Abstract
Neurotrophic factor signaling modulate cellular and behavioral responses to drugs of abuse. Chronic exposure to abused drugs, among other biochemical adaptations, decreases the expression of Insulin Receptor Substrate-2 (IRS-2; a protein involved in neurotrophic signaling) in the ventral tegmental area (VTA), a neural substrate for many drugs of abuse. Using viral-mediated gene transfer to locally alter the activity of IRS-2, we show that overexpression of IRS-2 in the VTA results in an enhanced preference for environments previously paired with cocaine, as measured by the place conditioning paradigm, while blockade of IRS-2 activity results in avoidance of cocaine-paired compartments. In addition, IRS-2 overexpression leads to enhanced cocaine-induced locomotor activity, while blockade of IRS-2 expression significantly blunts behavioral responses to cocaine. These results demonstrate that levels of IRS-2 in the VTA regulate responsiveness to the behavioral effects of cocaine.
Keywords: growth factors, neural plasticity, viral-mediated gene transfer, drug addiction, behavioral sensitization
Neurotrophic factors (NF) and their signaling pathways are best understood for promoting growth, differentiation and survival of neurons during development. More recently, these factors have been implicated as mediators of neuronal maintenance and plasticity in the adult nervous system (Lindsay, Weigand, Altar, & DiStefano, 1994; Lu & Figurov, 1997). Neurotrophins are a family of neurotrophic factors that are highly expressed in brain. Neurotrophins, acting in several brain regions, have been implicated in the modulation of neuronal excitability and synaptic transmission (Poo, 2001; Schuman, 1999), learning and memory (Alonso et al., 2002), mood disorders (Duman, 2002; Nestler et al., 2002; Russo-Neustadt, 2003) and the cellular and behavioral responses to drugs of abuse (Bolaños & Nestler, 2004).
Repeated exposure to drugs of abuse causes long-lasting cellular, molecular, and behavioral adaptations in the mesolimbic dopamine system, adaptations that have been implicated, at least in part, in the transition from drug abuse to addiction (Koob, Sanna, & Bloom, 1998; Nestler, 2001). This neural circuit is comprised of dopaminergic neurons in the ventral tegmental area (VTA) and their target regions in the limbic forebrain such as the nucleus accumbens (NAc), and is a major substrate for motivated behavior and responses to natural and drug reinforcers (Di Chiara & North, 1992; Kelley & Berridge, 2002).
Chronic exposure to morphine, among other adaptations, induces higher levels of tyrosine hydroxylase (TH) in the VTA, impairs the structural integrity of VTA dopamine neurons (Russo et al., 2007; Sklair-Tavron et al., 1996), and alters levels of several NF signaling proteins, including the down-regulation of the Insulin Receptor Substrate 2/phosphatidylinositol-3′-kinase (IRS2–PI3K) signaling pathway in the VTA (Nestler, Berhow, & Brodkin, 1996; Wolf, Numan, Nestler, & Russell, 1999). The functional consequences of changes in IRS-2 levels in the VTA are yet to be fully elucidated; however, we have shown that viral-mediated changes in expression of IRS-2 in the VTA regulates cell morphology and morphine reward (Russo et al., 2007) including responses to emotion-eliciting stimuli (Krishnan et al., 2008). Here we further investigated the influence of IRS2 in the VTA on behavioral responses to drugs of abuse by studying interactions between IRS-2 and cocaine, using locomotor activity and conditioned place preference (CPP) paradigms, after selectively increasing or blocking IRS-2 activity in the VTA by microinjecting a herpes simplex virus (HSV) vector encoding a wild type (HSV-IRS2wt) or a dominant negative mutant form (HSV-RS2dn) of this protein.
Adult male Sprague-Dawley rats (Charles River, Raleigh, NC), weighing 350–375 g at the start of the experiment, were used in this study. All rats were habituated to the animal facility for at least one week before experimental manipulation. Rats were double housed in clear polypropyline boxes containing wood shavings, in an animal colony maintained at 23–25°C on a 12-hr light/dark cycle in which lights were on between 07:00 and 19:00 hr. All rats were provided with food and water ad libitum. Experiments were conducted in compliance with the Guidelines for the Care and Use of Laboratory Animals (National Institute of Health, 1996), and approved by Florida State University animal care and use committee.
