Abstract
Maternal aggression is a robust type of aggression displayed by lactating female rats. Although arginine vasopressin (AVP) has been implicated in the control of male aggression, its involvement in maternal aggression has not been thoroughly investigated. Previous neuroanatomical studies suggest that AVP may mediate the display of aggression during lactation. In the current study, AVP and an AVP V1a receptor antagonist were centrally administered to primiparous rats on days 5 and 15 of lactation, and aggression, maternal behavior, and grooming were recorded. Although AVP did not affect the number of attacks or duration of aggression, it increased the latency to initiate aggression on day 5, in addition to decreasing maternal behavior and increasing grooming. Conversely, V1a antagonist treatment increased maternal aggression on both days of lactation, decreased maternal behavior on day 15, and decreased grooming on day 5. Thus, it appears that central AVP activity modulates maternal aggression, as well as maternal behavior and grooming behavior during lactation.
Keywords: AVP, V1a, maternal aggression, maternal behavior, grooming, lactation, anxiety, resident, intruder, sex steroids, agonistic behavior, thermoregulation
INTRODUCTION
Maternal aggression is a distinct form of aggression found in lactating females, and is most robust in rats during the first two weeks of lactation (Erskine et al., 1978). It is initiated shortly after parturition, and peaks on days 5–9 of lactation before declining (Erskine et al., 1978; Mayer et al., 1987). Whereas the endocrine dependent states of parturition and lactation are essential for the establishment of rat maternal aggression (Erskine et al., 1980b), hypophysectomy on day 5 postpartum fails to alter maternal aggression, indicating that the expression of maternal aggression may not be regulated by pituitary hormones during lactation (Erskine et al., 1980a). Other studies suggest that the maintenance of maternal aggression is dependent on olfactory cues from offspring (Ferreira et al., 1987), and aggression is not affected by the age of the mothers (Takahashi and Lore, 1982). Important neural areas implicated in the control of maternal aggression in rats include the lateral and ventrolateral caudal periaqueductal gray and the paraventricular nucleus (PVN) (Giovenardi et al., 1997; Giovenardi et al., 1998; Lonstein and Stern, 1998), since postpartum lesions of these areas facilitate maternal aggression. In contrast, lesions of the septum and ventral medial hypothalamus (Flannelly et al., 1986; Hansen, 1989) decrease maternal aggression. The PVN, as well as the central amygdala (CeA) also have been implicated in rat maternal aggression in dams selected for high and low anxiety behavior (Bosch et al., 2005). Modulation of central 5-HT activity in the median raphe, dorsal periaqueductal gray, corticomedial amygdala, and medial septum affects the display of maternal aggression as well (De Almeida and Lucion, 1994; De Almeida and Lucion, 1997). Neurohormones which may be involved in the modulation of maternal aggression include oxytocin, corticosterone releasing factor (CRF), and AVP.
Several studies suggest that oxytocin may be involved in the control of maternal aggression. Lesions to the PVN decrease oxytocin and increase maternal aggression, and oxytocin antisense infused into the PVN increases maternal aggression as well (Giovenardi et al., 1997; Giovenardi et al., 1998). These studies suggest that oxytocin neurons in the PVN may exert an inhibitory effect on aggression prior to parturition (Giovenardi et al., 1997). Other neurohormones which are found in the PVN and may be involved in the role of the PVN in maternal aggression are sex steroids and AVP. In lactating Wistar rats bred for high or low anxiety behavior (HAB or LAB), infusion of oxytocin antagonist into the PVN or CeA decreases maternal aggression in HAB dams, and exogenous OXT infused into the PV increases maternal aggression in LAB dams (Bosch et al., 2005). These results are supported by retrodialysis data indicating oxytocin release in the PVN of lactating Wistar rats during a maternal defense test (Bosch et al., 2004). Although previous study suggests that oxytocin antagonist (d(CH2)5, [Tyr(Me)2-Thr4-Tyr-NH29]–vasotocin) infusion into the CeA increases maternal aggression (Lubin et al., 2003), it is possible that the high dose of antagonist (1000ng) used in this study was also acting on V1a receptors (Chan et al., 1996). Furthermore, acute cocaine treatment of lactating rat dams alters oxytocin levels in the MPOA and amygdala, and it is postulated that these changes in oxytocin may be involved in the effects of cocaine on maternal aggression (Elliot et al., 2001). In female C57BL/6 mice, maternal separation decreases oxytocin immunoreactivity in the parvocellular PVN and decreases the latency to attack a novel male intruder (Veenema et al., 2007).
CRF has also been implicated in the control of maternal aggression, as exogenous CRF impairs maternal aggression in mice (Gammie et al., 2004). This finding was supported by the investigation of CRF receptor 2 KO mice, which display decreased maternal aggression, possibly due to the documented overproduction of CRF in these dams (Gammie et al., 2005). The effects of both CRF and AVP on rodent maternal aggression may not be surprising considering their colocalization in the PVN of lactating rats (Walker et al., 2001). However, further work is needed to determine if CRF modulates aggression in other rodent species.
Previous behavioral studies indicate that AVP mediates other forms of aggression in several rodent species (Compaan et al., 1993; Delville et al., 1996a; Delville et al., 1996b; Elkabir et al., 1990; Ferris et al., 1997; Ferris and Potegal, 1988; Stribley and Carter, 1999; Winslow et al., 1993). One species where the modulation of aggression by AVP has been thoroughly studied is the golden hamster. In male golden hamsters in vitro receptor autoradiography reveals binding sites for V1a and 5-HT1B receptors, and immunocytochemistry indicates there are 5-HT synapses on AVP neurons in the anterior hypothalamus (AH). Manipulations of this anatomical substrate demonstrate that AVP injection into the AH increases aggression and 5-HT decreases aggression, as peripheral fluoxetine inhibits resident-intruder aggression (Delville et al., 1996a; Ferris et al., 1997). This attenuation of inter-male aggression by 5-HT is thought to result from the modulation of the AVP system at the hypothalamus, as treatment with a V1a receptor antagonist also suppresses aggressive displays (Ferris and Potegal, 1988; Potegal and Ferris, 1990).
