtained for the Gad67 in situ hybridization profiles, removal of Nkx2-1 gene function at E12.5 had less of an effect on the overall numbers of EGFP-positive neocortical interneurons (Fig. 4E). Third, there was a dramatic increase in the absolute numbers of EGFP+, VIP+ and EGFP+, CR+ interneurons in the neocortex in Nkx2-1E12.5LOF animals (Fig 4E). Taken together, this suggested that when Nkx2-1 gene function is removed at E12.5, large numbers of interneuron precursors normally destined to develop into PV and SST-expressing cortical interneurons underwent a fate-switch and instead adopted profiles typical of CGE-derived interneurons. It was striking that in Nkx2-1E10.5LOF but not Nkx2-1E12.5LOF mice, we observed a marked increase in the number of EGFP-positive, DARPP-32-positive (Fig. 4F–H) striatal MSNs. This would explain the loss of GABAergic interneurons that we observed. This suggests that after E10.5 removal of Nkx2-1 a large number of neurons normally destined to adopt a cortical interneuron fate instead are reprogrammed to become striatal medium spiny projection neurons (a population normally derived from the LGE). In addition, we observed the virtual absence of MGE-derived striatal interneuron populations (Marin et al., 2000) in Nkx2-1E10.5LOF mice (data not shown). This is consistent with recent findings suggesting that the postmitotic expression of Nkx2-1 is required for interneurons to invade the striatum, possibly due to a requirement for this gene in suppressing Npl2 expression (Oscar Marin, personal communication). This suggests that the loss of Nkx2-1 results in MGE-derived neurons adopting chararacteristics normally restricted to neurons arising from non-Nkx2-1 expressing ventral regions (LGE and CGE).
Figure 4. The generation of MGE-derived interneuron subtypes is altered in both Nkx2-1E10.5LOF and Nkx2-1E12.5LOF mutant mice.
(A–C) In comparison to littermate controls, (A) the morphological and molecular profiles of cortical interneurons are altered in Nkx2-1E10.5LOF (B) and Nkx2-1E12.5LOF (C) mutant mice. In Nkx2-1E10.5LOF mutants (B) the pronounced loss of EGFP-expressing PV and Sst cells is accompanied by a proportional increase in the number of VIP- and CR-expressing interneurons. Nkx2-1E12.5LOF mutants (C) display the same shift towards VIP and CR profiles in the superficial layers (layers II, III and IV) but not in the deeper layers (layers V and VI) of cortex. Interestingly, in Nkx2-1E12.5LOF mutants the Sst population seems unaffected. (D) In Nkx2-1E10.5LOF mutants the shift in the overall molecular profile of EGFP-labeled interneurons is accompanied by a dramatic decrease in their number. However, even taking into account the reduction in the absolute numbers of genetically fate mapped cortical interneurons, there is a total net increase in the number of EGFP-labeled VIP and CR cells. This suggests that the loss of Nkx2-1 at this timepoint results in a fate switch in many of labeled MGE-derived neurons. This alteration in fate is even more apparent in Nkx2-1E12.5LOF mutants. In these mice there is a 5-fold and a 2-fold absolute increase in the numbers of EGFP-expressing VIP and CR cells, respectively. Moreover, in these same mice there is only a slight overall change in the total number of cortical interneurons. (E–G) In Nkx2-1E10.5LOF mutants where a net loss of fate mapped (EGFP+) cells destined to cortical regions is observed (E) there is a large increase in the number of EGFP-expressing neurons with morphologies, consistent with them being striatal medium spiny projections neurons. (G). These cells express DARPP-32 (F) supporting the notion tNkx2-1 is also required for the determination of the interneuron versus medium spiny projection neuron identity. Error bars in the histograms of B, C, E and H represent SEM. A single asterisk above the histogram indicates P<0.05, while two asterisks indicate P<0.01, as evaluated by a Student T-test.
The observed shift in the immunohistochemical profile of cortical interneurons suggests an alteration in the subtypes generated. However, these markers are present within a variety of interneuron subtypes and as such provide a relatively incomplete description of the diversity encountered in the cerebral cortex. To ascertain if the physiological profile of the cortical interneurons was altered as a result of the loss of Nkx2-1 gene function, we did a comparative whole cell patch clamp electrophysiological and morphological analysis of fate-mapped cortical interneurons in Nkx2-1E10.5LOF and Nkx2-1E12.5LOF compared this to control littermates. Due to the recombination kinetics of Z/EG and by focusing on the EGFP+ interneurons, we effectively restricted our analysis to interneuron populations that exited the VZ shortly after the loss of Nkx2-1 gene function (Miyoshi et al., 2007). Furthermore, while the distribution of immunohistochemical subtypes remains relatively constant from E10.5 to E12.5 in wildtype cortex (Fig. 3), we know that the physiological and morphological characteristics of EGFP+ labeled interneurons differ substantially between these two ages (Miyoshi et al., 2007).
