Abstract
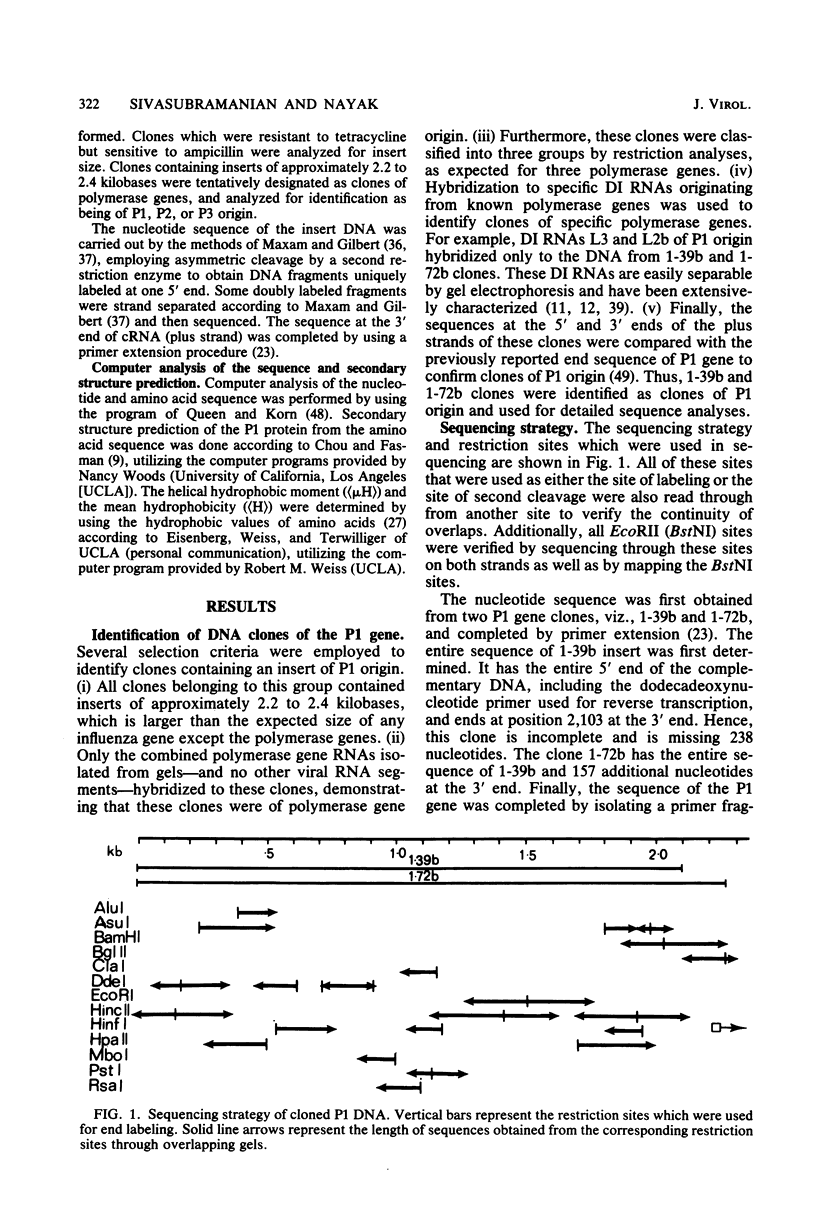
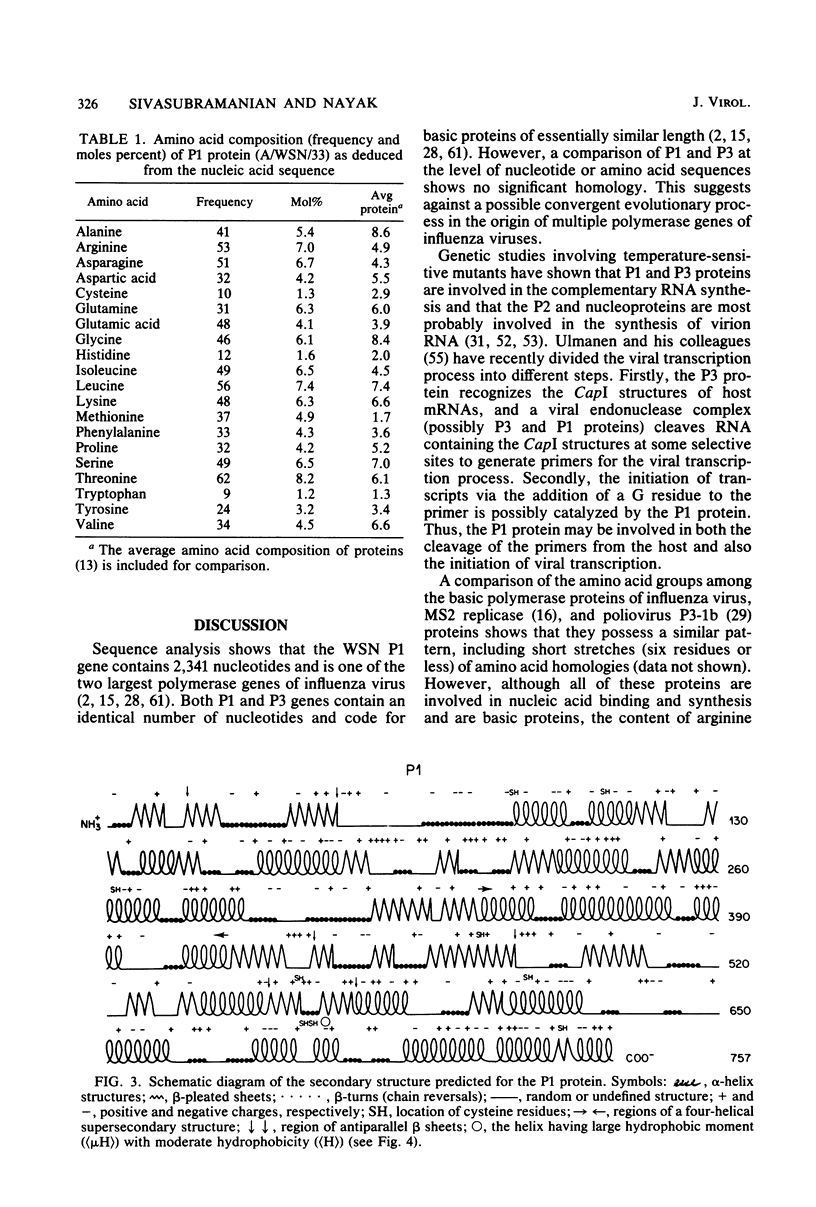
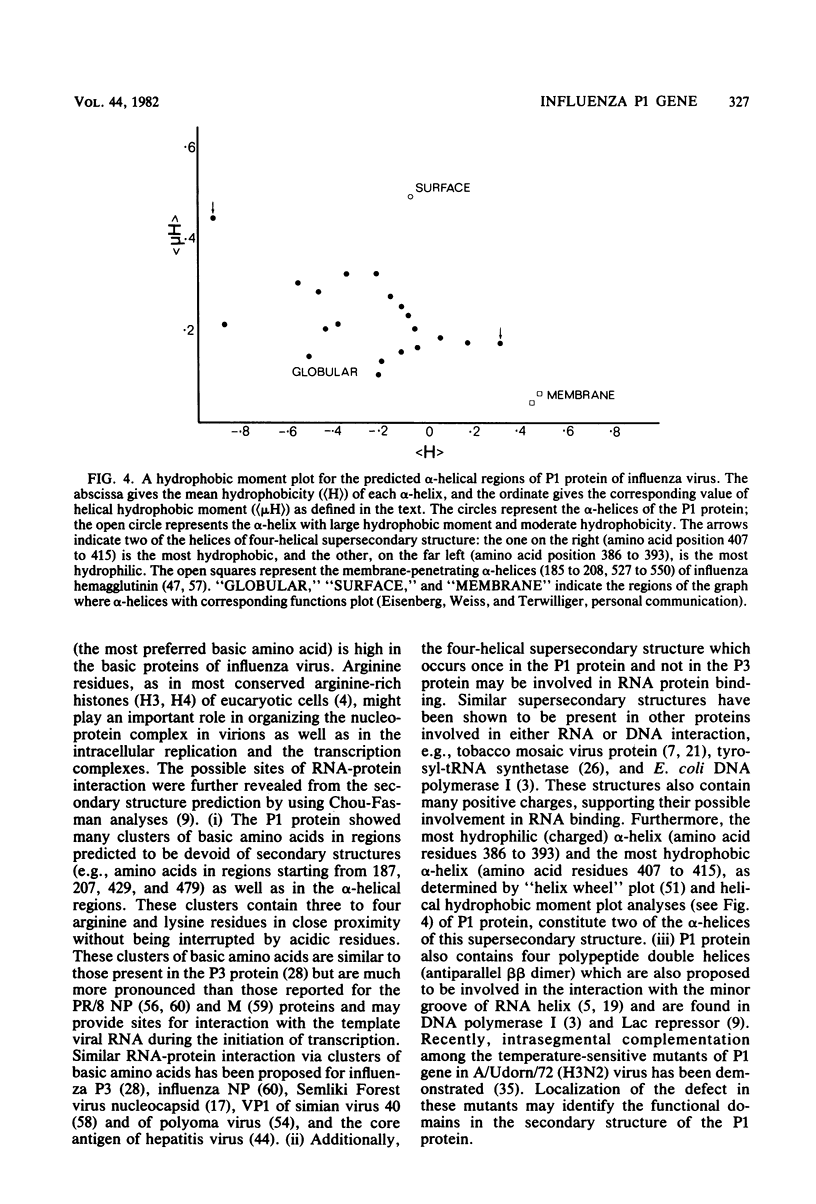
The nucleotide sequence of polymerase 1 (P1) gene of a human influenza virus (A/WSN/33) has been determined by using cDNA clones, except for the last 83 nucleotides, which were obtained by primer extension. The WSN P1 gene contains 2,341 nucleotides and codes for a protein of 757 amino acids (Mr = 86,500). P1 gene possesses a striking tandem repeat of 12 nucleotides (nucleotide position 2,188 to 2,199, 2,200 to 2,211) and a corresponding tandem repeat of tetrapeptide in the P1 protein. The deduced sequence of P1 protein is enriched in basic amino acids, particularly arginine. In addition, it also contains clusters of basic amino acids which may provide sites for the interaction with the template virion RNA capped primer as well as with other proteins involved in viral replication and transcription. A secondary structure prediction, using Chou and Fasman analyses (Annu. Rev. Biochem. 47:251-276, 1978), shows that the P1 protein possesses some unique features, viz., one "four-helical supersecondary structure" and four "polypeptide double helices" (antiparallel beta-pleated sheets) which are considered important in RNA binding.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Rossmann M. G., Johnson J. E. A four-helical super-secondary structure. Biochem Biophys Res Commun. 1977 Mar 7;75(1):83–86. doi: 10.1016/0006-291x(77)91292-x. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Huddleston J. A., Brownlee G. G. The complete sequence of RNA segment 2 of influenza A/NT/60/68 P1 protein. Nucleic Acids Res. 1982 Feb 25;10(4):1335–1343. doi: 10.1093/nar/10.