Rats were anesthetized with an intramuscular injection of a ketamine/xylazyne cocktail (80/10 mg/kg) and given atropine (0.25 mg/kg) subcutaneously to minimize bronchial secretions; afterward, rats were given unilateral microinjections (2.0 μl over 10 min of either HSV-GFP (control vector expressing green fluorescent protein), HSV-IRS2wt, or HSV-IRS2dn, or Sham surgery (lowered needle to targeted area but no volume injected), into the rostral region of VTA (AP: −4.9, Lat: +2.2, DV: − 7.6 mm below dura) (Paxinos & Watson, 1997) using a 32 gauge Hamilton syringe angled at 10° from the midline, to avoid piercing the sinus system. Construction of the viral vectors has been described previously (Neve, Howe, Hong, & Kalb, 1997). All behavioral experiments were commenced 3 days after viral surgery, a time at which maximal transgene expression is observed (Barrot et al., 2002; Carlezon et al., 1997). No detectable IRS-2 or GFP expression was seen in either efferent (e.g., the NAc) or afferent (e.g., the dorsal raphé) regions of the injected area, nor a week after viral infusion consistent with earlier findings (Barrot et al., 2002). Histological assessment of microinjections and transgene detection was performed as described previously (Russo et al., 2007). Half the rats utilized for these experiments received viral infusion in the left or right side of the VTA. Separate statistical analyses were done to control for side of infusion; no differences were detected. Only needle placements ranging from −4.9 and −5.5 mm from Bregma (i.e., rostral VTA) were included in this study.
Place conditioning was carried out exactly as described (Bolaños et al., 2003). Briefly, a three-compartment apparatus (FSU Psychology engineering group) was used, where compartments differed in floor texture, wall coloring, and lightning. On the preconditioning day (day 0), rats were allowed to freely explore the entire apparatus for 30 min to obtain baseline preference to any of the three compartments (side compartments: 35 × 27 × 25 cm; middle compartment: 10 × 27 × 25 cm). Only rats showing no spontaneous preference to either compartment were used (unbiased procedure); this accounted for more than 90% of all of the animals tested. Rats then received unilateral viral infusions, including Sham surgery, into the rostral VTA, and were allowed to recover for two days. After recovery, conditioning trials (two per day) were given on two consecutive days (day 3 and 4). During the conditioning trials, rats received an intraperitoneal (IP) saline injection (1 ml/kg) and were confined to one of the large size compartments of the apparatus for 1 hr. Then, 3 hr after the first conditioning session, rats received cocaine (10 or 20 mg/kg, IP) and were confined to the opposite side compartment for 1 hr. Conditioning trials were counterbalanced such that half the rats received drug in one compartment and the other half received the drug in the opposite compartment. On the final day (day 5), rats were again allowed to freely explore the entire apparatus for 30 min.
Locomotor activity was measured each day for 2 hr in automated (75 cm diameter × 15 cm wide, 4 photocell beams) circular activity chambers (Med Associates, St. Albans, VT). Rats were exposed to the chambers after one IP saline injection each day for 3 consecutive days and underwent surgery at the end of day 3 (as described above). On day 5, rats received an acute injection of saline to assess whether surgery itself changed baseline locomotion (see Fig. 2A). Rats were then treated with cocaine (5 or 15 mg/kg in 1 ml/kg, IP; Sigma-Aldrich, St. Louis, MO) once daily for 5 consecutive days (starting on day 6). One day after the last cocaine injection (day 11), rats were given an injection of saline to assess whether they were responding to the injection itself or to cocaine, and were rested for one week. At the end of the rest period (day 18), rats were challenged with either 5 or 15 mg/kg cocaine. Statistical significance was measured using mixed-design (between and within variables) analysis of variance (ANOVAs) followed by Tukey post-hoc tests. Data are expressed as the mean ± SEM. Statistical significance was defined as P< 0.05.