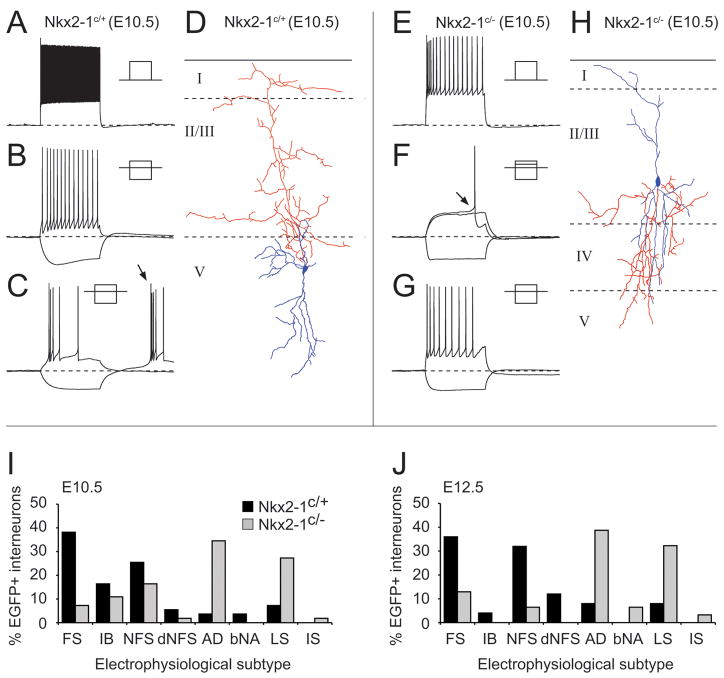
The profiles of the interneurons obtained from P13–P21 in whole cell recordings of Nkx2-1E10.5Ctrl and Nkx2-1E12.5Ctrl mice closely resembled that described in previously published data (Miyoshi et al., 2007)(Fig. 5A–D): (1) fast spiking interneurons (Fig. 5A) with basket cell morphologies were present at both ages consistent with the prolonged embryonic generation of this MGE-derived subclass; (2) temporal bias was most apparent in the non-FS interneuron populations, with the later age having increased numbers of non-fast spiking (NFS) (Fig. 5B) and delayed non-fast spiking (dNFS) interneuron subtypes and a reduced number of intrinsic burst spiking interneurons (IB; Fig. 5C) with Martinotti morphologies (Fig. 5D). Removal of Nkx2-1 resulted in EGFP+ cells with profiles previously attributed to interneurons from the CGE-derived interneuron progenitor pool (Butt et al., 2005). These included initial adapting interneurons (iAD)(Fig. 5E), late spiking (Fig. 5F) neurogliaform, as well as rapidly (rAD) and slowly (sAD; Fig. 5G) adapting, bi- and tripolar interneurons (Fig. 5H). The effect of Nkx2-1 loss-of-function varied in accordance with interneuron subtype. FS interneurons were the most severely affected in both Nkx2-1E10.5LOF and Nkx2-1E12.5LOF animals (Fig. 5I and Fig. 5J), in line with the dramatic reduction of PV-positive interneurons observed in the immunohistochemical analysis (Fig. 3). The SST-expressing populations of interneurons, including IB, NFS and dNFS subtypes, were also reduced in number at both time points. By contrast, the effect on NFS interneurons was most apparent in Nkx2-1E12.5LOF animals, consistent with the observation that this is the peak timepoint for the generation of this subtype (Miyoshi et al., 2007). Increased numbers of adapting (iAD, rAD) and late spiking interneuron subtypes in Nkx2-1c/− animals were observed at both ages examined. These results demonstrate a continued requirement for Nkx2-1 in progenitors for the generation of particular subclasses of cortical interneurons with MGE character.
Figure 5. Conditional loss of function Nkx2-1 at an early (E10.5) and later (E12.5) time point results in a shift in the electrophysiological profile of fate-mapped EGFP+ neocortical interneurons.