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. E., Stump K. H., Kelley W. S. Escherichia coli DNA polymerase I. Sequence characterization and secondary structure prediction. J Biol Chem. 1982 Feb 25;257(4):1965–1972. [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J. A proposed model for interaction of polypeptides with RNA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):283–287. doi: 10.1073/pnas.71.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton A. J., Robertson J. S. Structure of the host-derived sequences present at the 5' ends of influenza virus mRNA. Nucleic Acids Res. 1980 Jun 25;8(12):2591–2603. doi: 10.1093/nar/8.12.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champness J. N., Bloomer A. C., Bricogne G., Butler P. G., Klug A. The structure of the protein disk of tobacco mosaic virus to 5A resolution. Nature. 1976 Jan 1;259(5538):20–24. doi: 10.1038/259020a0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Adler A. J., Fasman G. D. Conformational prediction and circular dichroism studies on the lac repressor. J Mol Biol. 1975 Jul 25;96(1):29–45. doi: 10.1016/0022-2836(75)90180-1. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Hiti A. L., Nayak D. P. Construction and characterization of a bacterial clone containing the hemagglutinin gene of the WSN strain (HON1) of influenza virus. Gene. 1980 Aug;10(3):205–218. doi: 10.1016/0378-1119(80)90050-5. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Hiti A. L., Nayak D. P. Influenza defective interfering viral RNA is formed by internal deletion of genomic RNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):215–219. doi: 10.1073/pnas.77.