Figure 2.
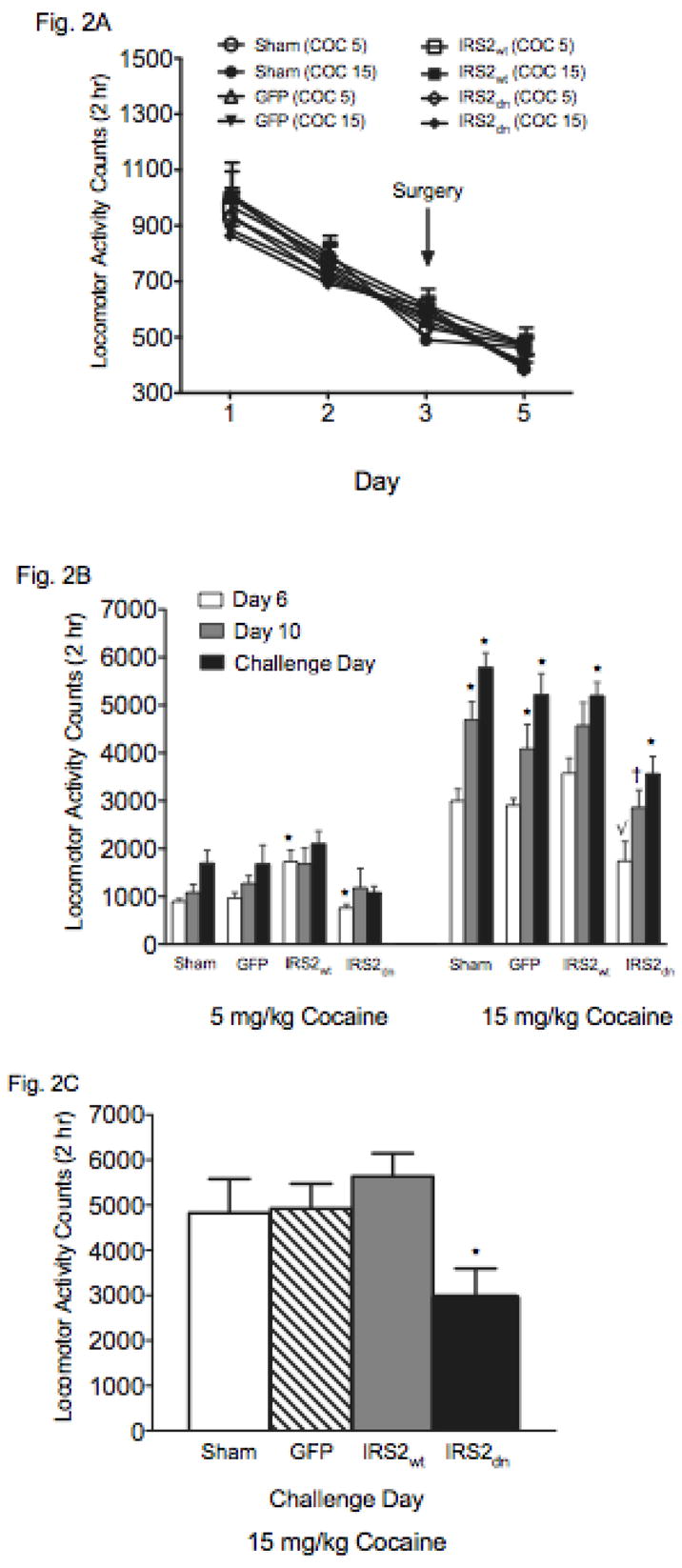
IRS-2 activity in the VTA regulates cocaine-induced locomotion. (A) Baseline locomotor activity for 3 consecutive days prior to and following 2 days after surgery. (B) Data representing all groups on day 6, 10, and 18 (N = 68). Repeated administration of cocaine enhanced behavioral responding in Sham, GFP, and IRS-2 groups (*p< 0.05: compared to day 6). Blockade of IRS-2 activity decreased sensitivity to cocaine (√p< 0.05: compared to Sham, HSV-GFP, and HSV-IRS2wt on day 6; †p= 0.07: compared to day 10). HSV-IRS2dn-treated rats show behavioral sensitization to cocaine on day 18 (*p< 0.05: compared with the group’s responsiveness on day 6 and 10). (C) HSV-IRS2dn significantly blunted behavioral responses to the cocaine challenge on day 18 (*p< 0.05; N = 36)
Figure 1A (left panel) shows the region of the VTA to which Sham surgeries or microinjections of HSV vectors (HSV-GFP, HSV-IRS-2wt, or HSV-IRS-2dn) were aimed. As previously reported, we found that expression of GFP (data not shown) and IRS-2 was maximal between days 3 and 4 after virus injection, significantly declining thereafter, and non-detectable 1 week after the microinjection (Barrot et al., 2002; Carlezon et al., 1997). In addition, no change in IRS-2 immunoreactivity was present in rats receiving Sham or HSV-GFP microinjections (Russo et al., 2007). Confocal microscopy (Fig. 1A; right panel) revealed that the percentage of TH-positive neurons overexpressing IRS-2 in the VTA (~50%) was similar to previous findings (Olson et al., 2005; Russo et al., 2007).
