Abstract
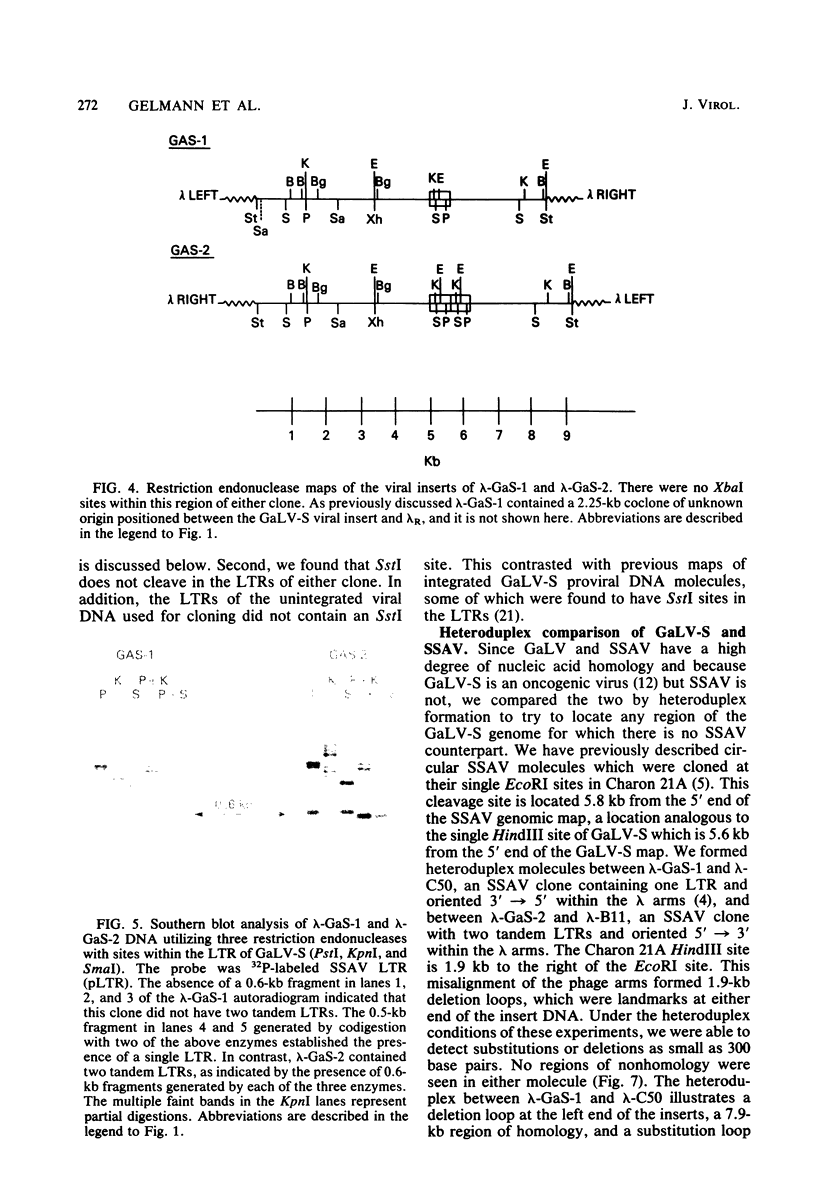
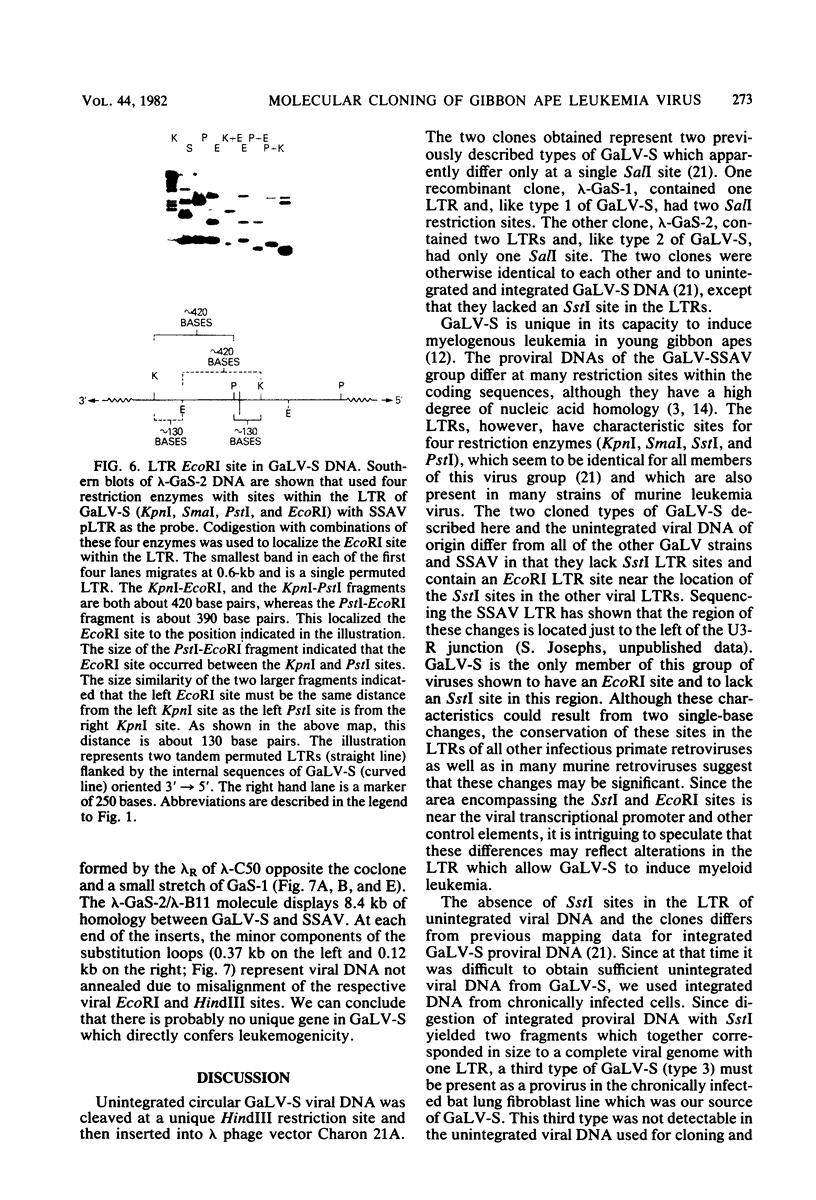
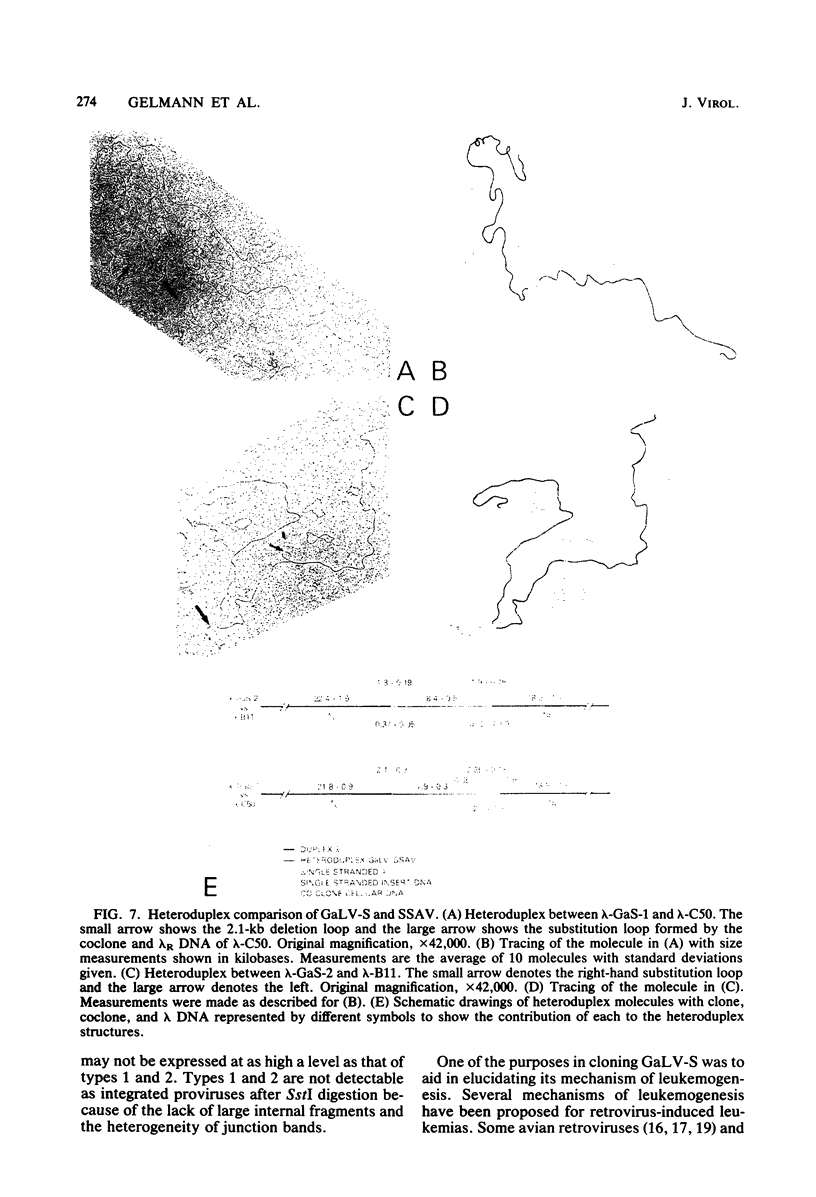
Closed circular unintegrated DNA of the SEATO strain of gibbon ape leukemia virus (GaLV-S) was isolated from canine thymus fibroblasts after cocultivation with chronically infected bat lung fibroblasts. Restriction endonuclease HindIII cleaves GaLV-S DNA once, thus allowing isolation and cloning of HindIII-digested unintegrated DNA in a permitted form. Two clones isolated in the vector, Charon 21A, were nearly identical by restriction enzyme mapping to each of the two types of GaLV-S previously observed. These two types differ at a single SalI site. Unlike previous maps of GaLV-S proviral DNA, however, both clones lack SstI sites in the long-terminal-repeat units. Both the GaLV-S clones and the major species of GaLV-S proviral DNA contain an EcoRI site in the long-terminal-repeat units. The presence of this EcoRI site and the absence of an SstI site in the GaLV-S long-terminal-repeat units differentiate it from all other known GaLV strains and from the closely related nononcogenic simian sarcoma-associated virus. Heteroduplex