(A–C) electrophysiological profiles and morphological reconstructions (D) of EGFP+ interneurons obtained from whole cell patch clamp recording performed on control (Nkx2-1C/+) acute in vitro brain slices. (A) high frequency non-adapting discharge of a fast spiking (FS) interneuron in response to a supra-threshold current injection (+0.6nA, 500ms). (B) non-fast spiking (NFS) interneuron displaying slow adaptation in spike frequency in response to spike threshold current injection (+150pA) superimposed on trace exhibiting prominent voltage sag but no rebound spike in response to hyperpolaring step (−100pA). (C) intrinsic burst spiking interneuron characterized by multiple spikes on rebound (arrow) from hyperpolarizing current step (−30pA) to −80mV and adapting spike frequency discharge at spike threshold (+20pA). (D), morphological reconstruction of the IB interneurons shown in panel (C); axon, red; dendrite, blue. (E–G) Recordings and morphology (H) of EGFP+ interneurons recorded from conditional loss-of-function animals (Nkx2-1C/−). (E) Initial adapting interneurons (iAD) exhibited pronounced adaptation in spike height and frequency when injected with supra-threshold current step (+375pA). (F) Profile of a late spiking interneuron characterized by a steady ramp depolarization in response to near spike threshold current injection (+36pA) and at threshold (+40pA) a significant delay to spike onset (arrow). (G) Slowly adapting (sAD) interneuron which were primarily defined on the basis that they showed adaptation in spike frequency and stopped firing prior to the cessation of the 500 ms suprathreshold current injection (+120pA; superimposed on −60pA step) similar to rAD interneurons (Butt et al., 2005). (H) reconstructed morphology of the cells whose electrophysiological profile is shown in panel (G). Complete profile of interneurons recorded from E10.5 (I) and E12.5 (J) control (blue histogram bars; E10.5 n=55, E12.5 n=25) and conditional loss of function (green; E10.5 n=55, E12.5 n=31) juvenile animals (P13–P21). FS, fast spiking interneuron; IB, intrinsic bursting interneuron; NFS, non-fast spiking interneuron; dNFS, delayed non-fast spiking interneuron; AD, adapting interneurons including iAD, sAD and rAD; bNA, burst nonadapting; LS, late spiking; IS, irregular spiking interneuron.
Discussion
We find that upon Nkx2-1 gene removal, MGE-progenitors alter their presumptive fate in a temporally regulated manner. Removal of Nkx2-1 gene function early during neurogenesis results in the ectopic production of striatal medium spiny projection neurons, and to a lesser extent CR and VIP-expressing interneurons (Butt et al., 2005; Xu et al., 2004). By contrast, later removal of Nkx2-1 results in near normal numbers of cortical interneurons. However many of the MGE-derived interneurons within these mice adopt mature profiles normally consistent with an CGE origin. These findings suggest that Nkx2-1 functions as a temporally regulated molecular switch in the determination of neuronal subclass identity.
Alteration in the fate of MGE-derived neurons after the early removal of Nkx2-1 gene function
The loss of Nkx2-1 gene function in Nkx2-1E10.5LOF mice favors the production of striatal medium spiny neurons from MGE-progenitors. This is consistent with previous observations demonstrating that in Nkx2-1−/− mutants the remnant MGE takes on an LGE-character (Sussel et al., 1999). With regard to cortical interneurons, in both Nkx2-1E9.5LOF and Nkx2-1E10.5LOF mice, we observe over a three-fold reduction in their numbers, strongly suggesting that progenitors normally giving rise to these cells instead become medium spiny neurons. Notably the small number of EGFP-expressing cortical interneurons still generated within the MGE of Nkx2-1E9.5LOF or Nkx2-1E10.5LOF mice are preferentially reprogrammed to assume characteristics consistent with CGE-derived populations. These results are likely explained by the varying temporal output of the re-specified region, with early-born populations generating largely ventral projection neurons and later-born cells yielding cortical interneurons. Our fate-mapping scheme allows us to observe the acute effects of gene removal on a temporally marked population, and as such the increase in MSN is likely the predominant effect of Nkx2-1 removal at this timepoint. Finally, in Nkx2-1E9.5LOF and Nkx2-1E10.5LOF mice we also observe a virtual absence of interneuron populations within the striatum (data not shown). Recent work has suggested that this phenotype is likely the result of the loss of the postmitotic expression of Nkx2-1 in interneuron populations normally destined to populate the striatum (Oscar Marin personal communication; results not shown).
The findings extend previous work in two discrete ways. First as a result of overcoming the perinatal lethality observed in straight Nkx2-1 mutants we are able to study the mature phenotype of cortical interneurons lacking Nkx2-1 gene function. Second, the previous study was unable to determine whether the expansion of LGE in Nkx2-1 null mutants was cell autonomous. Our study allows us to follow the fate of MGE precursors in Nkx2-1E9.5LOF or Nkx2-1E10.5LOF mice. As the initial EGFP-expressing populations in both loss-of-function and control animals is identical, the observation that they adopt different identities provides strong evidence for a cell autonomous mechanism.