Figure 1.
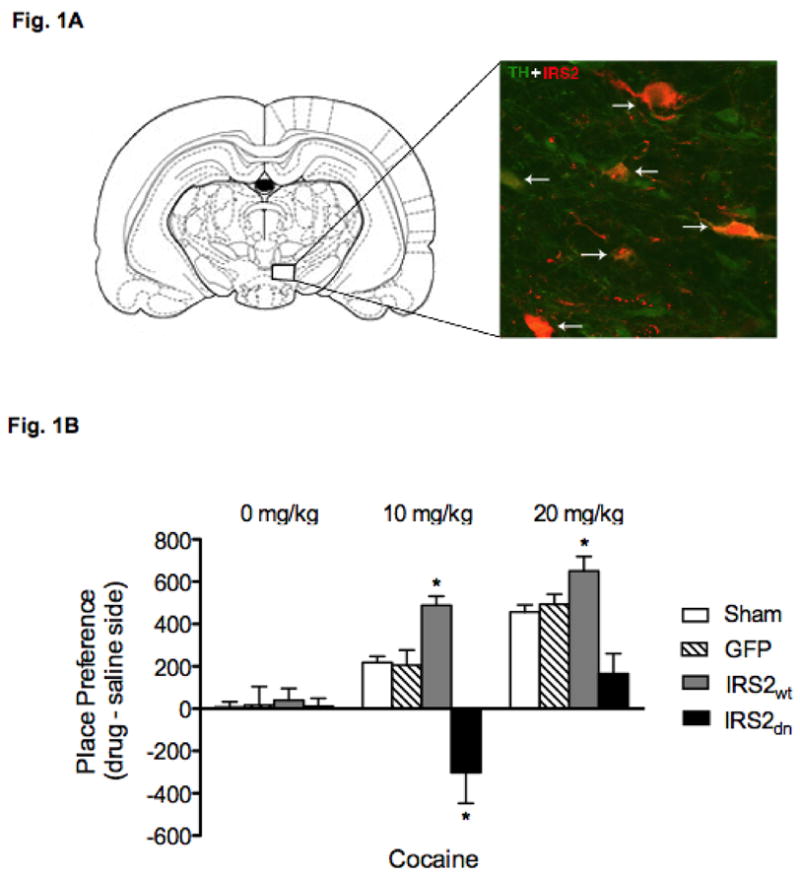
Viral-mediated gene transfer in the VTA. (A) Left panel: Rostral region of the VTA to which microinjections of HSV vectors and Sham surgery were targeted. Right panel: Merged confocal photomicrograph (magnification, 400X) of a representative brain slice from the rostral VTA (~5 mm caudal to Bregma) double-labeled for TH (green: Cy2) and IRS-2 (red: Cy3) fluorescence. Dual-labeled cells represented by orange fluorescence. Arrows indicate colabeled cells. (B): IRS-2 activity in the VTA regulates responses to cocaine CPP (N = 78). HSV-IRS2wtoverexpression enhanced sensitivity to 10 mg/kg of cocaine (*p< 0.05), whereas expression of HSV-IRS2dn resulted in place aversion (*p< 0.05). Sham, HSV–GFP, and HSV–IRS2wt rats treated with 20 mg/kg cocaine showed reliable place conditioning, while the HSV-IRS2dn rats did not show place conditioning or aversion to cocaine.