comparisons of each of the two clones to clones of simian sarcoma-associated virus show no obvious deletion or substitution loops. This suggests that the ability of GaLV-S to induce myeloid leukemia in gibbon apes in not due to an acquired onc gene.
Full text
PDF
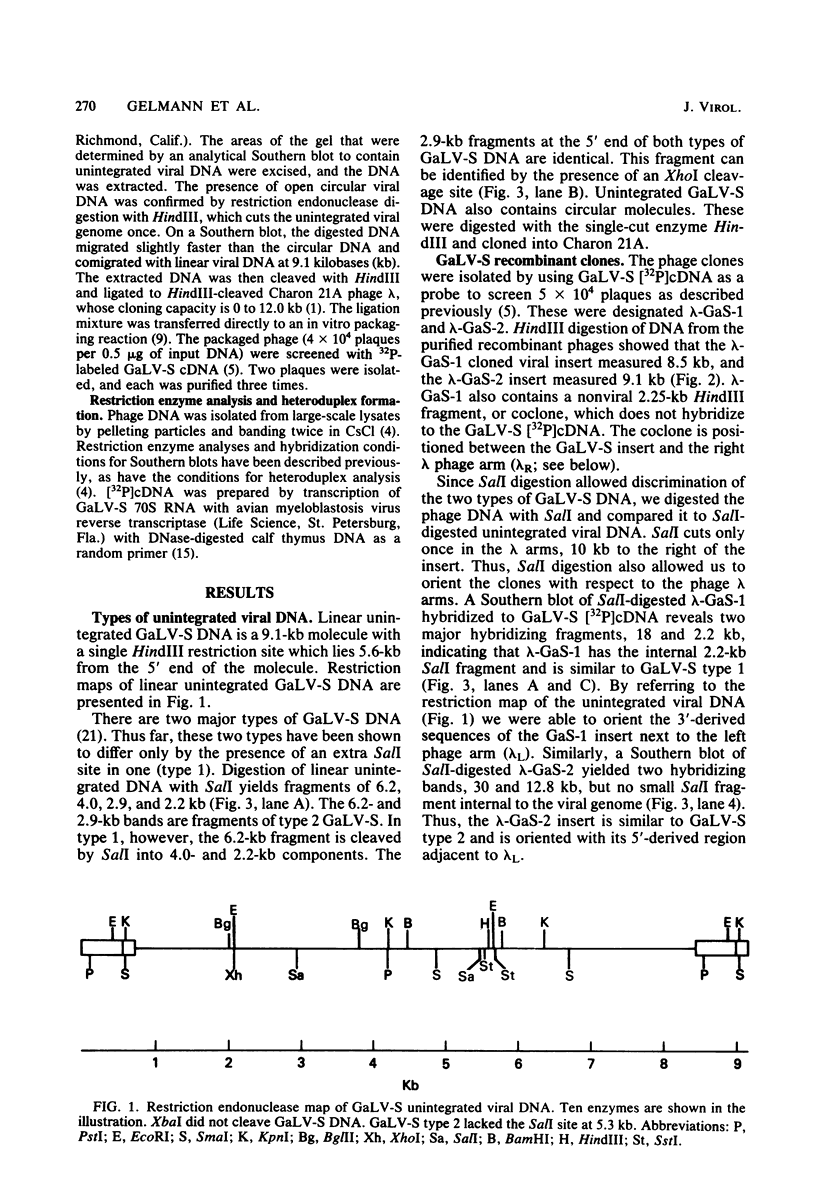
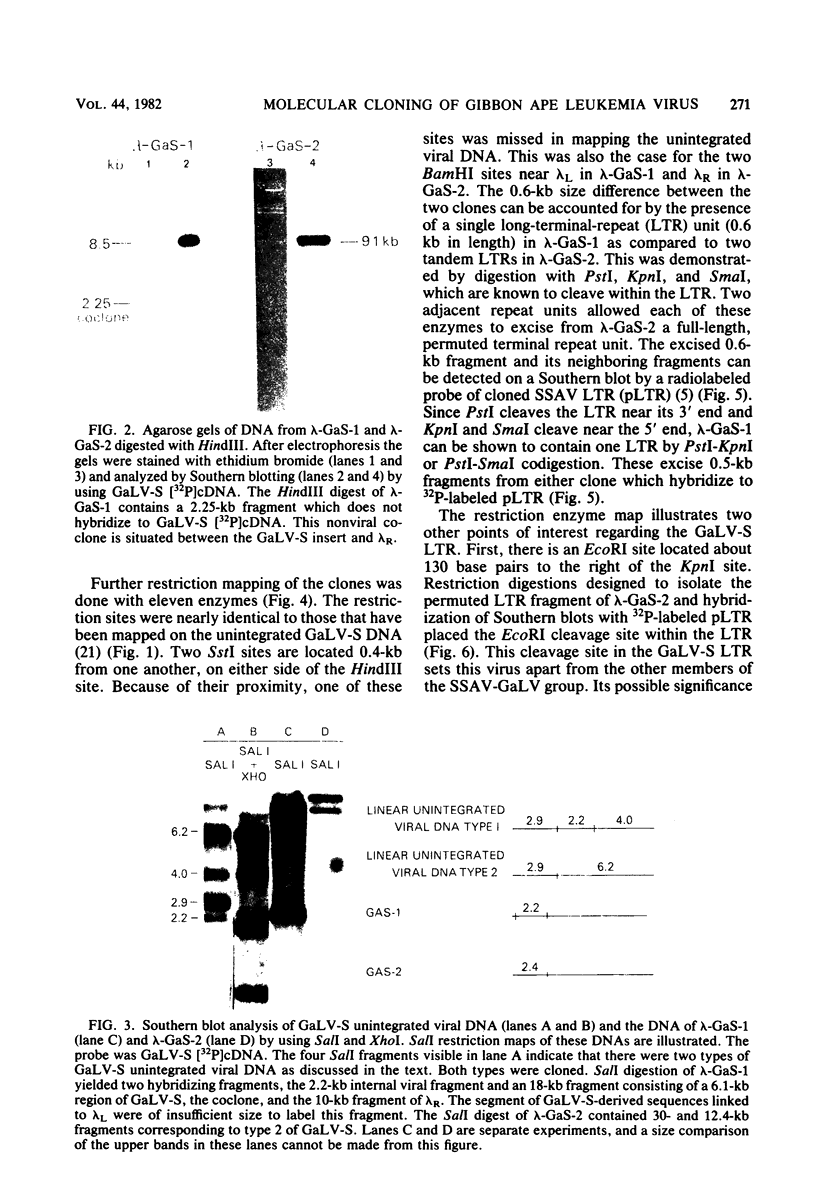

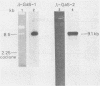
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gallo R. C., Gallagher R. E., Wong-Staal F., Aoki T., Markham P. D., Schetters H., Ruscetti F., Valerio M., Walling M. J., O'Keeffe R. T. Isolation and tissue distribution of type-C virus and viral components from a gibbon ape (Hylobates lar) with lymphocytic leukemia. Virology. 1978 Feb;84(2):359–373. doi: 10.1016/0042-6822(78)90255-6. [DOI] [PubMed] [Google Scholar]
- Gelmann E. P., Petri E., Cetta A., Wong-Staal F. Deletions of specific regions of the simian sarcoma-associated virus genome are found in defective viruses and in the simian sarcoma virus. J Virol. 1982 Feb;41(2):593–604. doi: 10.1128/jvi.41.2.593-604.