E12.5 removal of Nkx2-1 gene function results in class switching in the cortical interneuron subtypes generated
The expansion of genetically fate mapped cortical interneurons with CGE character in Nkx2-1E10.5LOF mice is several times larger than could be explained by the small number of CGE cells labeled at this age using our Olig2CreER;Z/EG fate mapping strategy. However, the substantial decrease in the total numbers of cortical interneurons observed in Nkx2-1E10.5LOF animals, coupled with the large increase in the numbers of striatal medium spiny neurons complicates the interpretation of these findings. In addition, the presence of dyskinesia and epilepsy in these animals raises the possibility that neurological dysfunction is impacting the normal development of the fate mapped populations. Indeed, in humans NKX2-1 haplioinsufficiency is manifested in hypotonia and dyskinesia (Breedveld et al., 2002; Krude et al., 2002; Moeller et al., 2003; Pohlenz et al., 2002;)
Removal of Nkx2-1 at E12.5 circumvents these difficulties. These mice have no overt motor defects or seizure activity. Moreover, they neither display the decrease in cortical interneuron number nor the ectopic generation of MGE-derived medium spiny striatal neurons observed in Nkx2-1E10.5LOF mice. Instead, we observe an increase in the production of VIP/CR interneuron subtypes that are typically derived from the CGE. This ectopic population, like their wild type counterparts, targets the superficial layers of the Nkx2-1E12.5LOF cortex. Importantly, these VIP/CR interneurons appear to be ectopically generated roughly in proportion to the loss of MGE-derived subtypes (fast spiking, PV-expressing basket cells and non-fast spiking, SST-expressing, Martinotti neurons). By contrast, genetically fate mapped cortical interneurons within the deep layers of Nkx2-1E12.5LOF mice, based on their immunohistochemical profile, appear to have no discernable alterations in their subtype identity.
One cause of this dichotomy may be that at this time-point our strategy fails to remove Nkx2-1 from cells destined to populate deep layers of cortex. For instance, perhaps our approach results in the mosaic removal of Nkx2-1 gene function. We think that this is unlikely as the Olig2CreER driver line maintains high levels of Cre expression throughout the MGE at this age. Another possible explanation is that Nkx2-1 expression prior to its removal is sufficient to determine subtype identity in later born MGE progenitor populations fated for deep cortical layers. However, if this were the case why then are these subtypes lost in the superficial layers of these same animals? One interpretation is that at mid-neurogenesis (approximately E12.5), Olig2 fate-mapped progenitors encompass two pools – one that becomes immediately postmitotic and resides in deeper layers and another that undergoes continuing limited proliferation in the SVZ and will generate more superficial interneuron populations. It would not be surprising in this case if the later populations would be disproportionately affected by the removal of both the Nkx2-1 gene and protein products.
Alternatively there may exist PV/SST populations that are generated independent of a requirement for Nkx2-1. However, recent work has demonstrated that the generation of both SST and PV cortical interneuron populations require Lhx6 gene function (Liodis et al., 2007) and Lhx6 expression in turn appears to be largely dependent on Nkx2-1 (Sussel et al., 1999; Du et al., 2008). Moreover, virtually all SST and PV cortical interneurons are derived from Lhx6-expressing lineages (Fogarty et al., 2007). Consistent with this, we observe relatively normal levels of Lhx6 expression in the deep cortical layers of Nkx2-1E12.5LOF mice. Hence for this hypothesis to be viable there must exist a population of progenitors at E12.5 that expresses Lhx6 independent of a requirement for Nkx2-1. One possiblility is that Nkx6-2, like Nkx2-1, can drive Lhx6 expression. Indeed, it has been demonstrated that some SST-expressing interneurons arise from the Nkx6-2-expressing progenitor domain within the dorsal MGE (Fogarty et al., 2007; Wonders et al., 2008). Furthermore given that the Nkx6-2 domain can generate, albeit in comparatively low numbers, a range of interneuron subtypes with immunohistochemical profiles previously associated with the CGE (Fogarty et al., 2007) the fate-switch observed at E10.5 may be a transformation to dorsal MGE rather than CGE identity (Butt et al., 2005; Xu et al., 2004). The precise shift in the regional identity of MGE-progenitors aside, these results suggest Nkx2-1 acts to temporally repress different genetic programs within the MGE at different developmental time-points.