In male rats, infusion of corticosterone releasing factor (CRF) and AVP into the lateral ventricles or amygdala has synergistic effects on aggression during social encounters (Elkabir et al., 1990). Additionally, AVP mRNA expression and AVP immunoreactivity are increased in the PVN and SON following a resident-intruder test, and 5-HT immunoreactivity in the AH and SON are negatively correlated with aggression (Veenema et al., 2006). Similar to its effects in male hamsters, 5-HT agonism decreases maternal aggression in rats when administered icv, or in specific nuclei (median raphe, dorsal periaqueductal gray, and corticomedial amygdala), but may also increase aggression at specific doses in the medial septal nuclei (De Almeida and Lucion, 1994; De Almeida and Lucion, 1997). Taken together, these data suggest that AVP may also mediate maternal aggression. While there are significant differences in the specific actions of AVP across vertebrate species, Insel and Young (2000) proposed that these differences are associated with variations in the distribution of V1 receptors in the brain. Lonstein and Gammie (2002) further suggest that since aggression in male and female prairie voles is similar and AVP modulates aggression in male prairie voles, it may have similar effects in females. More recent neuroanatomical studies of elevated maternal aggression observed in multiparous rats when compared to primiparous animals (Byrnes et al., 2008; Nephew et al., 2007) have revealed significant increases in V1a mRNA expression in the amygdala, supra optic nucleus, and lateral septum of the more aggressive multiparous females on day 5 of lactation. However, AVP levels in these animals have not been determined, and further investigation of the effects of parity on neural AVP is needed. Although other forms of aggression have been thoroughly investigated in the rat, and AVP mediates the display of aggression in several rodent species, there are few studies on the role of AVP in maternal aggression.
Recently, the AVP V1b receptor has been implicated in the social behavior roles of AVP. In male AVP V1b KO mice, both social memory (Wersinger et al., 2004) and aggression (Wersinger et al., 2002) are impaired. In further study of these KO mice, both inter-male and maternal aggression are decreased, but defensive behaviors are unaffected by the loss of AVP V1b. Surprisingly, when territorial aggression is measured following defensive behavioral testing, V1b KO mice increase aggression with repeated testing. It is suggested that the V1b receptor is involved in the appropriate pairing of social cues and behavioral responses (Wersinger et al., 2007a). In addition, resident-intruder aggression is decreased in male Syrian hamsters when orally treated with a selective V1b antagonist (Blanchard et al., 2005). V1b expression has been documented in the CA2 hippocampal neurons of mice, rats, and humans, suggesting that its roles in socials behavior may be conserved (Young et al., 2006). Wersinger et al. also assessed the social behavior of AVP V1a KO mice. Although preliminary study indicated that V1a KO mice had elevated aggression levels (Wersinger et al., 2003), more recent experiments found no effects on social aggression, anxiety-like behavior, or social recognition in males or females (Wersinger et al., 2007b). These authors hypothesize that other components in the neural circuitry of these social behaviors compensate for the loss of AVP V1a receptors. However, the V1a KO mice did have olfactory deficits, suggesting that pharmacological manipulations of the AVP system may act through a chemosensory mediated mechanism.
The goal of the present study was to determine whether AVP mediates the initiation, intensity, and/or duration of maternal aggression in lactating female rats. Maternal behavior and grooming behaviors were also recorded. We hypothesized that blocking central endogenous AVP signaling through the V1a receptor by acute intracerebroventricular (icv) administration of a V1a antagonist on day 5 and 15 of lactation would result in the attenuation of maternal aggression, and that icv infusion of exogenous AVP would increase maternal aggression on day 15, when aggression levels are typically lower. The results of the current study, in fact, failed to find an attenuation of maternal aggression after administration of a V1a antagonist. Rather, treatment of lactating rats with a V1a antagonist stimulated maternal aggression.
METHODS
Animals
Animals in this study were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council, and the research protocol was approved by the Tufts Institutional Animal Care and Use Committee. Sprague-Dawley rats (200–225g, Charles River, Kingston, NY) were triple-housed in a light- (on 0500–1900 h) and temperature- (21–24°C) controlled room with food and water available ad libidum. Following mating, pregnant rats were anesthetized with isoflurane on day 20 of gestation and implanted with unilateral guide cannulae directed into the right lateral ventricle. Females were allowed to recover in individual home cages, and following parturition, were kept with their litters throughout the experiment. Cannulae placements were confirmed at the end of the study by icv injection of India ink. Only animals that had successful surgeries were included in the statistical analyses.
General Procedure
On days 5 (d5) and 15 (d15) of lactation, implanted females were administered either (icv) saline vehicle (2μl) 10 minutes prior to testing, one of three AVP (Sigma) doses (0.5, 2.5, 12.5 ng in 2μl of saline) 10 minutes prior to testing, or one of three AVP V1a antagonist (Sigma) doses (5.0, 25.0, or 125.0 ng of d(CH2)5Tyr(Me)AVP in 2μl of saline) two hours prior to behavioral testing. All treatments were infused over 60s. These doses were based on reviews of the behavioral effects of AVP (Engelmann et al., 1996; Goodson and Bass, 2001) as well as personal communication with M. Manning. All treatments were randomized across both days such that each subject received one of the seven treatments on d5 and d15. The two hour delay in behavioral testing after the antagonist treatment was designed to avoid potential agonistic activity (Ferris et al., 1985). Since AVP V1a antagonist has been shown to delay aggression for 18 hours in prairie voles (Winslow et al., 1993), we were confident that we would not omit any behavioral effects due to the timing of the treatment injections. Furthermore, initial behavioral pilot studies indicated that handled animals return to typical undisturbed behavioral patterns within 10 minutes, so effects of the injection procedure on behavior was not expected. Behavioral testing was conducted between 1330 and 1630 h. A digital video camera (Panasonic PV-GS180) allowed for behavioral observation without human interference. Fifteen minute behavioral trials began when a slightly smaller intruder male (50–70 days old) was placed into the female’s clear plastic home cage. Rectal temperatures were recorded at the conclusion of the aggression trials to assess any potential physiological effects of the treatments (Banet and Wieland, 1985; Diamant and De Wied, 1993; Meisenberg and Simmons, 1984). Studies of the role of exogenous AVP and vasotocin on aggression indicate that behavioral effects of central AVP may be mediated by physiological actions, (Le Moal et al., 1984; Nephew et al., 2005). Pups were left with the females throughout the study.
Upon conclusion of the aggression trials, the digital videotapes were scored by an observer that was blind to the treatments using ODlog video analysis software (Macropod Inc.). The ODlog software records continuous data in 5 seconds bins, and also generates frequency and duration summaries for all behavioral measures over the 15 minute observation period. Treatment groups were combined within the saline, AVP, and V1a antagonist groups due to lack of significant dose effects following two way ANOVA (p > 0.05, saline n = 9, AVP n = 20, and V1a antagonist n = 26).
Behavioral Variables
Maternal aggression included the scoring of both frontal and lateral attacks. Attacks consisted of bites, pummeling with forelimbs or hindlimbs, and pinning the intruder to the floor of the cage. Latency to initiate, number, and duration of attacks were recorded. A single attack started upon contact between the male and female, and concluded when they separated. Grooming consisted of cleaning and/or manipulation of the dams own fur with mouth or paws. Maternal behavior included the retrieval and gathering of pups, nest building, pup licking, and crouching over the pups.
