Summary
Host parasitism by Trichomonas vaginalis is complex and in part mediated by adherence to vaginal epithelial cells (VECs). Four trichomonad surface proteins bind VECs as adhesins, and AP65 is a major adhesin with sequence identity to an enzyme of the hydrogenosome organelle that is involved in energy generation. In order to perform genetic analysis and assess the role of AP65 in T. vaginalis adherence, we silenced expression of ap65 using antisense RNA. The gene for ap65 was inserted into the vector pBS-neo in sense and antisense orientations to generate plasmids pBS-neoS (S) and pBS-neoAS (AS), respectively. Trichomonads were then transfected with S and AS plasmids for selection of stable transfectants using Geneticin, and the presence of plasmid in transfectants was confirmed by polymerase chain reaction of the neo gene. Reverse transcription polymerase chain reaction and Northern blot analysis showed decreased amounts of ap65 transcript in AS transfected parasites. Growth kinetics of the antisense-transfected and wild type organisms were similar, suggesting that silencing AP65 did not affect overall energy generation for growth. Immunoblot analysis using monoclonal antibody (mAb) to AP65 of AS transfectants showed decreased amounts of AP65 when compared to wild type or S transfectants. Not unexpectedly, this corresponded to decreased amounts of AP65 bound to VECs in a functional ligand assay. Reduction in parasite surface expression of AP65 was related to lower levels of adherence to VECs by AS-transfectants compared to control organisms. Antisense silencing of ap65 was not alleviated by growth of trichomonads in high iron, which up-regulates transcription of ap65. Our work reaffirms the role for AP65 as an adhesin, and in addition, we demonstrate antisense RNA gene silencing in T. vaginalis to study the contribution of specific genes in pathogenesis.
Introduction
Trichomonas vaginalis is an ancient protist without any canonical mitochondrial processes. In addition to glycolysis for core energy metabolism, the organism has a double membrane-bound organelle for decarboxylation of pyruvate for energy generation (Müller, 1993; Kulda, 1999). Trichomonosis (Kassai et al., 1988) is caused by T. vaginalis, the number one, non-viral sexually transmitted disease (STD). There are ∼8 million new T. vaginalis infections in the USA and 250-350 million worldwide (Cates, 1999; WHO, 2001; Weinstock et al., 2004). This sexually transmitted infection (STI) has consequences to women’s health, including adverse pregnancy outcomes (Cotch et al., 1991; 1997), predisposition to cervical cancer (Zhang and Begg, 1994; Yap et al., 1995; Zhang et al., 1995; Viikki et al., 2000) and increased susceptibility to HIV/AIDS (Wasserheit, 1992; Laga et al., 1993; Sorvillo and Kerndt, 1998; Sorvillo et al., 2001). Among other sequelae from trichomonosis are orchitis associated with oligoasthenoteratospermia and hypogonadism (Lloyd et al., 2003), newborn urinary tract infections with chronic lung disease (Hoffman et al., 2003), and lung coinfection by T. vaginalis and pneumocystis in a patient with AIDS (Dubougher et al., 2003). Men with trichomonosis may have a non-chlamydial, non-gonococcal urethritis (Kreiger et al., 1993), and symptomatic men with trichomonosis who are HIV-positive have higher concentrations of infectious HIV in semen, facilitating HIV transmission (Hobbs et al., 1999). This STI is a health disparities disease. The higher HIV rates of infection among African-Americans are in part because of the fact that trichomonosis expands the portal of exit in HIV-positive patients and expands the portal of entry for HIV-negative patients (Sorvillo et al., 2001). It is estimated that 24% of HIV infections are directly attributable to T. vaginalis infections. Therefore, control of this STD may be one of the most effective means for managing the HIV transmission risk worldwide.
Successful infection occurs despite immune surveillance and a complex host environment that is always changing. While host parasitism is complex and multifaceted, one key step in infection is the adherence of T. vaginalis to vaginal epithelial cells (VECs) (Alderete et al., 1988). Four surface proteins (AP65, AP51, AP33 and AP23) interact with the host cells via ligand-receptor interactions (Alderete and Garza, 1985; Arroyo et al., 1992; Garcia et al., 2003). There is a direct relationship between the level of cytoadherence, surface expression of adhesins (Arroyo et al., 1992), and binding of the adhesins to immortalized VECs (Garcia et al., 2003). AP65 appears to be a major adhesin and, as with AP51 and AP33, ap65 is a member of a multigene family (Alderete et al., 1988; O’Brien et al., 1996; Engbring and Alderete, 1998b). Interestingly, AP65 has sequence identity to the hydrogenosome decarboxylating malic enzyme (Alderete et al., 1995; 1998; Hrdý and Müller, 1995; O’Brien et al., 1996). Growth of trichomonads in a high iron medium is prerequisite for compartmentalization of the protein outside the hydrogenosome, and contact of parasites with immortalized VECs signals for morphologic transformation of parasites (Arroyo et al., 1993) concomitant with surface placement of adhesins (Garcia et al., 2003). We wanted to continue characterizing the structure-function properties of AP65 given its prominent role in VEC adherence. The development of stable transfection in other protozoan models permits for the use of genetic approaches to control expression of virulence genes (Zhang and Matlashewski, 1997; Ankri et al., 1999). Given the multigene copy nature of ap65 and the other adhesins, silencing of ap65 expression through antisense transfection was attempted over targeted gene replacement (Land et al., 2004). Using the antisense technology, we reduced the expression of ap65 in live, highly motile T. vaginalis organisms. Episomal expression of antisense mRNA decreased AP65 expression and gave lower adherence levels to VECs than S transfected parasites. The results presented in this report reaffirm a role for AP65 in adherence of the parasite to VECs.
Results
Antisense inhibition of ap65 expression
Given the multicopy nature of ap65 in T. vaginalis, we used antisense RNA to silence the expression of AP65, a prominent adhesin for VEC adherence. We hypothesized that decreased amounts of ap65 transcript lead to altered parasite adherence to immortalized VECs. The successful episomal expression of marker genes in T. vaginalis has been reported earlier by others (Delgadillo et al., 1997). Thus, we constructed plasmids containing the neo and DNA fragments representing the putative coding region of ap65-3, which were inserted in the sense (pBS-neoS) or antisense (pBS-neoAS) orientations, as shown in Fig. 1A. After electroporation of plasmids, trichomonads growing in 100 μg ml-1 Geneticin were obtained. Drug-resistant organisms from both pBS-neoS (S) and pBS-neoAS (AS) plasmid transfections were cloned in soft agar containing 100 μg ml-1 Geneticin. A representative S and AS transfected cloned population was characterized further. The DNA from the parental T. vaginalis T016 isolate and from the S and AS trichomonads were used as templates in a polymerase chain reaction (PCR) reaction to amplify the 795-bp putative coding region of neo. Not unexpectedly, Fig. 1B shows the PCR product detected in the S and AS transfectants but not in the wild type (Wt) parental organisms, confirming the presence of plasmids in the cloned drug-resistant parasites.