Nkx2-1 acts as a molecular switch that favors MGE fates over LGE and CGE character
The observation that loss of Nkx2-1 gene function results in the apparent trans-fating of presumptive fast spiking, PV+, basket cell or non-FS, SST+ cortical interneurons into either striatal medium spiny projection neurons or CGE-derived CR+/VIP+ interneuronal subtypes argues that this gene acts as a primary determinant of MGE subtype identity. While in some ways the Lhx6 gene has been argued to function in this capacity (Du et al., 2008; Liodis et al., 2007), Nkx2-1 appears to act higher in the hierarchy governing ventral cell fate. While SST and PV neurons are large absent in Lhx6 null mice, there is no obvious increase in CR or VIP neurons. Interestingly, the abnormality in radial migration seen in Lhx6 nulls was not evident in our mutants, perhaps because a complete fate transformation occurs. Hence by adopting a CGE character, they do not require Lhx6 for this aspect of their development.
An important issue to discern is whether the apparent trans-fating of MGE-derived progenitors is a complete or partial respecification. Determining the extent to which the loss of Nkx2-1 results in the production of bona fide medium spiny neurons or interneurons of CGE character is dependent on the stringency of our phenotypic analysis. The analysis of the cortical interneurons included evaluation of the morphology, marker expression and intrinsic physiological character. Within the limits of this analysis, we saw no indication that either of the mutant populations was distinguishable from the corresponding wild type LGE- or CGE-derived populations.
One interpretation of these results is that Nkx2-1 acts upon pre-existing populations of LGE/CGE-derived precursors and bestows them with additional properties that allow them to assume an MGE-derived identity. In this light, one might view the LGE/CGE phenotypes as a developmental “ground-state”, an interpretation that we do not favor. Rather we believe that evolution has selected distinct regulatory networks of gene expression for the cell types derived from each of the three ganglionic eminences. We believe that Nkx2-1 both activates MGE-specific differentiation programs and represses the expression of genes that would lead to either CGE or LGE-specific neurons. Therefore, we believe Nkx2-1 functions as a genetic switch to simultaneously induce MGE-specific patterns of gene expression, while concurrently repressing CGE/LGE-specific genetic programs. This model is reminiscent of findings in the spinal cord where type 1 genes (i.e. repressed by hedgehog-signaling) and type 2 genes (i.e. induced by hedgehog signaling) act competitively in the determination of ventral spinal cord cell types (Jessell, 2000).
While at present only a handful of the genes involved in either MGE versus CGE/LGE programs are known, our data indicates that the loss of Nkx2-1 gene function results in both the expansion and loss of particular genes. It is very likely that several other genes, whose eminence-specific expression patterns are presently unknown are also altered and play a role in the respecification that we observe. In our mutant analysis, we noted both an expansion of CoupTFII and Nkx6-2, and the loss of Lhx6 and Lhx7 expression, regardless of the time at which the function of this gene was removed. Our model therefore is consistent with the idea that Nkx2-1 can simultaneously act as an inducer and a repressor. Examination of the structure of Nkx2-1 gene suggests that it is well suited to such a role (Chen and Schwartz, 1995; De Felice et al., 1995; Harvey, 1996; Lints et al., 1993). At the N-terminal end of Nkx2-1 is a TN-domain that has been shown to be able to bind to Groucho, a known transcriptional co-repressor (Muhr et al., 2001). At the C-terminal end of this protein, it has been shown to exist an activator-domain (Chen and Schwartz, 1995; De Felice et al., 1995) regulated by the conserved SD-motif that functions to recruit transcriptional activators (Watada et al., 2000). We therefore envision that within MGE-precursors Nkx2-1 exists in two distinct forms, one in which it binds transcriptional repressors that act to suppress the expression of CGE/LGE genes, as well as a form that recruits activators capable of inducing the transcriptional activation of MGE genes. We are currently undertaking a structure function analysis of Nkx2-1 to explore whether this model is accurate.
Our experiments have explored the temporal requirement for Nkx2-1 gene function. We find that loss of this gene during neurogenesis results in a temporally regulated switch in the class of neurons generated at different developmental timepoints. As such, Nkx2-1 appears to play a critical role in the progressive specification of subpallial progenitors. Work in the retina (Cepko et al., 1996) and the cerebral cortex (Desai and McConnell, 2000) has provided evidence for progenitors attaining specific neuronal identities through progressive restrictions in their identity. The present work suggests that Nkx2-1 functions in the genetic hierarchy controlling the fate of cortical interneurons by directing MGE-derived progenitors to becoming predominantly either PV-expressing basket cell or SST-expressing Martinotti interneurons. Determination of genes both activated and suppressed by Nkx2-1 should be informative in providing insights into the molecular mechanism by which interneurons of specific subclasses are generated.