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmann E. P., Wong-Staal F., Kramer R. A., Gallo R. C. Molecular cloning and comparative analyses of the genomes of simian sarcoma virus and its associated helper virus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3373–3377. doi: 10.1073/pnas.78.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Kawakami T. G., Buckley P. M. Antigenic studies on gibbon type-C viruses. Transplant Proc. 1974 Jun;6(2):193–196. [PubMed] [Google Scholar]
- Kawakami T. G., Huff S. D., Buckley P. M., Dungworth D. L., Synder S. P., Gilden R. V. C-type virus associated with gibbon lymphosarcoma. Nat New Biol. 1972 Feb 9;235(58):170–171. doi: 10.1038/newbio235170a0. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Luczak J. C., Gallo R. C. Mapping of related and nonrelated sequences of RNA from wooly monkey virus and gibbon ape leukemia virus. Virology. 1979 Feb;93(1):48–56. doi: 10.1016/0042-6822(79)90274-5. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Voltin M., Gallo R. C. Characterization of a partial provirus from a gibbon ape naturally infected with gibbon ape leukemia virus. Virology. 1980 Jul 30;104(2):474–481. doi: 10.1016/0042-6822(80)90349-9. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Bishop J. M. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol. 1979 Aug;31(2):514–521. doi: 10.1128/jvi.31.2.514-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. P., Dungworth D. L., Kawakami T. G., Callaway E., Lau D. T. Lymphosarcomas in two gibbons (Hylobates lar) with associated C-type virus. J Natl Cancer Inst. 1973 Jul;51(1):89–94. doi: 10.1093/jnci/51.1.89. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Strommer J. N., Hillyard R. L., Komaromy M. C., Baluda M. A. Cellular sequences are present in the presumptive avian myeloblastosis virus genome. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5177–5181. doi: 10.1073/pnas.77.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Kawakami T. G. Isolation and identification of lymphocytic and myelogenous leukemia-specific sequences in genomes of gibbon oncornaviruses. J Virol. 1980 Aug;35(2):400–408. doi: 10.1128/jvi.35.2.400-408.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor C. D., Wong-Staal F., Reitz M. S., Jr Comparative restriction endonuclease maps of proviral DNA of the primate type C simian sarcoma-associated virus and gibbon ape leukemia virus group. J Virol. 1982 Jan;41(1):298–308. doi: 10.1128/jvi.41.1.298-308.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe L. G., Deinhardt F., Theilen G. H., Rabin H., Kawakami T., Bustad L. K. Induction of tumors in marmoset monkeys by simian sarcoma virus, type 1 (Lagothrix): a preliminary report. J Natl Cancer Inst. 1971 Nov;47(5):1115–1120. [PubMed] [Google Scholar]
- Wong-Staal F., Dalla-Favera R., Gelmann E. P., Manzari V., Szala S., Josephs S. F., Gallo R. C. The v-sis transforming gene of simian sarcoma virus is a new onc gene of primate origin. Nature. 1981 Nov 19;294(5838):273–275. doi: 10.1038/294273a0. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Reitz M. S., Jr, Gallo R. C. Retrovirus sequences in a leukemic gibbon and its contact: evidence for partial provirus in the nonleukemic gibbon. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2032–2036. doi: 10.1073/pnas.76.4.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Daniels D. L., Schroeder J. L., Williams B. G., Denniston-Thompson K., Moore D. D., Blattner F. R. Restriction maps for twenty-one Charon vector phages. J Virol. 1980 Jan;33(1):401–410. doi: 10.1128/jvi.33.1.401-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]