Fig. 1.
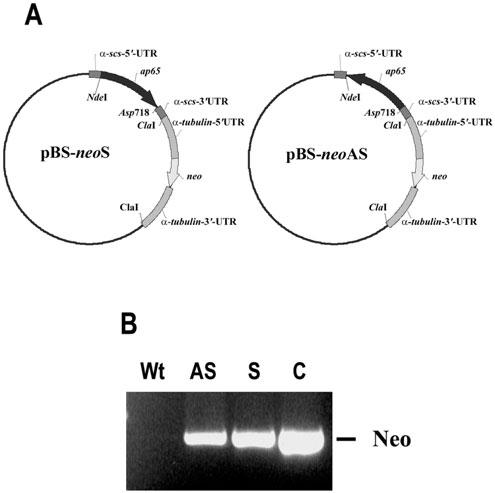
Constructs for expression of ap65 sense (S) and antisense (AS) RNA (A) and PCR amplification of the neo coding region in transfected parasites (B) to demonstrate the presence of plasmids. Part A shows the individual plasmids with the ap65-3 gene in the sense (left plasmid) vs. the antisense (right) orientation. The parent plasmid pBS-FDHAHA-2neo with the 5′- and 3′-α-succinyl coA synthetase untranslated region (UTR) and the neomycin (neo) gene flanked by the 5′- and 3′-α-tubulin UTR was engineered to carry the ap65-3 open reading frame in the S and AS orientations using the NdeI and ASP718 restriction sites. A description of the origin of the plasmid is described in Experimental procedures. Part B presents results of a PCR reaction to amplify the 795-bp neo gene from DNA derived from trichomonads transfected by electroporation with the respective plasmids (lanes labelled AS and S) and compared to the PCR product from the plasmid alone as control (lane C). Total genomic DNA was used as template for the PCR reactions using S and AS primers specific to the 795-bp neo coding region. The lane labelled Wt is of control wild type parasites to confirm the absence of plasmid or cross-hybridizing DNA.
Episomal expression of antisense ap65 mRNA modulates ap65 transcript levels
We next compared steady-state levels of mRNA of the Wt parental isolate trichomonads to transcript levels of the S and AS transfectant parasites. Northern analysis was performed in total RNA hybridized with a DIG-labelled ap65 specific probe. Fig. 2A shows a reduction of up to 62% of ap65 mRNA in AS parasites when compared to levels of transcript for Wt organisms. Further, AS transfected parasites had no detectable ap65 antisense transcript by Northern analysis. Parasites from S transfectants did not show any decrease in levels of endogenous ap65 transcript and had one larger hybridizing band representing the ap65 transcript derived from the S plasmid. The slightly higher intensity of ap65 transcript in the sense transfectant is the result of slightly higher amounts of total DNA added to the well, as evidenced by the increased amount of rRNA.
Fig. 2.
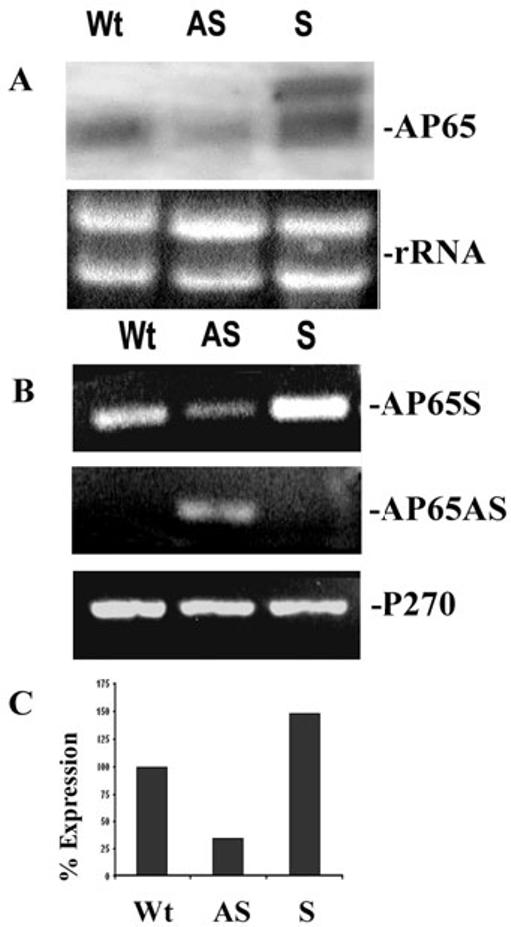
Levels of ap65 mRNA in antisense (AS) transfected trichomonads is less than transcript in the sense (S) and wild type (Wt) organisms.
A. Northern analysis was performed to detect levels of ap65 mRNA. The larger band in the S transfectants is of the ap65 transcript derived from the S plasmid. Using the Scion image β program and densitometric scanning of the bands indicates a 62% reduction of transcript in the AS transfectants. The rRNA bands are included as controls to show equivalent amounts of RNA added to the blots; however, the slightly increased intensity of the S ap65 transcript is the result of slightly higher amounts of sample added to the well. In this experiment, 10 μg total RNA in each lane was electrophoresed in 1.2% agarose-formaldehyde gels and blotted onto Hybond-P membranes. The blot was probed with DIG-labelled ap65 that hybridized to an approximately 2-kb endogenous ap65 transcript.
B. A representative experiment showing RT-PCR products for the ap65 sense transcript (AP65S) and the episomally expressed antisense transcript (AP65AS). RT-PCR was also performed using primers to amplify a 300-bp region of the p270 gene (P270) as a control. Equal volumes of the PCR reactions were separated on 1% agarose followed by staining with ethidium bromide.
C. Quantitation of amounts of PCR products in part A for the ap65 transcript in AS transfected parasites compared to S transfected and Wt organisms. The bar graph shows the relative amounts of the RT-PCR products for ap65. The amount of Wt ap65 transcript was normalized to 100%. As for Part A, quantitation was done using the Scion image β program and densitometric scanning.