Materials and Methods
Animal methods and genotyping
All mice used in these studies were maintained according to protocols approved by the Institutional Animal Care and Use Committee of the New York University School of Medicine. Triple heterozygote male mice (Nkx2-1+/− (Kimura et al., 1996); Olig2CreER/+ (Takebayashi et al., 2002); Z/EG+/− (Novak et al., 2000)) were intercrossed with Nkx2-1 conditional homozygote females (Nkx2-1C/C) (Kusakabe et al., 2006) to generate experimental control (Nkx2-1C/+; Olig2CreER/+; Z/EG+/−) and mutant (Nkx2-1C/−; Olig2CreER/+; Z/EG+/−) mice. Pregnant females were administered four milligrams tamoxifen (Sigma, St. Louis, MO) (20mg/ml dissolved in corn oil (Sigma)) at 12-2pm at either E9.5, E10.5 or E12.5 by gavaging with silicon-protected needles. When pregnant mothers did not deliver pups by noon of E19.5, a cesarean section was performed and pups were fostered. PCR genotyping of the Nkx2-1−, Nkx2-1C and Olig2CreER and alleles was performed using the following sets of primers: Nkx2-1− -forward, TCGCCTTCTATCGCCTTCTTGACGAG; Nkx2-1− -reverse, TCTTGTAGCGGTGGTTCTGGA; Nkx2-1C -forward, TGCCGTGTAAACACGAGGAC; Nkx2-1− -reverse, GACTCTCAAGCAAGTCCATCC; Olig2CreER-forward, TCGAGAGCTTAGATCATCC; Olig2CreER-reverse, CACCGCCGCCCAGTTTGTCC; Olig2CreER-mutant reverse, GGACAGAAGCATTTTCCAGG. The Z/EG allele was genotyped by using primers specific to the enhanced green fluorescent protein (EGFP) or by using β–gal staining solution to an animal’s body part.
In Situ Hybridization
E12.5 and E14.5 embryos were dissected in cold phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde (PFA)/PBS solution overnight at 4°C. Postnatal P15–P17 brains were fixed by transcardiac perfusion followed by 4h to overnight postfixation with 4% PFA/PBS solution at 4°C. Embryos and brain tissue was rinsed with PBS and cryoprotected using 30%sucrose/PBS solution overnight at 4°C. Tissues were embedded in Tissue Tek, frozen on dry ice and sectioned at 12μm. Section in situ hybridizations were performed as previously described (Hanashima et al., 2002) using non-radioactive DIG-labeled probes. The cDNA probes used included Nkx2-1, Nkx6-2, Dlx2, Gad67, Lhx6, Lhx7, Shh, Gli1, CoupTF-II, Islet1, Cre and Olig2.
Immunohistochemistry
E12.5 embryos were dissected in cold phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde (PFA)/PBS solution 40 minutes at 4°C. Postnatal P15–P17 brains were fixed by transcardiac perfusion followed by 1h postfixation with 4% PFA/PBS solution at 4°C. Embryos and brain tissue were rinsed with PBS and cryoprotected using 30% sucrose/PBS solution overnight at 4°C. Tissues were embedded in Tissue Tek, frozen on dry ice and sectioned at 12μm (for molecular expression profile analysis) and 50μm (for cell imaging). Sections for immunohistochemistry analysis were processed using 3% normal goat serum/0.1% Triton X-100 in all procedures except washing steps where only PBS was used. Sections were blocked for 30 min followed by incubation with the primary antibodies overnight at 4°C. After one wash with PBS, secondary antibodies (Molecular probes) were applied for 60 min at room temperature. Nuclear counterstaining was performed with 100ng/ml 4′,6-diamidino-2-phenylindole (DAPI) solution in PBS for 15 minutes. The following antibodies were used in distinct combinations: rabbit anti-GFP (1:1000; Molecular Probes), rat anti-GFP (1:1000; Nacalai Tesque, Kyoto, Japan), mouse anti-Parvalbumin (1:1000; Sigma), rat anti-Somatostatin (1:500; Chemicon, Temecula, CA), rabbit anti-Neuropeptide Y (1:500; Incstar, Stillwater, MN) mouse anti-Calretinin (1:1000; Chemicon), rabbit anti-VIP (1:300; Incstar), mouse anti-Nkx2-1 (TTF-1) (1:200; Progen, Heidelberg, Germany), rabbit anti-Lhx6 (1:1000). Fluorescent images were captured using a cooled CCD camera (Princeton Scientific Instruments, Trenton, NJ) or a Zeiss LSM 510 META laser scanning microscope (Carl Zeiss MicroImaging, Inc.).