Statistics
Lactation day 5 and 15 data were analyzed separately by one-way ANOVA for treatment followed by Tukey’s post-hoc tests for pairwise multiple comparisons if significant treatment and/or day effects were identified (SigmaStat 2.03). If the data were not normally distributed, a Kruskal-Wallis one-way ANOVA on ranks was used, followed by Dunn’s pairwise comparisons. T-tests were used to compare d5 and d15 treatments. Due to an absence of dose effects, the individual AVP and V1a antagonist doses were combined into overall AVP and antagonist treatments. All results are presented as means ± SEM, and the level of statistical significance was p < 0.05.
RESULTS
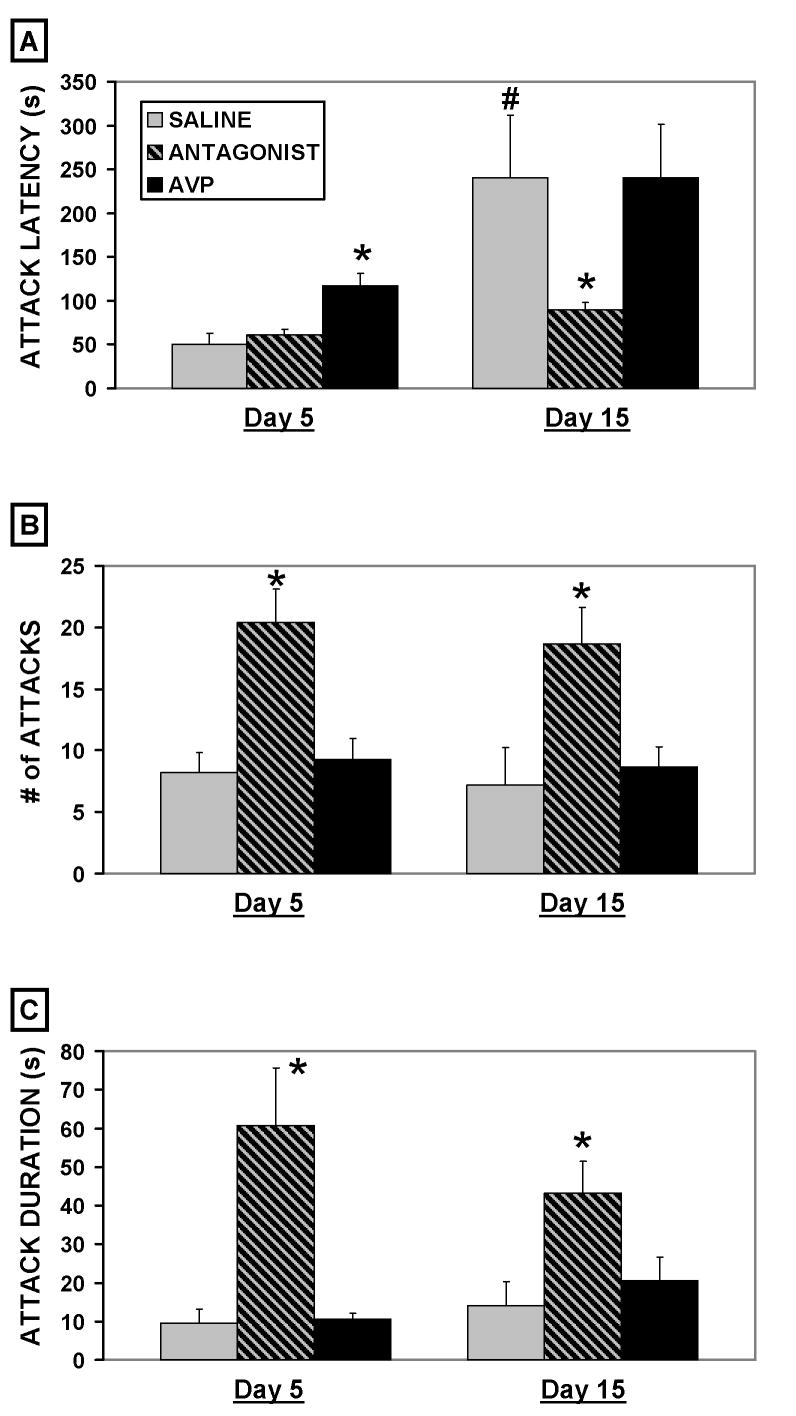
There were significant effects of treatment on d5 (F2,54 = 8.9, p < 0.01) and d15 (F2,54 = 3.2, p < 0.05) latencies to initiate aggression (fig. 1A). Latencies to initiate aggression in saline treated control animals were longer on d15 of lactation compared to d5 ( t-test, p < 0.05). When compared to saline treated controls, exogenous AVP prolonged attack latencies on d5 of lactation (p < 0.05) and the V1a antagonist conversely shortened latencies on d15 (p < 0.05). Despite longer latencies on d15, there were no significant differences between d5 and d15 in number or duration of attacks in saline-treated animals (fig. 1B–C). However, treatment with V1a antagonist increased the number of attacks on both d5 (F2,54 = 8.0, p < 0.01) and d15 (F2,54 = 5.7, p < 0.01) compared to corresponding saline controls (fig. 1B). Acute exogenous V1a antagonist also increased attack duration on both d5 (F2,54 = 6.1, p < 0.01) and d15 (F2,54 = 3.8, p < 0.05) compared to corresponding saline controls (fig. 1C). During the attacks bites were frequently directed at vulnerable body parts, such as the neck and belly, though physical injury due to bites was rare.
Figure 1.
Figure 1A–C. Mean (+SEM) seconds(s) for attack latency, number of attacks, and cumulative seconds spent attacking during 15 minute maternal aggression trials on days 5 and 15 of lactation following icv injections of saline (n = 9), an AVP V1a receptor antagonist (n = 26), or AVP (n = 20). * indicates a significant difference when compared to same day saline controls following ANOVA (p < 0.05). # indicates a significant difference when compared to day 5 saline controls following ANOVA (p < 0.05).