Figure 1B shows the effects of HSV treatments on a model of drug-seeking behavior, namely the cocaine CPP paradigm. Place preference conditioning has been widely utilized to assess the rewarding or aversive properties of drugs. In this behavioral assay, animals learn to prefer environments previously associated with rewarding, while avoiding environments associated with aversive drug effects (Carlezon, 2003; Hoffman, 1989). As can be seen in Figure 1B, time spent in the cocaine-paired compartments varied by viral treatment and drug dose (virus X drug dose interaction: F(6,66)= 4.40; p< 0.0009). Rats receiving the various viral-vector treatments and conditioned to saline did not show preferences for either side of the CPP compartments. Conversely, rats receiving HSV-IRS2wt microinjections into the VTA spent significantly more time in environments paired with a moderate dose (10 mg/kg) of cocaine (p< 0.05), while rats with HSV-IRS2dn microinjections did not consistently approach the cocaine-paired environments. In fact, microinjecting HSV-IRS2dn into the VTA resulted in avoidance of the cocaine-paired compartments (p< 0.05). The Sham, HSV–GFP, and HSV–IRS2wt groups treated with 20 mg/kg cocaine showed robust place conditioning, while IRS2dn expressing rats did not show reliable conditioning to the cocaine-paired compartments.
We next assessed the effect of IRS-2 manipulation on locomotor activity using a behavioral sensitization paradigm. Figure 2A shows that baseline locomotion was similar between the groups before and 2 days after surgery, thus indicating that surgery alone, or the virus itself, did not affect spontaneous locomotor activity. Data presented in Figure 2B compares behavioral responding to 5 or 15 mg/kg cocaine on day 6 (first cocaine exposure), day 10 (last day of cocaine before rest period) and day 18 (challenge day) in all groups. These rats also received a saline injection on day 11 to assess for potential conditioned locomotion effects. No differences in behavioral responding to saline in any of the groups were revealed (data not shown). A three-factor ANOVA yielded significant main effects of viral treatment (F(3,180)= 17.9; p< 0.0001), drug (F(1,180)= 309.4; p< 0.0001), and day (F(2,238)= 27.9; p< 0.0001). Behavioral responses to cocaine also varied as a function of viral treatment and drug (virus X drug dose interaction: F(3,180)= 5.7; p< 0.0009), and day (drug dose X day interaction: F(3,180)= 9.8; p< 0.0001). Post hoc analyses revealed significant differences for 5 mg/kg cocaine dose on day 6, indicating that behavioral responses were significantly higher for the HSV-IRS2wt-treated, and lower for the HSV-IRS2dn-tretaed rats (p< 0.05). As expected for the higher cocaine dose, analysis indicated that the Sham and HSV-GFP groups had enhanced locomotor responding on days 10 and 18 when compared to day 6 (p< 0.05), indicating the development and expression of locomotor sensitization, respectively. Further analysis revealed that rats receiving HSV-IRS2wt had greater overall behavioral responding to cocaine than the Sham, HSV-GFP, and HSV-IRS2dn groups on day 6, findings which indicate that overexpression of IRS2wt increases behavioral sensitivity to acute cocaine. In addition, these rats showed enhanced sensitivity to the stimulant on days 10, and 18, as compared to their behavioral responding on day 6 (p< 0.05), thus indicating that overexpression of IRS2wt in the VTA results in enhanced behavioral responding. However, the magnitude of their behavioral responding did not differ from that observed in the Sham and HSV-GFP groups on day 18. Moreover, no significant differences in behavioral responding were evident in the HSV-IRS2wt group between day 10 and 18. Conversely, a different behavioral profile was observed in rats microinjected with IRS2dn (Fig. 2B). For instance, no significant differences were apparent amongst IRS2dn expressing rats when their behavioral responding was compared on days 6 and 10 (p> 0.05). In fact, these rats showed significantly blunted responses to cocaine when compared to the other groups on days 6 and 10. However, a significant increase in locomotor activity was observed in these animals on day 18 (p< 0.05), although their behavioral responding to the cocaine challenge injection was still significantly lower than that observed in the other treatment groups (Fig. 2B).