The results of a reverse transcription polymerase chain reaction (RT-PCR) from a representative experiment performed on total RNA isolated from S, AS and Wt T. vaginalis parasites also show a relationship between antisense RNA and amounts of transcribed ap65 (Fig. 2B, top). The primers used amplify 631-bp of the ap65 coding region. There was less PCR product in the reaction with AS trichomonads (top). Furthermore, as no antisense was detected by Northern blots (Fig. 2A), we performed RT-PCR using primers designed specific to the antisense mRNA. Not surprisingly, only the AS transfectants yielded a product from RT-PCR using primers (middle panel) to amplify 488-bp of the antisense transcript. As a control to show equal amounts of RNA, PCR reactions were performed with primers specific to p270 (bottom panel), a prominent immunogen that is constitutively expressed in T. vaginalis under these experimental conditions (Dailey and Alderete, 1991). Finally, parasites expressing ap65 antisense mRNA decreased the amounts of endogenous ap65 transcript by 66% (Fig. 2C) when compared to the intensity of mRNA band of Wt control organisms, which was normalized at 100%. Quantitation of the RT-PCR products was by the Scion image β program. These combined Northern blot and RT-PCR data demonstrate that antisense mRNA expression indeed downregulated the amounts of endogenous ap65 mRNA.
Decreased amounts of total AP65 and AP65 bound to VECs follow ap65 antisense mRNA expression
We wanted to confirm that diminished levels of ap65 transcript led to lower amounts of the adhesin. Further, it was important to assess the binding of AP65 to immortalized VECs by the ligand assay. An immunoblot was performed using the monoclonal antibody (mAb) DM116 to AP65 (Garcia et al., 2003) on total proteins. As can be seen from Fig. 3A, only lanes with total proteins of Wt and S organisms had readily detectable AP65. In contrast, the mAb F5 to adhesin AP33 shows equal amounts of the protein for each of the samples. The AP33 serves as an internal control to show equivalent parasite numbers added to each lane. Equally noteworthy and not surprisingly, immunoblots with mAb DM116 of proteins bound to immortalized VECs after the ligand assay gave only a weak band for AP65 from the AS parasites when compared to Wt and S trichomonads. Densitometric scanning as above indicated that the AS organisms had an 89% decrease in amount of AP65 bound to host cells (Fig. 3B). These results show that antisense inhibition efficiently downregulates endogenous AP65 expression, which is proportional to the amount of AP65 bound to HeLa cells as determined by the ligand assay. Finally, as indicated in Experimental procedures, AS transfected trichomonads were grown in the absence of G418 to attempt to remove the plasmid and to re-express AP65. In data not shown we indeed saw increased amounts of transcript by RT-PCR and correspondingly elevated amounts of AP65 by immunoblot with mAb DM116 when compared with the AS parasites with drug pressure.
Fig. 3.
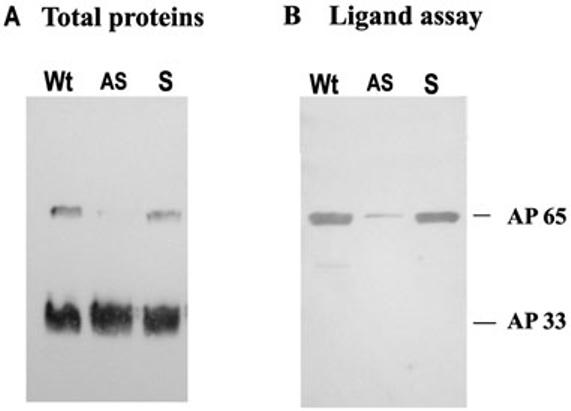
Immunoblots with specific mAb DM116 detecting the amount of AP65 in total protein preparations (A) and bound to HeLa cells in a ligand assay (B) from equal numbers of S and AS transfected trichomonads compared to control Wt organisms.
A. Total proteins from 107 trichomonads were subjected to SDS-PAGE on 10% acrylamide before blotting onto Hybond-P membranes. As a control to show equivalent protein amounts in each lane, the blot was also probed with mAb F5 to the AP33 adhesin (Engbring and Alderete, 1998a; 1998b).
B. AP65 bound to HeLa cells were solubilized and electrophoresed for blotting and probing with mAb DM116 as in part A. Total proteins and the ligand assay were as described in Experimental procedures.
Decreasing AP65 has no effect on the growth of parasites
We measured the activity of malic enzyme, and not unexpectedly, AS trichomonads had 11.8% decarboxylase activity when compared to Wt parasites. As such, it was important to examine whether decreased amounts of AP65 and therefore energy metabolism adversely affected the parasite thereby influencing the property of adherence. We examined growth kinetics as a property influenced by ATP synthesis. Fig. 4 illustrates the growth curves for the S, AS and Wt parasites. There was no readily discernable difference in the growth rates and cell densities between S, AS and Wt organisms. The S, AS and Wt trichomonads remained highly motile throughout all of these growth studies. Thus, the absence of any change in the growth kinetics of AS parasites in comparison to S and Wt trichomonads suggests that the reduced AP65 expression in AS transfected parasites does not alter the energy availability essential for demonstrable growth and maintenance of parasite integrity under these conditions of batch culture.
Fig. 4.
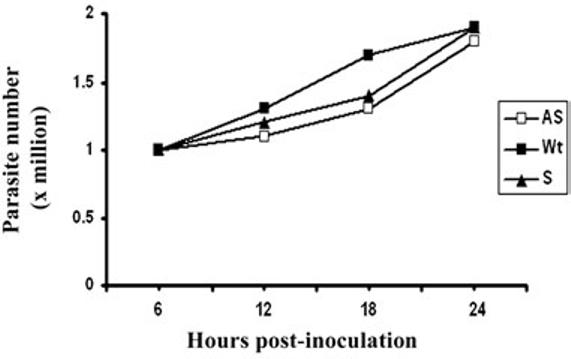
Growth kinetics of wild type and transfected trichomonads. The starting density was 105 organisms. Numbers of parasites were enumerated using a Neubauer hemocytometer at the different time points. The results from four different growth experiments were averaged, and cell numbers did not differ by more than 5% of the values given.