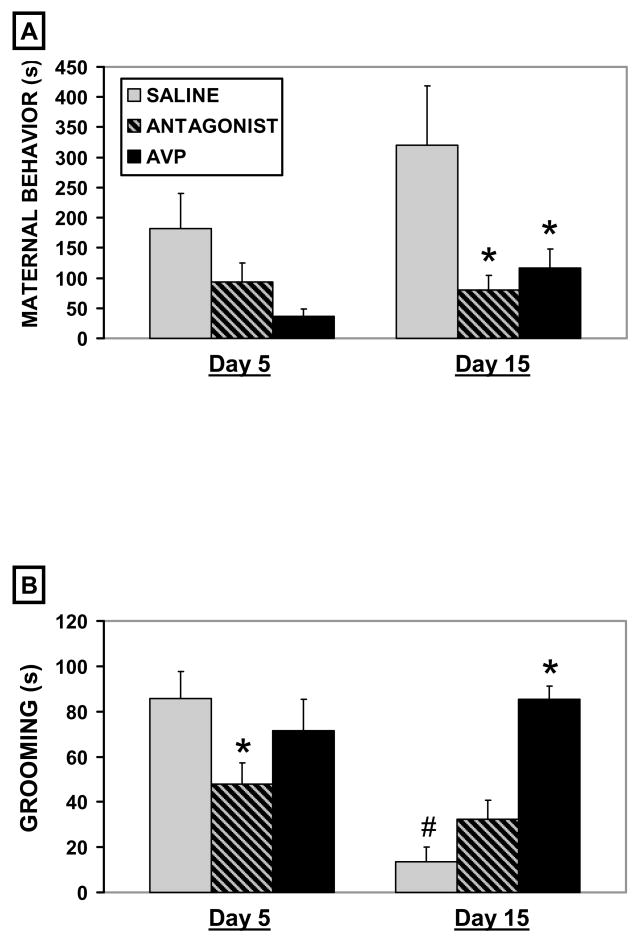
There was a significant effect of treatment on maternal behavior during the resident-intruder trials (F2,54 = 6.5, p < 0.01) with both the V1a antagonist and AVP decreasing the duration of maternal behavior on d15 (p < 0.05, fig. 2A). There was also a significant effect of treatment on grooming behavior (fig. 2B). Saline treated control females spent less time grooming on d15 compared to d5 (t-test, p < 0.01), V1a antagonist treatment decreased grooming duration on d5 (H2 = 6.2, p < 0.05), and AVP treatment increased the duration of grooming on d15 (H2 = 10.2, p < 0.01) compared to corresponding saline controls.
Figure 2.
Figure 2. A–B. Mean (+SEM) cumulative seconds (s) of grooming and maternal behavior during 15 minute maternal aggression trials on days 5 and 15 of lactation following icv injections of saline (n = 9), an AVP V1a receptor antagonist (n = 26), or AVP (n = 20). * indicates a significant difference when compared to saline controls following ANOVA (p < 0.05). # indicates a significant difference when compared to day 5 saline controls following ANOVA (p < 0.05).
As shown in figure 3, there was a significant effect of treatment on d5 resident-intruder trial induced changes in body temperature (F2,54 = 8.3, p < 0.01). Acute central AVP significantly suppressed the increase in body temperature associated with aggression testing on d5 (P < 0.05). Behavioral data for separate dose treatment groups are presented in table 1.
Figure 3.
Mean (+SEM) elevation in body temperature (°C) during 15 minute maternal aggression trials on days 5 and 15 of lactation following icv injections of saline (n = 9), an AVP V1a receptor antagonist (n = 26), or AVP (n = 20). * indicates a significant difference when compared to saline controls following ANOVA (p < 0.05).
Table 1.
Means ± SEM behavioral and temperature change data for separate dose treatment groups. Behavioral durations are presented in seconds (s).
| Day 5 | |||||||
|---|---|---|---|---|---|---|---|
| SALINE (N = 9) | V1a 5.0ng (N = 8) | V1a 25ng (N = 10) | V1a 125ng (N = 8) | AVP 0.5ng (N = 6) | AVP 2.5ng (N = 7) | AVP 12.5ng (N = 7) | |
| Latency (s) | 50 ± 13.1 | 63.8 ± 10.3 | 54.0 ± 8.6 | 65.0 ± 19.7 | 126.7 ± 34.8 | 115.7 ± 24.9 | 109.3 ± 22.6 |
| # of Attacks | 8.22 ± 1.6 | 20.3 ± 3.8 | 18.6 ± 3.4 | 22.9 ± 6.8 | 7.2 ± 2.3 | 7.6 ± 2.9 | 12.7 ± 3.5 |
| Attack Duration (s) | 9.5 ± 3.6 | 58.1 ± 13.3 | 76.0 ± 34.8 | 44.0 ± 20.2 | 14.9 ± 7.2 | 6.0 ± 1.6 | 11.2 ± 3.4 |
| Grooming (s) | 85.7 ± 12.1 | 40.3 ± 12.0 | 53.3 ± 18.3 | 49.1 ± 17.7 | 63.1 ± 21.1 | 69.4 ± 23.6 | 80.8 ± 29.7 |
| Maternal Behavior (s) | 181.6 ± 58.7 | 142.0 ± 80.1 | 63.4 ± 24.7 | 83.2 ± 58.3 | 12.0 ± 9.3 | 53.2 ± 22.3 | 40.7 ± 24.7 |
| ▴Temperature (C) | 1.5 ± 0.2 | 1.68 ± 0.2 | 1.9 ± 0.3 | 1.3 ± 0.3 | 0.8 ± 0.3 | 0.9 ± 0.2 | 0.8 ± 0.2 |
| Day 15 | |||||||
| Latency (s) | 240.6 ± 71.8 | 79.3 ± 13.6 | 112.9 ± 15.3 | 78.9 ± 14.2 | 183.1 ± 106. | 121.1 ± 19.4 | 431.0 ± 141.1 |
| # of Attacks | 7.2 ± 3.0 | 20.3 ± 4.6 | 13.9 ± 1.6 | 21.0 ± 5.7 | 9.9 ± 2.8 | 8.2 ± 3.1 | 8.1 ± 2.8 |
| Attack Duration (s) | 14.0 ± 6.4 | 50.1 ± 17.7 | 18.5 ± 3.4 | 57.1 ± 14.6 | 38.7 ± 16.4 | 12.1 ± 4.5 | 11.7 ± 4.1 |
| Grooming (s) | 13.6 ± 5.2 | 46.0 ± 12.0 | 21.3 ± 19.5 | 30.6 ± 15.2 | 47.7 ± 19.0 | 48.9 ± 12.1 | 164.0 ± 54.9 |
| Maternal Behavior (s) | 320.4 ± 98.6 | 122.4 ± 69.7 | 89.5 ± 38.9 | 38.2 ± 11.8 | 92.3 ± 49.0 | 97.9 ± 32.6 | 161.4 ± 80.7 |
| ▴Temperature (C) | 0.8 ± 0.2 | 1.4 ± 0.2 | 1.0 ± 0.3 | 0.7 ± 0.2 | 1.3 ± 0.4 | 1.4 ± 0.3 | 1.3 ± 0.3 |
DISCUSSION
The acute manipulations of central AVP in this study support the hypothesis that central AVP V1a receptors mediate the display of maternal aggression in lactating female rats. AVP antagonism stimulated aggression, whereas exogenous vasopressin delayed the display of maternal aggression. In addition to effects on aggression, these AVP manipulations also significantly attenuated maternal behavior and core body temperature responses, and modulated grooming. Overall, the present results suggest that the central vasopressinergic system may mediate maternal aggression, maternal behavior, and grooming in lactating rats.