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. R., Nayak D. P. Sequence relationships among defective interfering influenza viral RNAs. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3092–3096. doi: 10.1073/pnas.76.7.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Chanock R. M., Lai C. J. Nonviral oligonucleotides at the 5' terminus of cytoplasmic influenza viral mRNA deduced from cloned complete genomic sequences. Cell. 1980 Sep;21(2):495–500. doi: 10.1016/0092-8674(80)90486-9. [DOI] [PubMed] [Google Scholar]
- Fields S., Winter G. Nucleotide sequences of influenza virus segments 1 and 3 reveal mosaic structure of a small viral RNA segment. Cell. 1982 Feb;28(2):303–313. doi: 10.1016/0092-8674(82)90348-8. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Duerinck F., Haegeman G., Iserentant D., Merregaert J., Min Jou W., Molemans F., Raeymaekers A., Van den Berghe A. Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature. 1976 Apr 8;260(5551):500–507. doi: 10.1038/260500a0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. The capsid protein of Semliki Forest virus has clusters of basic amino acids and prolines in its amino-terminal region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6376–6380. doi: 10.1073/pnas.77.11.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene P. J., Gupta M., Boyer H. W., Brown W. E., Rosenberg J. M. Sequence analysis of the DNA encoding the Eco RI endonuclease and methylase. J Biol Chem. 1981 Mar 10;256(5):2143–2153. [PubMed] [Google Scholar]