Expression of antisense reduces surface AP65
We have available mAbs that react by immunofluorescence with AP65 on the surface of non-permeabilized trichomonads (mAb 12G4) and with AP65 within hydrogenosomes (mAb F11) (Garcia et al., 2003). These mAbs to AP65 were recently characterized and are to different epitopes (Garcia et al., 2003). In Fig. 5 the mAb 12G4 was strongly reactive with surface AP65 both in Wt and S parasites (A and C) compared to the weak intensity of fluorescence in AS organisms (B). Similarly, with permeabilized parasites, F11 gave weaker fluorescence in AS transfected organisms (panel E) compared to S transfected and Wt trichomonads (panels D and F). This result corresponds with the earlier data on the decreased amounts of total AP65 in AS trichomonads.
Fig. 5.
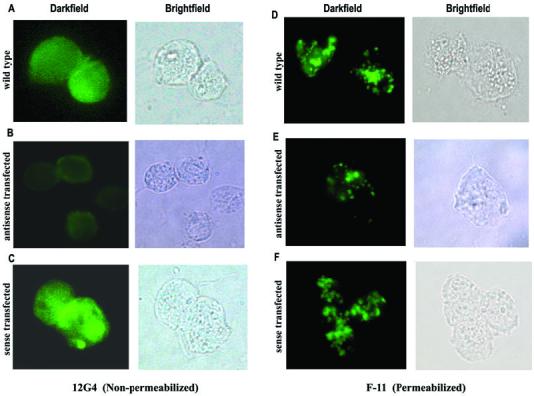
Immunofluorescence and corresponding brightfield microscopy showing decreased surface (non-permeabilized) and non-surface (permeabilized) AP65 in antisense transfected (B and E) compared to wild type (A and D) and sense transfected (C and F) trichomonads. The fluorescence patterns seen in permeabilized organisms are expected and represent the protein within the hydrogenosome organelles.
The AP65 plays a role in cytoadherence
We felt that using this genetic approach of gene silencing, we now were positioned to examine for a function of AP65 as an adhesin. In a representative experiment using quadruplicate samples shown in Fig. 6, the level of adherence to immortalized VECs was reduced by 50% for parasites expressing AS compared to Wt and S organisms. This extent of decreased attachment to VECs was reproducible on at least three separate occasions under identical conditions. This per cent decrease is consistent with earlier reports on the inhibition of adherence using specific polyclonal anti-AP65 serum IgG, and lack of complete abolishment of adherence is likely the result of the role of the other adhesins interacting with VECs (Arroyo et al., 1992; Garcia et al., 2003).
Fig. 6.
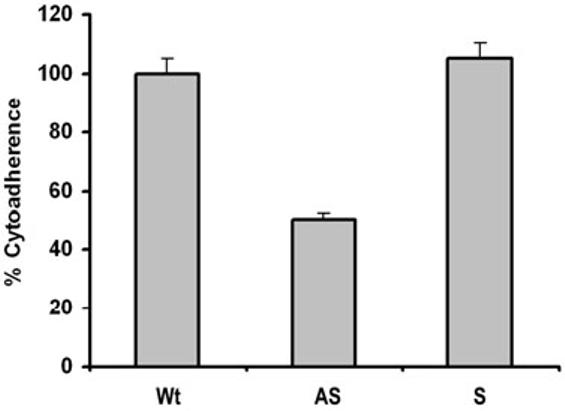
Antisense (AS) transfected T. vaginalis organisms have lower levels of adherence to immortalized MS-74 vaginal epithelial cells compared to sense transfected (S) and wild type (Wt) trichomonads. The extent of adherence by Wt parasites was normalized to 100% for comparative purposes, as before (Arroyo et al., 1992; Garcia et al., 2003). The results are the average and standard deviations from four different experiments. Each experiment was performed with quadruplicate samples.
From the earlier studies we know that iron regulates the amounts of adhesins and the corresponding levels of adherence of T. vaginalis (Lehker et al., 1991; Garcia et al., 2003). We then tested whether antisense inhibition of AP65 synthesis was reversible by growing parasites in iron-replete medium when compared to iron-depleted medium. Fig. 7 illustrates the expected low levels of adherence of the low-iron-grown Wt and S trichomonads. Not surprisingly based on past work (Lehker et al., 1991; Arroyo et al., 1993), levels of adherence were elevated when iron-depleted organisms were cultivated overnight in a iron-replete medium. Interestingly, the AS parasites in iron-replete medium did not increase levels of adherence to those of Wt, suggesting that antisense is efficiently inhibiting ap65 gene translation, and this was confirmed by immunoblots (Fig. 3). The level of adherence of AS organisms in iron-replete medium was similar to that seen when AS parasites were grown in normal TYM-serum medium. Interestingly, AS trichomonads in iron-depleted medium had additional decreased levels of adherence, reinforcing a role for the other adhesins and the fact that low iron downregulates synthesis of all adhesins (Lehker et al., 1991; Garcia et al., 2003).
Fig. 7.
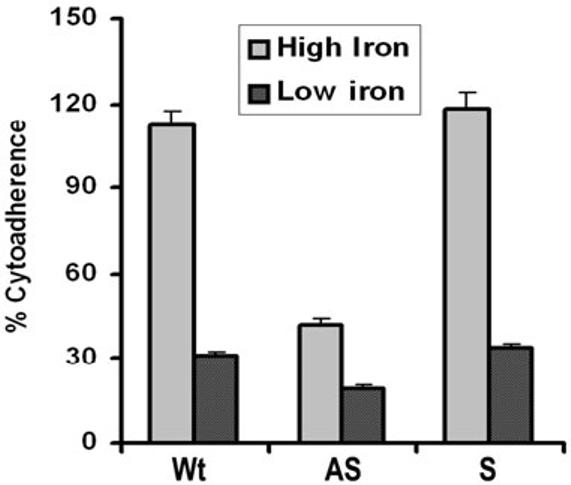
High iron does not restore levels of adherence in antisense transfected T. vaginalis organisms to those seen for Wt and S transfected trichomonads. The extent of adherence by Wt parasites grown in iron-replete medium was normalized to 100% for comparative purposes. The results are the average and standard deviations from four different experiments, and each experiment was performed with quadruplicate samples.