To better understand the role IRS-2 plays in mediating enhanced behavioral responding to cocaine, we conducted a third experiment (Figure 2C) in which rats received the drug regimen as described above. Briefly, rats were placed in the locomotor chambers after receiving a saline injection for 5 days. Starting on day 6, rats received one daily cocaine injection (15 mg/kg) for 5 consecutive days and were placed in the locomotor chambers. After the last cocaine injection, rats were rested for a week. On day 15, rats were anesthetized and received viral infusions as described above. Only rats showing enhanced behavioral responding to cocaine on the last day of cocaine treatment (day 10) were assigned to the various viral conditions. Three days after surgery (day 18) rats were challenged with 15 mg/kg cocaine. Figure 2C shows that rats treated with HSV-IRSdn had a diminished response to cocaine, whereas the other groups showed the expected behavioral responding to the cocaine challenge (F(3,32) = 10.39; p< 0.0001). Further tests confirmed that blockade of IRS2 activity significantly blunted the cocaine-induced behavioral effects (HSV-IRS2dn versus HSV-IRS2wt, p = 0.007; HSV-IRS2dn versus HSV-GFP, p = 0.0009).
Previous reports have implicated neurotrophins in the cellular and behavioral adaptations occurring in the VTA after prolonged exposure to drugs of abuse (see Introduction). In the present study, we increased or decreased levels of IRS-2, a protein known to mediate and amplify PI3K activity (Fisher & White, 2004), in the VTA by using viral-mediated gene transfer to locally increase or block IRS-2 activity levels in this brain region. We demonstrate here that increased levels of IRS-2 in the VTA leads to enhanced reward and locomotor sensitivity to cocaine, while decreasing the levels of this protein caused opposite effects. Together, these data suggest that IRS-2 activity in the VTA regulates behavioral responsiveness to cocaine.
Enhancing IRS-2 activity resulted in increased sensitivity to the rewarding effects of cocaine because rats treated with HSV-IRS2wt reliably approached environments previously paired with doses of cocaine that did not induce place conditioning in control rats. In contrast, HSV-IRS2dn–treated rats avoided environments paired with a threshold dose of cocaine, while showing no reliable preference or avoidance to environments paired with the higher dose of cocaine. These findings are in agreement with demonstrations that molecular manipulations that reduce the rewarding effects of cocaine often lead to cocaine-induced place aversions (Barot, Ferguson, & Neumaier, 2007; Carlezon et al., 1997; Olson et al., 2005). The biphasic properties of cocaine are well documented, and it is possible that the aversion is caused by a decrement in cocaine’s rewarding effects (i.e., tolerance to the rewarding effects of the drug), which, in turn, unmasks other aversive effects of the drug (Ettenberg, 2004; Koob & Le Moal, 2008; Solomon & Corbit, 1974). A parallel pattern of behavioral reactivity was observed when the effects of IRS-2 activity levels on locomotor activity were assessed. Increasing IRS-2 activity lead to an enhancement in the locomotor-activating effects induced by acute and repeated cocaine exposure, while blocking IRS-2 activity blocked the behavioral responding to cocaine. However, HSV-IRS2dn-treated rats still showed a modest, though significant, increase in locomotor activity, on the challenge day. Given that no viral expression can be detected a week after virus infusion (Barrot et al., 2002; Carlezon et al., 1997), these data suggest that IRS-2 activity may be important for the expression of drug-induced behavioral sensitization (Izzo, Martin-Fardon, Koob, Weiss, & Sanna, 2002; Russo et al., 2007). This is likely the case because rats previously sensitized to the drug and treated with HSV-IRS2dn three days before the drug challenge showed a significantly blunted response to cocaine.