The novel effects on maternal aggression contrast with the documented stimulatory effects of AVP on male rodent aggression (Compaan et al., 1993; Delville et al., 1996a; Delville et al., 1996b; Elkabir et al., 1990; Ferris et al., 1997; Ferris and Potegal, 1988; Stribley and Carter, 1999; Winslow et al., 1993). However, in male mice selected for short attack latencies, AVP projections to the lateral septum form the BNST, and BNST AVP neuronal density are decreased compared to long attack latency selected mice (Compaan et al., 1993). In addition, male rats selected for low anxiety are highly aggressive and have decreased septal AVP during a resident intruder test (Beiderbeck et al., 2007). Unexpectedly, in these selected rat lines, the septal infusion of either AVP or V1a antagonist did not affect intermale aggression. The authors conclude that although AVP septal release fluctuates with male aggression, local AVP release does not directly affect aggression, but may affect aggression through its’ role in social and anxiety-related behaviors. Furthermore, highly aggressive male prairie voles have decreased AVP activity in the BNST and MeA, and decreased AVP receptor levels in the lateral septum when compared to less aggressive meadow voles (Insel et al., 1994; Wang, 1995; Young et al., 1997). Although V1a KO mice seem to have normal aggression (Wersinger et al., 2007b), preliminary studies indicated that these mice may have elevated aggression (Wersinger et al., 2003), and V1b KO mice have impaired aggression (Wersinger et al., 2007a; Wersinger et al., 2002). Taken together, these studies in rats and mice suggest that the display of aggression may be linked to decreased central AVP activity (for additional review, see (Veenema and Neumann, 2007)). The current data showing increased maternal aggression following V1a antagonism support this hypothesis.
Contrary to our original hypothesis, antagonism of central V1a receptors significantly increased maternal aggression on both d5 and d15 of lactation. It should be noted that even though we used a relatively low dosage range, there were no significant differences between V1a antagonist dosages (see table 1), indicating that the antagonist was effective at low concentrations. While the lack of specific control injection for the V1a antagonist treatments is a potential confound due to handling effects, pilot studies and comparisons with the aggression levels of animals in untreated, non-handled control animals (Byrnes et al., 2008) indicate that handling stress during the injection protocol 10 minutes prior to behavioral observation does not affect maternal aggression. The current data suggest that endogenous AVP inhibits maternal aggression by acting at the V1a receptor, and support preliminary work showing similar effects when the same V1a antagonist was injected into the anterior hypothalamus of virgin adult female Syrian hamsters (Gutzler et al., 2007), as well as prior lesion studies that indicate AVP may act centrally to inhibit aggression in lactating females (Giovenardi et al., 1997). Additional neuroanatomical studies indicate that AVP gene expression is reduced following parturition (Crowley et al., 1993). In recent neuroanatomical investigations of the increase in maternal aggression in multiparous females compared to primiparous females (Nephew et al., 2007), it appears that the more aggressive multiparous females have increased V1a receptor mRNA expression in the central and medial amygdala, lateral septum, and supraoptic nucleus on d5 of lactation. However, further investigations of AVP and V1a receptor expression in maternal aggression associated nuclei are needed to elucidate the role of central vasopressinergic activity in the control of maternal aggression. Based on the current behavioral data, we hypothesize that AVP activity is low during the early and middle stages of lactation when aggression levels are high. A decreased activation of the central vasopressinergic circuits should allow for the display of maternal aggression, such that treatment with a V1a antagonist enhances this display. The increased latencies following icv AVP infusions in the current study offer additional support for this hypothesis.
Administering exogenous AVP had the opposite effects of V1a antagonism on latency to initiate aggression by significantly increasing latencies on d5. However, despite doubling the attack latencies, AVP had no effects on number of attacks or overall attack duration. This lack of effects may be due to the relatively low aggression levels at this time. The data suggest that a mechanism exists that ensures the adequate display of maternal aggression despite the AVP-induced increase in latencies. It is unlikely that our AVP doses were too low to significantly lower aggression levels, as the doses used were based on behaviorally active treatments found in several similar rodent studies on aggression (Bester-Meredith et al., 2005; Ferris et al., 1997; Parker and Lee, 2001), and there was a significantly attenuated increase in body temperature on d5 in AVP-treated mothers in our study. This thermal effect indicates that higher doses may introduce a physiological effect which could confound potential behavioral actions. Further study on virgin, pregnant, and lactating rats is needed to clarify AVP’s role in the regulation of maternal aggression. The use of a multiparous model exhibiting elevated levels of aggression will provide a useful construct for further testing of AVP effects maternal aggression.
In the present study, although the latencies to initiate maternal aggression in saline control animals were three minutes longer on d15 compared to d5 of lactation, there were no significant between day differences in either the number or duration of attacks. The similar levels of aggression despite significant differences in latencies indicate that the display of aggression in saline control females was not constrained by the length of the behavioral observation period. While other studies have reported an overall decrease in maternal aggression at this stage in lactation, it may be that the females were tested prior to a significant attenuation of aggression. It is also possible that subjecting experimental females to aggression on d5 primed their response for the resident-intruder challenge on d15. However, if this hypothesis was valid, the latencies to attack would be expected to be similar as well, if not shorter on d15. This was not observed. Another possibility is that overall aggression was reduced by olfactory recognition due to the housing of the females and intruder males in the same room, and this decrease on d5 eliminated the potential for documenting a further decrease on d15.
In addition to modulating aggression, AVP also affected grooming and maternal behavior. The actions of V1a antagonist and AVP on grooming support previous studies on the effects of AVP on grooming in both rats (Caldwell et al., 1986; Elkabir et al., 1990) and mice (Lumley et al., 2001), where exogenous AVP increased grooming behavior. The lack of enhanced grooming following AVP on d5 may be due to the significantly attenuated increase in body temperature following this treatment. Since it is established that grooming increases in response to elevated body temperature (Yanase et al., 1991), an attenuating effect of AVP on body temperature may have suppressed potential effects on grooming. The lack of significant V1a antagonist effects on d15 grooming may be a “floor” effect, considering the lower baseline levels of grooming expressed by the saline controls at this time point. As well as confirming earlier investigations on the effects of AVP on grooming, these data provide a positive control that the dams were receiving behaviorally active doses.