Discussion
Recent work by us reaffirmed the importance of AP65 in adherence to VECs during host infection (Garcia et al., 2003). In this paper we describe the use of antisense transfection for silencing the expression of ap65 to provide genetic evidence for the role of AP65 in adherence. We felt this was necessary because the adhesin has sequence identity to decarboxylating malic enzyme found in the double membrane-bound hydrogenosome, the organelle where the oxidative decarboxylation of pyruvate takes place for energy generation (Müller, 1993; Hrdý and Müller, 1995; Kulda, 1999). Importantly, we wanted to determine if the antisense approach can be used as an alternative to gene replacement through homologous recombination, especially in the case where putative virulence factors are members of multigene families. This is the case for the adhesin genes where there exist six ap65, three ap51 and three ap33 genes in the trichomonad genome (Alderete et al., 1995; 1998; O’Brien et al., 1996; Engbring and Alderete, 1998b). We now report on the successful use of antisense for gene silencing of T. vaginalis, and the data support earlier experimental evidence that AP65 has functional diversity as an adhesin and hydrogenosome enzyme (Alderete et al., 2001).
To modulate expression of the family of ap65 genes, T. vaginalis parasites were successfully and stably transfected with the plasmids containing ap65 in sense and antisense orientations. Amplification by PCR of the neo gene confirmed the presence of the episomal plasmids. The parasites expressing ap65 antisense mRNA silenced expression of the native ap65 genes, confirming the use of antisense RNA in modulating gene expression in trichomonads, as has been shown for other parasites (Zhang and Matlashewski, 1997; Ankri et al., 1999). Importantly, decreased transcription of ap65 was reflected quantitatively on the amounts of VEC-binding proteins and diminished parasite adherence to VECs.
As AP65 is decarboxylating malic enzyme in hydrogenosomes (Hrdý and Müller, 1995; Kulda, 1999), we measured for activity of malic enzyme and found that AS transfectants had 11.8% of the activity compared to Wt organisms. Thus, we wanted to examine whether reduced amounts of enzyme altered properties affected by energy metabolism. Further, it is conceivable that lower ATP levels, if this in fact occurs with decreased AP65, might also be reflected in changes in adherence to host cells. Thus, we monitored the property of trichomonal growth and multiplication. Comparative growth studies, however, showed that decreased AP65 did not adversely affect trichomonal multiplication rates and overall cell densities (Fig. 4). Indeed, any slight difference between transfected parasites compared to the wild type organisms is likely to be because of the drug G418. It is not surprising that decreased amounts or elimination of AP65 would not affect growth and energy metabolism given the availability of another malic enzyme, albeit requiring NADP in lieu of NAD, present in the cytoplasm that also converts malate to pyruvate (Müller, 1993; Kulda, 1999). Moreover, the recent findings of other enzymes possibly contributing to alternative energy-generating pathways (Brown et al., 1999) may be another reason why loss of this particular enzyme pathway would not be detrimental to trichomonads. Finally, it is well established that the drug resistant MR100 created in the laboratory is deficient in synthesis of hydrogenosome enzymes, including malic enzyme, and this parasite can be maintained in batch culture (Kulda, 1999).
Consistent with earlier reports (Lehker et al., 1991; Arroyo et al., 1992; Garcia et al., 2003), T. vaginalis organisms grown in iron-replete medium had fivefold higher levels of adherence compared to trichomonads grown overnight in iron-depleted medium (Fig. 7). The fact that levels of attachment to VECs by AS transfected organisms grown in iron-replete medium were not elevated shows the efficient inhibition of translation of the ap65 transcript. Further, it was not unexpected that AS parasites had lower levels of adherence when grown in iron-depleted medium. This would result from the downregulation of expression of the endogenous ap65 and the other adhesin genes (Lehker et al., 1991; Arroyo et al., 1992; Garcia et al., 2003).
This study now shows that demonstration of the use of antisense silencing of expression of ap65 permits us to dissect the relative contribution of AP51 and AP33 adhesins to adherence, especially as these are also members of multigene families (Alderete et al., 1995; 1998; O’Brien et al., 1996). Of interest will be the extent of adherence abrogation upon silencing each individually or, if possible, abolishing expression of multiple adhesins simultaneously, especially because the adhesins are coordinately transcribed and expressed on the surface. Equally importantly, once the trichomonad surface proteins for binding basement membrane components like fibronectin (Crouch and Alderete, 1999; Crouch et al., 2001) and laminin (Costa e Silva-Filho et al., 1988) are identified, this approach will be invaluable should gene replacement not be achievable. Finally, the establishment of a stable transfection system, as shown here, has more recently permitted us to express heterologous proteins in T. vaginalis. Even more exciting is the use of this transfection system to express in Tritrichomonas foetus the AP65 adhesin with demonstrable increased adherence to immortalized VECs (data not shown). Altogether, these approaches will allow for structure-function characterization of virulence factors in the future.
Experimental procedures
Parasite culture
T. vaginalis isolate T016 was grown in trypticase-yeast extract-maltose (TYM) medium with 5% serum (Diamond, 1957). For iron-replete parasites, TYM-serum was supplemented with 200 μM ferrous ammonium sulphate (Sigma Chemical Co.), and iron-depleted parasites were obtained by cultivation in medium depleted of iron with 50 μM 2,2-dipyridyl (Sigma) (Lehker et al., 1991).
Generation of sense (S) and antisense (AS) plasmids with ap65 coding region
The S and AS plasmids were constructed by cloning the coding region of AP65-3 gene in forward (S primer 5′-GTCCAGCATATGATGCTCGCATCTTCAGTC-3′ and AS primer 5′-GTCCACGGTACCTTAGTAGAGTTGCTCGTATTC-3′) and reverse orientation (S primer 5′-GTCCACGGTACCATGCTCGCATCTTCAGTC-3′ and AS primer 5′-GTCCAGCATATGTTAGTAGAGTTGCTCGTATTC-3′). The original plasmid pBS-FdHAHA-neo was kindly provided by Dr Patricia Johnson (UCLA). The ferredoxin gene in the plasmid was removed by partial digestion, and the ap65-3 gene of 1.7-kb was cloned into the NdeI and Asp718 sites of the plasmid. The S and AS ap65 plasmids were confirmed by sequencing. Plasmid DNA for transfection was purified using maxi prep columns (Qiagen, Inc.).