The effects of manipulating IRS-2 expression in the VTA on measures of cocaine-induced reward and locomotor reactivity are in agreement with previous evidence demonstrating PI3K activity as necessary for enhancement of opiate and natural reward (Krishnan et al., 2007; Russo et al., 2007), as well as for the expression of behavioral sensitization to cocaine (Izzo et al., 2002). The mechanism(s) underlying these effects are not fully known. The initiation of behavioral sensitization occurs at the level of the VTA, while its expression is mediated at the level of the NAc (Pierce & Kalivas, 1997). In the Izzo et al study (2002), rats received intracerebroventricular infusions of the PI3K inhibitor LY294002 and, thus, it is conceivable that the inhibitor was acting in any of numerous brain regions (e.g., NAc) to block the expression of behavioral sensitization, while in the present study, we specifically targeted rostral portions of the VTA to regulate IRS-2 activity. Given that topographical differences within the VTA have been shown to mediate differential responding to the rewarding and locomotor-activating properties of drugs (Bolaños, Neve, & Nestler, 2005; Bolaños et al., 2003; Carlezon et al., 2000; Ikemoto, Kohl, & McBride, 1997; Olson et al., 2005), it is conceivable that distinct populations of dopamine neurons within the VTA might mediate the effects observed in this study (Carr &Sesack, 2000; Di Chiara, 1997; Ikemoto, 2007; Olson & Nestler, 2007). Though anatomical characterization of the VTA is not yet complete, this assumption is consistent with findings suggesting that dopamine neurons from rostral portions of the VTA innervate primarily, but not exclusively, the NAc shell (i.e., mesolimbic), while dopamine neurons from more caudal VTA regions project predominantly, but not exclusively, to cortical areas (i.e., mesocortical regions) (Emson & Koob, 1978). These neural projections also show differential responding to drugs of abuse by increasing extracellular dopamine levels in the NAc shell as compared to prefrontal cortical areas (Bassareo, Tanda, Petromilli, Giua, & Di Chiara, 1996). Thus, it is conceivable, within this framework, that increasing IRS-2 activity within dopamine neurons in rostral VTA increases sensitivity to cocaine, resulting in increased reward and locomotor responding, while resulting in opposite effects when IRS-2 activity is blocked in this brain region.
Together, these results are in agreement with studies showing that other NF signaling pathways (e.g., MAP/ERK, bFGF, PI-3-K, PLCγ1, Akt) also regulate behavioral responding to drugs of abuse (Bolaños & Nestler, 2004; Pierce & Bari, 2001). Results from the present study further establish the functional importance of drug-induced regulation of IRS-2 expression in the VTA. Further assessment of the mechanisms underlying this IRS2-induced behavioral effects will lead to a better understanding of the neural and molecular basis of drug addiction.