Both V1a antagonist and AVP decreased maternal behavior on d15. One possible explanation for the similar effects of both treatments is that the antagonist-induced increase in aggression and the AVP-induced increase in grooming indirectly affected maternal behavior. However, since maternal behavior was not temporally constrained by these increases in aggression or grooming, it is also possible that AVP and the antagonist affect maternal behavior through different mechanisms. Interestingly, both acute increases and decreases in plasma glucocorticoid concentrations elevate aggression levels in male rats through neural mechanisms (Halasz et al., 2002; Haller et al., 1997; Haller et al., 2001). We are currently examining the effects of central V1a antagonist infusion on maternal behavior. Preliminary data support the current evidence that central V1a antagonist disrupts maternal behavior (Nephew and Bridges, 2007).
The present data do not support the hypothesis that AVP stimulates maternal aggression, which differs from the stimulatory actions of this neuropeptide on other forms of aggression in multiple male rodent species. However, the results do correlate with preliminary investigations of V1a mice (Wersinger et al., 2003), which display increased aggression, as well as correlational neuroanatomical studies in male rats selected for low anxiety behavior (Beiderbeck et al., 2007). The data also parallel recent work indicating that corticosteroid releasing factor (CRF) inhibits mouse maternal aggression. Gammie et al. report that the expression of maternal aggression in mice requires a decrease in central CRF activity (Gammie et al., 2004). They hypothesized that the effects of CRF on maternal aggression are related to its anxiogenic properties, and that CRF induced decreases in fear and anxiety mediate the expression of aggression in lactating mice. The relationship between CRF and AVP in the PVN has been studied in detail. To summarize, both neuropeptides are increasingly co-localized in the parvocellular PVN during lactation, and that the increased production of AVP in this area leads to increased sensitivity to immunotargeted lesions (Walker et al., 2001). Since AVP is also involved in the expression of anxiety (Keck et al., 2002; Murgatroyd et al., 2004; Wigger et al., 2004), in addition to being colocalized in a behaviorally relevant nuclei, it is possible that AVP mediates rat maternal aggression through a sex specific anxiety-mediated inhibitory mechanism. This possibility awaits study. Similar to the differential role of AVP across vertebrate species (Goodson and Bass, 2001), the present data suggest that the effects of AVP on aggression are sexually dimorphic. A comparable neuropeptidergic mechanism has been studied in prairie voles, where the neural control of partner preference is sex dependent. Oxytocin seems to be more important in the control of female partner preference, whereas AVP is the significant neuropeptide in males (Young and Wang, 2004).
Recent work has implicated adrenal function in the regulation of maternal aggression. While several studies have noted the apparent hyporesponsiveness of the hypothalamo-pituitary-adrenal axis to stressors in pregnant and lactating rats (Lightman and Young, 1989; Neumann et al., 2003; Neumann et al., 1998), recent studies by Haller et al. suggest that chronic exposure to glucocorticoid deficiency (induced by adrenalectomy and chronic treatment with low concentrations of glucocorticoids) contributes to the display of abnormal aggression in male rats (Halasz et al., 2002; Haller et al., 2004; Haller and Kruk, 2006; Haller et al., 2001). Although these investigations focus on abnormal aggression in male rats, the ethogram of abnormal aggression is similar to the maternal attacks observed in the present study. These similarities consist of preferential attacks towards the head, throat, and belly. In addition, adrenalectomized and low glucocorticoid replacement male rats have increased c-Fos activity in the central amygdala (Halasz et al., 2002), an area where AVP V1a receptor mRNA expression is up regulated in aggressive multiparous rats during lactation (Nephew and Bridges, 2007). Based on these studies, both glucocorticoid-induced abnormal aggression and maternal aggression have been postulated to be associated with anxiety. However, while these similarities are interesting, it is unknown whether the hyporesponsive HPA axis in pregnant and maternal rats parallels the adrenalectomy, low glucocorticoid replacement treatment protocol in male rats.
In summary, the data support the hypothesis that central AVP activity mediates the expression of maternal aggression, maternal behavior, and grooming in lactating rats. Recent investigation of AVP neuroanatomy in lactating rats has revealed that V1a receptor mRNA is up regulated in the amygdala, supraoptic nucleus, and lateral septum in multiparous females compared to primiparous females (Nephew et al., 2007); these neuroanatomical differences correlate with significant differences in maternal aggression (Byrnes et al., 2008). These behavioral and anatomical findings indicate that the expression and modulation of maternal aggression may involve differential vasopressinergic activity at one or more of these behaviorally relevant nuclei. Further investigations of the neuroanatomical and neurochemical differences in the vasopressin systems of males and females will provide insight into the neural control of aggression. (Insel and Young, 2000; Lonstein and Gammie, 2002)
Acknowledgments
Supported by NIH grants R37 HD19789 to RSB, and F32 HD 048103 to BCN. We thank Dr. Elizabeth Byrnes for comments in the preparation of this manuscript, and Dr. Phyllis Mann for statistical advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banet M, Wieland U. The effect of intraseptally applied vasopressin on thermoregulation in the rat. Brain Res Bull. 1985;14:113–116. doi: 10.1016/0361-9230(85)90070-x. [DOI] [PubMed] [Google Scholar]
- Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. European Journal of Neuroscience. 2007;26:3597–3605. doi: 10.1111/j.1460-9568.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Martin PA, Marler CA. Manipulations of Vasopressin alter aggression differently across testing conditions in monogamous and non-monogamous Peromyscus mice. Aggressive Behavior. 2005;31:189–199. [Google Scholar]
- Blanchard RJ, Griebel G, Farrokhi C, Markham C, Yang M, Blanchard DC. AVP V1b selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacology Biochemistry and Behavior. 2005;80:189–194. doi: 10.1016/j.pbb.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. Release of Oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defense. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain Oxytocin Correlates with Maternal Aggression: Link to Anxiety. J Neuroscience. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EM, Nephew BC, Bridges RS. Neuroendocrine and behavioral adaptations following reproductive experience in the female rat. In: Bridges R, editor. Neurobiology of the Parental Mind. Elsevier; San Francisco: 2008. [Google Scholar]