Stable transfection and selection for G418 resistance
Transfection of T. vaginalis cells was carried out by electroporation (Tsai et al., 2002). Parasites at early logarithmic phase of growth were used for transfection. Briefly, 4 × 107 parasites were centrifuged at 1800 r.p.m. at 4°C and the pellet resuspended in 400 μl fresh TYM before transferring into a 4 mm gap cuvette (BTX®, Genetronics) with 25 μg of plasmid DNA. Electroporation was performed at 320 V, 1000 microfarads and 725 ohms using ECM 630 Electro cell manipulator (BTX®). Following the pulse, cells were placed on ice for 10 min and transferred into two T25 flasks with 50 ml of fresh TYM-serum medium. The cells were grown free of drug for 24 h followed by the addition of Geneticin (G418) (Invitrogen-Life Technologies) at 100 μg ml-1. Single cells were cloned using soft-agar plates containing 25 μg ml-1 Geneticin (Delgadillo et al., 1997). The DNA was isolated from single cell cultures using DNAzol (Invitrogen) and further purified by phenol-chloroform extraction. The presence of plasmid in single cell clones was confirmed by PCR amplification of the neo gene using the neo-sense primer 5′-GATCGGTACCATGATTGATTGAACAAGATGGATTG-3′, and neo-antisense primer 5′-CTTTAGACCAAGTTCGTGTCAGAAGAACTCGTCAAG-3′. Finally, transfected parasites were grown in the absence of G418 and monitored for loss of plasmid and reexpression of AP65 protein. This was done for only a period of 3 weeks as prolonged batch cultivation of fresh isolates may result in downregulation of expression of the adhesins (Lehker et al., 1991).
RNA isolation, Northern hybridization, and RT-PCR analysis
Total RNA was isolated using the Trizol reagent (Invitrogen). RNA 10 μg lane-1 was separated on 1.2% (w/v) formaldehyde agarose gels and transferred onto Hybond N+ membrane (Amersham Pharmacia Biotech). The ap65 transcript was detected using DIG DNA labelling and detection kit (Roche Diagnostic Co.). Northern hybridization was carried out as per the manufacturer’s recommendations.
For RT-PCR, 1 μg of total RNA was reverse transcribed using SuperScript II RNase H- Reverse Transcriptase (Invitrogen). Then, 10% of the reverse transcribed cDNA was used as template for the PCR reactions. The primers used for PCR amplifications of the ap65 transcript were as follows: ap65-sense primer 5′-CAGTCAGTCGACCAGTTAGATATGGGTACAGAC-3′, ap65-antisense primer 5′-GTGACAGGATCCCGCTCGCAGTTAGCGCATGTAG-3′. The p270-sense primer was 5′-GTTGATAGAGAAGGTAGGGATAAC-3′ and p270-antisense primer was 5′-TATATTTATAATAAATTAGACTTCAACTCC-3′. The forward and reverse primers for PCR of the antisense transcript were 5′-GTAGACATTGCTGCTACGTC-3′ and 5′-GTCCAGCATATGATGCTCGCATCTTCAGTC-3′, respectively. As the abundance of antisense transcript was low in steady state because of binding to ap65 mRNA, it was necessary to run 30 cycles of the PCR.
Immunoblot detection of AP65 and AP33
Total proteins of 107 T. vaginalis organisms were obtained as before using trichloroacetic acid (TCA) (Alderete, 1983) for analysis by sodium dodecylsulphate-polyacrylamide gel (SDS-PAGE) electrophoresis (Laemmli, 1970) prior to blotting onto Hybond-P membranes (Amersham) for immunoblot detection with mAbs to AP65 (mAb DM116) and AP33 (mAb F5) (Garcia et al., 2003). TCA-precipitated proteins were solubilized using electrophoresis dissolving buffer (Laemmli, 1970). Electrophoresis was carried out using 10% acrylamide gels. The mAbs and epitope reactivity have been described before (Engbring and Alderete, 1998a; Garcia et al., 2003). Following reactivity with the mAb probes, the bands were visualized by the chemiluminescence assay with horseradish peroxidase as the colour developer (Bio-Rad Laboratories).
Ligand assay to determine the amounts of adhesins on the surface
The ligand assay to detect adhesins that bind the host cells was carried out as before (Arroyo et al., 1992). Briefly, after fixation of HeLa cells with glutaraldehyde and processing, 106 cells were incubated with a trichomonal detergent extract derived from 2 × 107 solubilized parasites. After incubation, cells were vigorously washed to remove unbound and loosely associated trichomonad proteins. Cells were boiled in electrophoresis dissolving buffer to elute the HeLa cell-binding proteins followed by SDS-PAGE in 10% acrylamide. The gels were further stained with Coomassie brilliant blue for visualization, and duplicate gels were blotted onto Hybond-P membranes for immunoblot analysis using the mAb DM116 to AP65.
Immunofluorescence detection of AP65 on the surface
Immunofluorescence of AP65 on the surface and in hydrogenosomes of trichomonads was carried out using a modification of a recently described procedure (Garcia et al., 2003). Briefly, 1 × 106 logarithmic phase organisms were washed twice with cold PBS and fixed with 4% paraformaldehyde for 30 min at RT. Fixed cells were washed in PBS and permeabilized with 1% NP-40 for 45 min at RT. Trichomonads were then blocked with 5% BSA for 1 h at RT prior to incubation for 1 h at RT with hybridoma supernatants of mAb 12G4 (1:100) and mAb F11 (1:1). Parasites were washed with PBS and incubated for 1 h at 37°C with fluoresceine isothiocyanate-conjugated antimouse IgG (Sigma) diluted 1:100. Finally, parasites were washed twice with PBS and observed under 1000× magnification using the Olympus BX41 microscope.
VEC adherence assay
Immortalized human MS-74 VECs (Klumpp et al., 2002) were used for the adherence assay as recently described (Garcia et al., 2003). Briefly, 2 × 104 MS-74 VECs were seeded onto individual wells of 96-well Costar flatbottom plates (Corning Inc.) and grown for 24 h in D-MEM supplemented with 10% fetal bovine serum. VECs were then washed twice with a medium mixture of D-MEM:TYM (2:1; v/v) without serum. Sense and antisense transfected and Wt trichomonads were labelled with [3H]thymidine for 18 h, washed three times with DMEM-TYM and resuspended. Then, 4 × 105 tritium-labelled parasites were added to the individual wells of the 96-well plate with a confluent MS-74 VEC monolayer and incubated for 1 h at 37°C. Cells were then washed thoroughly with the DMEM-TYM. Individual wells were placed in mini-scintillation vials and radioactivity measured. The assay was performed with quadruplicate samples, and the experiment was repeated four times.