Electrocorticographic recordings
Postnatal day P13 mice were anesthetized with a ketamine and xylazine (KX) mixture and stainless steel electrodes (Pinnacle Part #8209 & 8212; Pinnacle Technology Inc., Lawrence, KS) positioned in a miniature headmount (Pinnacle Part #8201) placed on the skull were implanted bilaterally into the neocortex. After two days of recovery, digital EEG recordings of awake, freely moving mice were obtained during a 5hr period taken at P15 and P16 using a Model 4100 USB DACS (Pinnacle Technology Inc., Lawrence, KS).
Acute in vitro cortical slice electrophysiology
Whole Cell patch clamp electrophysiology recordings were performed on EGFP+ cells in acute in vitro slices from control (Nkx2-1c/+) and conditional loss of function Nkx2-1 (Nkx2-1c/−) animals (age: postnatal day (P)13–P21) similar to that previously published (Butt et al., 2005; Miyoshi et al., 2007;). The majority of interneurons recorded were located in the primary somatosensory neocortex or more medial cortices.
Supplementary Material
Acknowledgments
We wish to thank Dr. Hirohide Takebayashi for providing the Olig2 CreER™ mouse. cDNA in situ probe templates were kindly provided by J. Ericson (Nkx6-2), A. Joyner (Cre, Gli1, Nkx2-1, Shh), V. Pachnis (Lhx6), J. Rubenstein (Dlx2), M. Studer (CoupTF-II).
The polyclonal rabbit anti-Lhx6 antibody was a kind gift from V. Pachnis. We would also like to thank for excellent technical help from Lihong Yin and Jiali Deng. SJBB is a recipient of a Human Frontier Science Program Organization Long-Term Fellowship; JHL is a recipient of a postdoctoral fellowship from the Swedish Brain Foundation (Hjärnfonden); GM is supported by a grant from the Japan Society for the Promotion of Science. Research in the Fishell laboratory is supported by National Institutes of Health – National Institute of Mental Health –National Institute of Neurological Disorders and Stroke Grants R01MH068469 and R01NS039007. SK is supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;1:103–115. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- Breedveld GJ, van Dongen JW, Danesino C, Guala A, Percy AK, Dure LS, Harper P, Lazarou LP, van der Linde H, Joosse M, Grüters A, MacDonald ME, de Vries BB, Arts WF, Oostra BA, Krude H, Heutink P. Mutations in TITF-1 are associated with benign hereditary chorea. Hum Mol Genet. 2002;11:971–979. doi: 10.1093/hmg/11.8.971. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2-2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Schwartz RJ. Identification of novel DNA binding targets and regulatory domains of a murine tinman homeodomain factor, nkx-2.5. J Biol Chem. 1995;270:15628–15633. doi: 10.1074/jbc.270.26.15628. [DOI] [PubMed] [Google Scholar]
- Chu H, Parras C, White K, Jimenez F. Formation and specification of ventral neuroblasts is controlled by vnd in Drosophila neurogenesis. Genes Dev. 1998;12:3613–3624. doi: 10.1101/gad.12.22.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Long JE, Thwin MT, Rubenstein JL. Cellular patterns of transcription factor expression in developing cortical interneurons. Cereb Cortex. 2006;16(Suppl 1):i82–88. doi: 10.1093/cercor/bhk003. [DOI] [PubMed] [Google Scholar]
- De Felice M, Damante G, Zannini M, Francis-Lang H, Di Lauro R. Redundant domains contribute to the transcriptional activity of the thyroid transcription factor 1. J Biol Chem. 1995;270:26649–26656. doi: 10.1074/jbc.270.44.26649. [DOI] [PubMed] [Google Scholar]
- Desai AR, McConnell SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- Du T, Xu Q, Ocbina PJ, Anderson SA. NKX2-1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkouli A, Hearn C, Errington M, Cooke S, Grigoriou M, Bliss T, Stylianopoulou F, Pachnis V. Loss of forebrain cholinergic neurons and impairment in spatial learning and memory in LHX7-deficient mice. Eur J Neurosci. 2005;21:2923–2938. doi: 10.1111/j.1460-9568.2005.04141.x. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- Gutin G, Fernandes M, Palazzolo L, Paek H, Yu K, Ornitz DM, McConnell SK, Hebert JM. FGF signalling generates ventral telencephalic cells independently of SHH. Development. 2006;133:2937–2946. doi: 10.1242/dev.02465. [DOI] [PubMed] [Google Scholar]
- Hanashima C, Shen L, Li SC, Lai E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J Neurosci. 2002;22:6526–6536. doi: 10.1523/JNEUROSCI.22-15-06526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Harvey RP. NK-2 homeobox genes and heart development. Dev Biol. 1996;178:203–216. doi: 10.1006/dbio.1996.0212. [DOI] [PubMed] [Google Scholar]