- Caldwell JD, Drago F, Prange AJJ, Pedersen CA. A comparison of grooming behavior potencies of neurohypophyseal peptides. Regulatory Peptides. 1986;14:261–272. doi: 10.1016/0167-0115(86)90009-1. [DOI] [PubMed] [Google Scholar]
- Chan WY, Wo NC, Cheng LL, Manning M. Isosteric substitution of Asn5 in antagonists of oxytocin and vasopressin leads to highly selective and potent oxytocin and V1a receptor antagonists: new approaches for the design of potential tocolytics for preterm labor. J Pharmacol Exp Ther. 1996;277:999–1003. [PubMed] [Google Scholar]
- Compaan JC, Buijs RM, Pool CW, De Ruiter JH, Koolhaas JM. Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice. Brain Res Bull. 1993;30:1–6. doi: 10.1016/0361-9230(93)90032-7. [DOI] [PubMed] [Google Scholar]
- Crowley RS, Insel TB, O’Keefe JA, Amico JA. Cytoplasmic oxytocin and vasopressin gene transcripts decline postpartum in the hypothalamus of the lactating rat. Endocrinology. 1993;133:2704–2710. doi: 10.1210/endo.133.6.7612074. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Lucion AB. Effects of intracerebroventricular administration of 5-HT receptor agonists on the maternal aggression of rats. Eur J Pharm. 1994;264:445–448. doi: 10.1016/0014-2999(94)00548-6. [DOI] [PubMed] [Google Scholar]
- De Almeida RM, Lucion AB. 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but in the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology. 1997;134:392–400. doi: 10.1007/s002130050476. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Serotonin blocks vasopressin-facilitated offensive aggression: interactions within the ventrolateral hypothalamus of golden hamsters. Physiol and Behav. 1996a;59:813–816. doi: 10.1016/0031-9384(95)02166-3. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol and Behav. 1996b;60:25–29. doi: 10.1016/0031-9384(95)02246-5. [DOI] [PubMed] [Google Scholar]
- Diamant M, De Wied D. Differential effects of centrally injected AVP on heart rate, core temperature, and behavior in rats. Am J Physiol Regul Integ Comp Physiol. 1993;264:51–61. doi: 10.1152/ajpregu.1993.264.1.R51. [DOI] [PubMed] [Google Scholar]
- Elkabir DR, Wyatt ME, Vellucci SV, Herbert J. The effects of separate or combined infusions of corticotrophin releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behavior in the rat. Regulatory Peptides. 1990;28:199–214. doi: 10.1016/0167-0115(90)90018-r. [DOI] [PubMed] [Google Scholar]
- Elliot JC, Lubin DA, Walker CH, Johns JM. Acute cocaine alters oxytocin levels in the medial preoptic area and amygdala in lactating rat dams: implications for cocaine-induced changes in maternal behavior and maternal aggression. Neuropeptides. 2001;35:127–134. doi: 10.1054/npep.2001.0854. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Neuman I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: Focus on learning and memory. Neuroscience and Biobehav Rev. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behavioral Biology. 1978;23:206–218. doi: 10.1016/s0091-6773(78)91814-x. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Postpartum aggression in rats: I. Effects of hypophysectomy. J Comp and Physiol Psych. 1980a;94:484–494. doi: 10.1037/h0077686. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Barfield RJ, Goldman BD. Postpartum aggression in rats: II. Dependence on maternal sensitivity to young and effects of experience with pregnancy and parturition. J Comp and Physiol Psych. 1980b;94:495–505. doi: 10.1037/h0077677. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Dahlof LG, Hansen S. Olfactory mechanisms in the control of maternal aggression, appetite, and fearfulness: effects of lesions to olfactory receptors, mediodorsal thalamic nucleus, and insular prefrontal cortex. Behavioral Neuroscience. 1987;101:709–717. doi: 10.1037//0735-7044.101.5.709. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. Neuroscience. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Pollock J, Albers HE, Leeman SE. Inhibition of flank marking behavior in golden hamsters by microinjection of a vasopressin antagonist into the hypothalamus. Neurosci Letters. 1985;55:239–243. doi: 10.1016/0304-3940(85)90027-8. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol and Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- Flannelly KJ, Kemble ED, Caroline Blanchard D, Blanchard RJ. Effects of septal-forebrain lesions on maternal aggression and maternal care. Behavioral and Neural Biology. 1986;45:17–30. doi: 10.1016/s0163-1047(86)80002-4. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Hasen NS, Stevenson SA, Bale TL, D’Anna KL. Elevated stress sensitivity in corticotrophin-releasing factor receptor 2 deficient mice decreases maternal, but not intermale aggression. Behavioural Brain Research. 2005;160:169–177. doi: 10.1016/j.bbr.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behavavioral Neuroscience. 2004;118:105–114. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus, oxytocin, and maternal aggression in rats. Annals of the New York Academy of Sciences. 1997;807:606–609. doi: 10.1111/j.1749-6632.1997.tb51981.x. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: Effects of Ibotenic acid lesion and oxytocin antisense. Physiol and Behav. 1998;63:351–359. doi: 10.1016/s0031-9384(97)00434-4. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin system in vertebrates. Brain Research Reviews. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Albers HE. A vasopressin (V1a) receptor antagonist stimulates aggression in female Syrian hamsters, but not during behavioral estrus. Soc. for Neurosci; San Diego, CA: 2007. [Google Scholar]
- Halasz J, Liposits Z, Kruk MR, Haller J. Neural background of glucocorticoid dysfunction-induced abnormal aggression in rats: involvement of fear- and stress-related structures. European Journal of Neuroscience. 2002;15:561–569. doi: 10.1046/j.0953-816x.2001.01883.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Albert I, Makara GB. Acute Behavioural Effects of Corticosterone Lack Specificity but Show Marked Context-Dependency. J Neuroendocrinology. 1997;9:515–518. doi: 10.1046/j.1365-2826.1997.00603.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Halasz J, Mikics E, Kruk MR. Chronic Glucocorticoid Deficiency-Induced Abnormal Aggression, Autonomic Hypoarousal, and Social Deficit in Rats. Journal of Neuroendocrinology. 2004;16:550–557. doi: 10.1111/j.1365-2826.2004.01201.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Kruk MR. Normal and abnormal aggression: human disorders and novel laboratory models. Neuroscience & Biobehavioral Reviews. 2006;30:292–303. doi: 10.1016/j.neubiorev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Haller J, van de Schraaf J, Kruk MR. Deviant Forms of Aggression in Glucocorticoid Hyporeactive Rats: A Model for ‘Pathological’ Aggression? Journal of Neuroendocrinology. 2001;13:102–107. doi: 10.1046/j.1365-2826.2001.00600.x. [DOI] [PubMed] [Google Scholar]