Enzyme assay
The activity of decarboxylating malic enzyme was measured spectrophotometrically by the rate of reduction of NAD+, as detailed before (Drmota et al., 1996).
Nucleotide sequence accession number
The nucleotide sequence of ap65-3 has been assigned Gen-Bank accession number U35243. The other two ap65-1 and ap65-2 genes characterized by us (Alderete et al., 1995) have sequence accession numbers of U18346 and U18347.
Acknowledgements
This work was supported by Public Health Service grants AI43940 and AI45429 from the National Institutes of Health. Members of the laboratory are also acknowledged for their suggestions and discussion of our work. Some results were omitted and presented as ‘data not shown’, and we will make available such data upon request.
References
- Alderete JF. Identification of immunogenic and antibody-binding proteins on the membrane of pathogenic Trichomonas vaginalis. Infect Immun. 1983;40:284–291. doi: 10.1128/iai.40.1.284-291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Demeš P, Gombošova A, Valent M, Fabušova M, Janoœka A, et al. Specific parasitism of purified vaginal epithelial cells by Trichomonas vaginalis. Infect Immun. 1988;56:2558–2562. doi: 10.1128/iai.56.10.2558-2562.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Engbring J, Lauriano CM, O’Brien JL. Only two of the Trichomonas vaginalis triplet AP51 adhesins are regulated by iron. Microb Pathog. 1998;23:1–16. doi: 10.1006/mpat.1997.0167. [DOI] [PubMed] [Google Scholar]
- Alderete JF, Garza GE. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun. 1985;50:701–708. doi: 10.1128/iai.50.3.701-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Millsap KW, Lehker MW, Benchimol M. Enzymes on microbial pathogens and Trichomonas vaginalis: molecular mimicry and functional diversity. Cell Microbiol. 2001;3:1–13. doi: 10.1046/j.1462-5822.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- Alderete JF, O’Brien JL, Arroyo R, Engbring JA, Musatovova O, Lopez O, et al. Cloning and molecular characterization of two genes encoding adhesion proteins involved in Trichomonas vaginalis cytoadherence. Mol Microbiol. 1995;17:69–83. doi: 10.1111/j.1365-2958.1995.mmi_17010069.x. [DOI] [PubMed] [Google Scholar]
- Ankri S, Padilla-Vaca F, Stolarsky T, Koole L, Katz U, Mirelman D. Antisense inhibition of expression of the light subunit (35 kDa) of the Gal/GalNac lectin complex inhibits Entamoeba histolytica virulence. Mol Microbiol. 1999;33:327–337. doi: 10.1046/j.1365-2958.1999.01476.x. [DOI] [PubMed] [Google Scholar]
- Arroyo R, Engbring J, Alderete JF. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol. 1992;6:853–862. doi: 10.1111/j.1365-2958.1992.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Arroyo R, Gonzalez-Robles A, Martinez-Palomo A, Alderete JF. Signalling of Trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol Microbiol. 1993;7:299–309. doi: 10.1111/j.1365-2958.1993.tb01121.x. [DOI] [PubMed] [Google Scholar]
- Brown DM, Upcroft JA, Dodd HN, Chen N, Upcroft P. Alternative 2-keto acid oxidoreductase activities in Trichomonas vaginalis. Mol Biochem Parasitol. 1999;98:203–214. doi: 10.1016/s0166-6851(98)00169-8. [DOI] [PubMed] [Google Scholar]
- Cates W. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex Trans Dis. 1999;26:S2–S7. doi: 10.1097/00007435-199904001-00002. [DOI] [PubMed] [Google Scholar]
- Costa e Silva-Filho F, de Souza W, Lopes JD. Presence of laminin-binding proteins in trichomonads and their role in adhesion. Proc Natl Acad Sci USA. 1988;85:8042–8046. doi: 10.1073/pnas.85.21.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotch MF, Pastorek JG, II, Nugent RP, Hillier SL, Gibbs RS, Martin DH, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Trans Dis. 1997;24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- Cotch MF, Patorek JG, II, Nugent RP, Yerg DE, Martin DH, Eschenbach DA. Demographic and behavioral predictors of Trichomonas vaginalis infection among pregnant women. Obstet Gynecol. 1991;78:1087–1092. [PubMed] [Google Scholar]
- Crouch ML, Alderete JF. Trichomonas vaginalis interactions with fibronectin and laminin. Microbiology. 1999;145:2835–2843. doi: 10.1099/00221287-145-10-2835. [DOI] [PubMed] [Google Scholar]
- Crouch ML, Benchimol M, Alderete JF. Binding of fibronectin by Trichomonas vaginalis is influenced by iron and calcium. Microb Pathog. 2001;31:1–14. doi: 10.1006/mpat.2001.0455. [DOI] [PubMed] [Google Scholar]
- Dailey DC, Alderete JF. The phenotypically variable surface protein of Trichomonas vaginalis has a single, tandemly repeated immunodominant epitope. Infect Immun. 1991;59:2083–2088. doi: 10.1128/iai.59.6.2083-2088.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgadillo MG, Liston DR, Niazi K, Johnson PJ. Transient and selectable transformation of the parasitic protist Trichomonas vaginalis. Proc Natl Acad Sci USA. 1997;94:4716–4720. doi: 10.1073/pnas.94.9.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond LS. The establishment of various Trichomonas of animals and man in axenic cultures. J Parasitol. 1957;43:488–490. [PubMed] [Google Scholar]
- Drmota T, Proost P, Van Ranst M, Weyda F, Kulda J, Tachezy J. Iron-ascorbate cleavable malic enzyme from hydrogenosomes of Trichomonas vaginalis: purification and characterization. Mol Biochem Parasitol. 1996;83:221–234. doi: 10.1016/s0166-6851(96)02777-6. [DOI] [PubMed] [Google Scholar]
- Dubougher C, Christophe N, Durand-Joly I, Gerbod D, Delgado-Viscogliosi P, Jouveshomme S, et al. Pulmonary coinfection by Trichomonas vaginalis and Pneumocystis sp. as a novel manifestation of AIDS. Hum Pathol. 2003;34:508–511. doi: 10.1016/s0046-8177(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Engbring JA, Alderete JF. Characterization of Trichomonas vaginalis AP33 adhesin and cell surface interactive domains. Microbiology. 1998a;144:3011–3018. doi: 10.1099/00221287-144-11-3011. [DOI] [PubMed] [Google Scholar]