Acknowledgments
This work was supported by grants R03 DA020089 (CAB) and R01 DA014133 (EJN) from the National Institute on Drug Abuse (NIDA), a NARSAD Young Investigator Award, and FYAP and CRC grants from Florida State University to CAB. Sergio D. Iñiguez was supported by a McKnight Neuroscience Fellowship from the State of Florida, and a Neuroscience Fellowship from Florida State University.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/bne/
References
- Alonso M, Vianna MR, Depino AM, Mello e Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I, Medina JH. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12(4):551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- Barot SK, Ferguson SM, Neumaier JF. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur J Neurosci. 2007;25(10):3125–3131. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99(17):11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Tanda G, Petromilli P, Giua C, Di Chiara G. Non-psychostimulant drugs of abuse and anxiogenic drugs activate with differential selectivity dopamine transmission in the nucleus accumbens and in the medial prefrontal cortex of the rat. Psychopharmacology (Berl) 1996;124(4):293–299. doi: 10.1007/BF02247433. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5(1):69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Neve RL, Nestler EJ. Phospholipase C gamma in distinct regions of the ventral tegmental area differentially regulates morphine-induced locomotor activity. Synapse. 2005;56(3):166–169. doi: 10.1002/syn.20136. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Perrotti LI, Edwards S, Eisch AJ, Barrot M, Olson VG, Russell DS, Neve RL, Nestler EJ. Phospholipase Cgamma in distinct regions of the ventral tegmental area differentially modulates mood-related behaviors. J Neurosci. 2003;23(20):7569–7576. doi: 10.1523/JNEUROSCI.23-20-07569.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA., Jr Place conditioning to study drug reward and aversion. Methods Mol Med. 2003;84:243–249. doi: 10.1385/1-59259-379-8:243. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, Nestler EJ. Sensitization to morphine induced by viral-mediated gene transfer. Science. 1997;277(5327):812–814. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, Nestler EJ. Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci. 2000;20(5):RC62. doi: 10.1523/JNEUROSCI.20-05-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20(10):3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Cortical and limbic dopamine (on opiate addiction): do not mix before use! Trends Pharmacol Sci. 1997;18(3):77–78. [PubMed] [Google Scholar]
- Di Chiara G, North RA. Neurobiology of opiate abuse. Trends Pharmacol Sci. 1992;13(5):185–193. doi: 10.1016/0165-6147(92)90062-b. [DOI] [PubMed] [Google Scholar]
- Duman RS. Synaptic plasticity and mood disorders. Mol Psychiatry. 2002;7(Suppl 1):S29–34. doi: 10.1038/sj.mp.4001016. [DOI] [PubMed] [Google Scholar]
- Emson PC, Koob GF. The origin and distribution of dopamine-containing afferents to the rat frontal cortex. Brain Res. 1978;142(2):249–267. doi: 10.1016/0006-8993(78)90634-0. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27(8):721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Fisher TL, White MF. Signaling pathways: the benefits of good communication. Curr Biol. 2004;14(23):R1005–1007. doi: 10.1016/j.cub.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Hoffman DC. The use of place conditioning in studying the neuropharmacology of drug reinforcement. Brain Res Bull. 1989;23(4–5):373–387. doi: 10.1016/0361-9230(89)90224-4. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Kohl RR, McBride WJ. GABA(A) receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem. 1997;69(1):137–143. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- Izzo E, Martin-Fardon R, Koob GF, Weiss F, Sanna PP. Neural plasticity and addiction: PI3-kinase and cocaine behavioral sensitization. Nat Neurosci. 2002;5(12):1263–1264. doi: 10.1038/nn977. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21(3):467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Weigand SJ, Altar CA, DiStefano PS. Neurotrophic factors: from molecules to man. Trends in Neuroscience. 1994;17:182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Lu B, Figurov A. Role of neurotrophins in synapse development and plasticity. Rev Neurosci. 1997;22:295–318. doi: 10.1515/revneuro.1997.8.1.1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2(2):119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Berhow MT, Brodkin ES. Molecular mechanisms of drug addiction: adaptations in signal transduction pathways. Mol Psychiatry. 1996;1(3):190–199. [PubMed] [Google Scholar]
- Neve RL, Howe JR, Hong S, Kalb RG. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience. 1997;79(2):435–447. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- Olson VG, Nestler EJ. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse. 2007;61(2):87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25(23):5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. San Diego: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci. 2001;12(2):95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25(2):192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10(1):93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A. Brain-derived neurotrophic factor, behavior, and new directions for the treatment of mental disorders. Semin Clin Neuropsychiatry. 2003;8(2):109–118. doi: 10.1053/scnp.2003.50014. [DOI] [PubMed] [Google Scholar]
- Schuman EM. Neurotrophin regulation of synaptic transmission. Curr Opin Neurobiol. 1999;9(1):105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93(20):11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81(2):119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Wolf DH, Numan S, Nestler EJ, Russell DS. Regulation of phospholipase Cgamma in the mesolimbic dopamine system by chronic morphine administration. J Neurochem. 1999;73(4):1520–1528. doi: 10.1046/j.1471-4159.1999.0731520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