- Hansen S. Medial Hypothalamic Involvement in Maternal Aggression of Rats. Behavioral Neuroscience. 1989;103:1035–1046. doi: 10.1037//0735-7044.103.5.1035. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neuroscience. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young LJ. Neuropeptides and the evolution of social behavior. Current Opinion in Neurobiology. 2000;10:784–789. doi: 10.1016/s0959-4388(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Keck ME, Wigger A, Welt T, Muller MB, Gesing A, Reul JM, Holsboer F, Landgraf R, Neumann ID. Vasopressin mediates the response of the combined dexamethasone/CRH test in hyper-anxious rats: Implications for pathogenesis of affective disorders. J Neuroscience. 2002;24:7762–7770. doi: 10.1016/S0893-133X(01)00351-7. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Dantzer R, Mormede P, Baduel A, Lebrun C, Ettenberg A, Van der Kooy D, Wenger J, Deyo Scott KFG, Bloom FE. Behavioral effects of peripheral administration of arginine vasopressin: a review of our search for a mode of action and a hypothesis. Psychoneuroendocrinology. 1984;9:319–341. doi: 10.1016/0306-4530(84)90042-8. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Young WSd. Lactation inhibits stress-mediated secretion of corticosterone and oxytocin and hypothalamic accumulation of corticotrophin-releasing factor and enkephalin messenger ribonucleic acids. Endocrinology. 1989;124:2358–2364. doi: 10.1210/endo-124-5-2358. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural controls of maternal aggression in laboratory rodents. Neuroscience and Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal and aggressive behaviors in rats. Brain Research. 1998;804:21–35. doi: 10.1016/s0006-8993(98)00642-8. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Elliot JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behavioral Neuroscience. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley LA, Robison CL, Chen WK, Mark B, Meyerhoff JL. Vasopressin into the preoptic area increases grooming behavior in mice. Physiol and Behav. 2001;73:451–455. doi: 10.1016/s0031-9384(01)00501-7. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Reisbick S, Siegel HI, Rosenblatt JS. Maternal aggression in rats: Changes over pregnancy and lactation in a Sprague-Dawley strain. Aggress Behav. 1987;13:29–43. [Google Scholar]
- Meisenberg G, Simmons WH. Hypothermia induced by centrally administered vasopressin in rats. A structure-activity study Neuropharm. 1984;23:1195–1200. doi: 10.1016/0028-3908(84)90239-9. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Wigger A, Frank E, Singewald N, Bunck M, Holsboer F, Landgraf R, Spengler D. Impaired repression at a vasopressin promoter polymorphism underlies overexpression of vasopressin in a rat model of trait anxiety. J Neuroscience. 2004;24:7762–7770. doi: 10.1523/JNEUROSCI.1614-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Animal Behavior. Burlington, VT: 2007. Arginine vasopressin antagonism disrupts the retention of maternal behavior in lactating rats. [Google Scholar]
- Nephew BC, Bridges RS, Byrnes EM. Central vasopressin V1a and oxytocin receptor levels in primiparous and multiparous lactating rats. World Congress on Neurohypophysial Hormones; Regensburg, Germany. 2007. [Google Scholar]
- Nephew BC, Reed LM, Romero LM. A potential cardiovascular mechanism for the behavioral effects of central and peripheral arginine vasotocin. General and Comparative Endocrinology. 2005;144:156–166. doi: 10.1016/j.ygcen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Bosch OJ, Toschi N, Torner L, Douglas AJ. No Stress Response of the Hypothalamo-Pituitary-Adrenal Axis in Parturient Rats: Lack of Involvement of Brain Oxytocin. Endocrinology. 2003;144:2473–2479. doi: 10.1210/en.2003-0037. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol. 1998;508:289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Lee TM. Central vasopressin administration regulates the onset of facultative paternal behavior in Microtus pennsylvanicus (Meadow voles) Horm Behav. 2001;39:285–294. doi: 10.1006/hbeh.2001.1655. [DOI] [PubMed] [Google Scholar]
- Potegal M, Ferris CF. Intraspecific aggression in male hamsters is inhibited by intrahypothalamic vasopressin receptor antagonist. Aggressive Behavior. 1990;15:311–320. [Google Scholar]
- Stribley JM, Carter CS. Developmental exposure to vasopressin increases aggression in adult prairie voles. Proc Natl Acad Sci. 1999;96:12601–12604. doi: 10.1073/pnas.96.22.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Lore RK. Intermale and maternal aggression in adult rats tested at different ages. Physiol and Behav. 1982;29:1013–1018. doi: 10.1016/0031-9384(82)90292-x. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur J Neurosci. 2006;24:1711–20. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32:437–450. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Neurobiological mechanisms of aggression and stress coping: a comparative study in mouse and rat selection lines. Brain Behav Evol. 2007;70:274–85. doi: 10.1159/000105491. [DOI] [PubMed] [Google Scholar]
- Walker CD, Tilders FJH, Burlet A. Increased Colocalization of Corticotropin-Releasing Factor and Arginine Vasopressin in Paraventricular Neurones of the Hypothalamus in Lactating Rats: Evidence from Immunotargeted Lesions and Immunohistochemistry. Journal of Neuroendocrinology. 2001;13:74–85. doi: 10.1046/j.1365-2826.2001.00589.x. [DOI] [PubMed] [Google Scholar]
- Wang Z. Species differences in the vasopressin-immunoreactive pathways in the bed nucleus of the stria terminalis and medial amygdaloid nucleus in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Behavavioral Neuroscience. 1995;109:305–311. doi: 10.1037//0735-7044.109.2.305. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Christiansen M, Young WS. Disruption of the vasopressin 1b receptor gene impairs the attack component of aggressive behavior in mice. Genes, Brain and Behavior. 2007a;6:653–660. doi: 10.1111/j.1601-183X.2006.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS. Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes, Brain and Behavior. 2007b;6:540–551. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Christiansen M, Hu SB, Gold PW, Young WS. Aggression is reduced in vasopressin 1b receptor null mice but is elevated in vasopressin 1a receptor null mice. Society for Neuroscience Annual Meeting; New Orleans, LA. 2003. [Google Scholar]
- Wersinger SR, Ginnns EI, O’Carroll AM, Lolait SJ, Young WS., III Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Molecular Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wersinger SRR, Kelliher K, Zufall F, Lolait SJ, O’Carroll AM, Young WS., III Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Hormones and Behavior. 2004;46:638–645. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wigger A, Sanchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neuman I, Holsboer F, Plotsky PM, Landgraf R. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharm. 2004;29:1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Yanase M, Kanosue K, Yasuda H, Tanaka H. Salivary secretion and grooming behavior during heat exposure in freely moving rats. J Physiolgy. 1991;432:585–592. doi: 10.1113/jphysiol.1991.sp018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V(1)a receptor gene expression in monogamous and nonmonogamous voles: Behavioral consequences. Behavioral Neuroscience. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]