- Engbring JA, Alderete JF. Three genes encode distinct AP33 proteins involved in Trichomonas vaginalis cytoadherence. Mol Microbiol. 1998b;28:305–313. doi: 10.1046/j.1365-2958.1998.00784.x. [DOI] [PubMed] [Google Scholar]
- Garcia AF, Chang T-H, Benchimol M, Klumpp DJ, Lehker MW, Alderete JF. Iron and contact with host cells induce expression of adhesins on surface of Trichomonas vaginalis. Mol Microbiol. 2003;47:1207–1224. doi: 10.1046/j.1365-2958.2003.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs MM, Kazembe P, Reed AW, Miller WC, Nkata E, Zimba D, et al. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sex Trans Dis. 1999;26:381–387. doi: 10.1097/00007435-199908000-00003. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Brown GD, Wirth FH, Gebert BS, Bailey, Anday EK. Urinary tract infection with Trichomonas vaginalis in a premature newborn infant and the development of chronic lung disease. J Perinatol. 2003;23:59–61. doi: 10.1038/sj.jp.7210819. [DOI] [PubMed] [Google Scholar]
- Hrdý I, Müller M. Primary structure of the hydrogenosomal malic enzyme of Trichomonas vaginalis and its relationship to homologous enzymes. J Euk Microbiol. 1995;42:593–603. doi: 10.1111/j.1550-7408.1995.tb05913.x. [DOI] [PubMed] [Google Scholar]
- Kassai T, Cordero del Campillo M, Euzeby J, Gaafar S, Hiepe T, Himonas CA. Standardized nomenclature of animal parasitic diseases (SNOAPAD) Vet Parasitol. 1988;29:299–326. doi: 10.1016/0304-4017(88)90148-3. [DOI] [PubMed] [Google Scholar]
- Klumpp DJ, Forrestal SG, Karr JE, Mudge CS, Anderson BE, Schaeffer AJ. Epithelial differentiation promotes the adherence of type 1-piliated Escherichia coli to human vaginal cells. J Infect Dis. 2002;186:1631–1638. doi: 10.1086/345557. [DOI] [PubMed] [Google Scholar]
- Kreiger JN, Jenny C, Verdon M, Siegel N, Springwater R, Critchlow CW, Holmes KK. Clinical manifestations of trichomoniasis in men. Ann Intern Med. 1993;118:844–849. doi: 10.7326/0003-4819-118-11-199306010-00003. [DOI] [PubMed] [Google Scholar]
- Kulda J. Trichomonads, hydrogenosomes and drug resistance. Int J Parasitol. 1999;29:199–212. doi: 10.1016/s0020-7519(98)00155-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laga M, Manoka A, Kivuvu M, Malele B, Tulza M, Nzila N, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- Land MK, Delgadillo-Correa MG, Tachezy J, Vanacova S, Hsieh LC, Sutak R, Johnson PJ. Targeted gene replacement of a ferredoxin gene in Trichomonas vaginalis does not lead to metronidazole resistance. Mol Microbiol. 2004;51:115–122. doi: 10.1046/j.1365-2958.2003.03791.x. [DOI] [PubMed] [Google Scholar]
- Lehker MW, Arroyo R, Alderete JF. The regulation by iron of the synthesis of adhesins and cytoadherence levels in the protozoan Trichomonas vaginalis. J Exp Med. 1991;174:311–318. doi: 10.1084/jem.174.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd GL, Case JR, De Frias D, Brannigan RE. Trichomonas vaginalis orchitis with associated severe oligoasthenoteratospermia and hypogonadism. J Urol. 2003;170:924. doi: 10.1097/01.ju.0000080375.18547.cc. [DOI] [PubMed] [Google Scholar]
- Müller M. The hydrogenosome. J Gen Microbiol. 1993;139:2879–2889. doi: 10.1099/00221287-139-12-2879. [DOI] [PubMed] [Google Scholar]
- O’Brien JL, Lauriano CM, Alderete JF. Molecular characterization of a third malic enzyme-like ap65 adhesin gene of Trichomonas vaginalis. Microb Pathog. 1996;20:335–349. doi: 10.1006/mpat.1996.0032. [DOI] [PubMed] [Google Scholar]
- Sorvillo F, Kerndt P. Trichomonas vaginalis and amplification of HIV-1 transmission [letter] Lancet. 1998;351:213–214. doi: 10.1016/S0140-6736(05)78181-2. [DOI] [PubMed] [Google Scholar]
- Sorvillo F, Smith L, Kerndt P, Ash L. Trichomonas vaginalis, HIV, and Africans. Emerg Infect Dis. 2001;7:927–932. doi: 10.3201/eid0706.010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C-D, Liu H-W, Tai J-H. Characterization of an iron-responsive promoter in the protozoan pathogen Trichomonas Vaginalis. J Biol Chem. 2002;277:5153–5162. doi: 10.1074/jbc.M110234200. [DOI] [PubMed] [Google Scholar]
- Viikki M, Pukkala E, Nieminen P, Hakama M. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol. 2000;39:71–75. doi: 10.1080/028418600431003. [DOI] [PubMed] [Google Scholar]
- Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Trans Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- Weinstock H, Berman S, Cates W. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections. Overview and Estimates . WHO; Geneva, Switzerland: 2001. [Google Scholar]
- Yap EH, Ho TH, Chan YC, Thong TW, Ng GC, Ho LC, Singh M. Serum antibodies to Trichomonas vaginalis in invasive cervical cancer patients. Genitourin Med. 1995;71:402–404. doi: 10.1136/sti.71.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Begg CB. Is Trichomonas vaginalis a cause of cervical neoplasia? Results from a combined analysis of 24 studies. Int J Epidemiol. 1994;23:682–690. doi: 10.1093/ije/23.4.682. [DOI] [PubMed] [Google Scholar]
- Zhang ZF, Graham S, Yu SZ, Marshall J, Zielezny M, Chen YX, et al. Trichomonas vaginalis and cervical cancer. A prospective study in China. Ann Epidemiol. 1995;5:325–332. doi: 10.1016/1047-2797(94)00101-x. [DOI] [PubMed] [Google Scholar]
- Zhang W-W, Matlashewski G. Loss of virulence in Leishmania donovani deficient in an amastigote-specific protein. A2 Proc Natl Acad Sci USA. 1997;94:8807–8811. doi: 10.1073/pnas.94.16.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]