- Jeong Y, El-Jaick K, Roessler E, Muenke M, Epstein DJ. A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development. 2006;133:761–772. doi: 10.1242/dev.02239. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kohtz JD, Baker DP, Corte G, Fishell G. Regionalization within the mammalian telencephalon is mediated by changes in responsiveness to Sonic Hedgehog. Development. 1998;125:5079–5089. doi: 10.1242/dev.125.24.5079. [DOI] [PubMed] [Google Scholar]
- Krude H, Schütz B, Biebermann H, von Moers A, Schnabel D, Neitzel H, Tönnies H, Weise D, Lafferty A, Schwarz S, DeFelice M, von Deimling A, van Landeghem F, DiLauro R, Grüters A. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J Clin Invest. 2002;109:475–480. doi: 10.1172/JCI14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe T, Kawaguchi A, Hoshi N, Kawaguchi R, Hoshi S, Kimura S. Thyroid-specific enhancer-binding protein/NKX2-1 is required for the maintenance of ordered architecture and function of the differentiated thyroid. Mol Endocrinol. 2006;20:1796–1809. doi: 10.1210/me.2005-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:419–431. doi: 10.1242/dev.119.2.419. [DOI] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Anderson SA, Rubenstein JL. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Holbrook S, Isshiki T, Weiss J, Doe CQ, Mellerick DM. Dorsoventral patterning in the Drosophila central nervous system: the vnd homeobox gene specifies ventral column identity. Genes Dev. 1998;12:3603–3612. doi: 10.1101/gad.12.22.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller LC, Kimura S, Kusakabe T, Liao XH, Van Sande J, Refetoff S. Hypothyroidism in thyroid transcription factor 1 haploinsufficiency is caused by reduced expression of the thyroid-stimulating hormone receptor. Mol Endocrinol. 2003;17:2295–2302. doi: 10.1210/me.2003-0175. [DOI] [PubMed] [Google Scholar]
- Muhr J, Andersson E, Persson M, Jessell TM, Ericson J. Groucho-mediated transcriptional repression establishes progenitor cell pattern and neuronal fate in the ventral neural tube. Cell. 2001;104:861–873. doi: 10.1016/s0092-8674(01)00283-5. [DOI] [PubMed] [Google Scholar]
- Nery S, Corbin JG, Fishell G. Dlx2 progenitor migration in wild type and Nkx2-1 mutant telencephalon. Cereb Cortex. 2003;13:895–903. doi: 10.1093/cercor/13.9.895. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Pohlenz J, Dumitrescu A, Zundel D, Martiné U, Schönberger W, Koo E, Weiss RE, Cohen RN, Kimura S, Refetoff S. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109:469–473. doi: 10.1172/JCI14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallu M, Corbin JG, Fishell G. Parsing the prosencephalon. Nat Rev Neurosci. 2002;3:943–951. doi: 10.1038/nrn989. [DOI] [PubMed] [Google Scholar]
- Sander M, Paydar S, Ericson J, Briscoe J, Berber E, German M, Jessell TM, Rubenstein JL. Ventral neural patterning by Nkx homeobox genes: Nkx6-1 controls somatic motor neuron and ventral interneuron fates. Genes Dev. 2000;14:2134–2139. doi: 10.1101/gad.820400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2-1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K, Nabeshima Y. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- Wang HF, Liu FC. Developmental restriction of the LIM homeodomain transcription factor Islet-1 expression to cholinergic neurons in the rat striatum. Neuroscience. 2001;103:999–1016. doi: 10.1016/s0306-4522(00)00590-x. [DOI] [PubMed] [Google Scholar]
- Watada H, Mirmira RG, Kalamaras J, German MS. Intramolecular control of transcriptional activity by the NK2-specific domain in NK-2 homeodomain proteins. Proc Natl Acad Sci U S A. 2000;97:9443–9448. doi: 10.1073/pnas.97.17.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JB, Von Ohlen T, Mellerick DM, Dressler G, Doe CQ, Scott MP. Dorsoventral patterning in the Drosophila central nervous system: the intermediate neuroblasts defective homeobox gene specifies intermediate column identity. Genes Dev. 1998;12:3591–3602. doi: 10.1101/gad.12.22.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Marin O, Hermesz E, Powell A, Flames N, Palkovits M, Rubenstein JL, Westphal H. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci U S A. 2003;100:9005–9010. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.