- Gutte B., Däumigen M., Wittschieber E. Design, synthesis and characterisation of a 34-residue polypeptide that interacts with nucleic acids. Nature. 1979 Oct 25;281(5733):650–655. doi: 10.1038/281650a0. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Herz C., Stavnezer E., Krug R., Gurney T., Jr Influenza virus, an RNA virus, synthesizes its messenger RNA in the nucleus of infected cells. Cell. 1981 Nov;26(3 Pt 1):391–400. doi: 10.1016/0092-8674(81)90208-7. [DOI] [PubMed] [Google Scholar]
- Hiti A. L., Davis A. R., Nayak D. P. Complete sequence analysis shows that the hemagglutinins of the H0 and H2 subtypes of human influenza virus are closely related. Virology. 1981 May;111(1):113–124. doi: 10.1016/0042-6822(81)90658-9. [DOI] [PubMed] [Google Scholar]
- Hiti A. L., Nayak D. P. Complete nucleotide sequence of the neuraminidase gene of human influenza virus A/WSN/33. J Virol. 1982 Feb;41(2):730–734. doi: 10.1128/jvi.41.2.730-734.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M. A. The large P proteins of influenza A viruses are composed of one acidic and two basic polypeptides. Virology. 1980 Nov;107(1):302–305. doi: 10.1016/0042-6822(80)90296-2. [DOI] [PubMed] [Google Scholar]
- Hélène C., Lancelot G. Interactions between functional groups in protein-nucleic acid associations. Prog Biophys Mol Biol. 1982;39(1):1–68. doi: 10.1016/0079-6107(83)90013-5. [DOI] [PubMed] [Google Scholar]
- Irwin M. J., Nyborg J., Reid B. R., Blow D. M. The crystal structure of tyrosyl-transfer RNA synthetase at 2-7 A resolution. J Mol Biol. 1976 Aug 25;105(4):577–586. doi: 10.1016/0022-2836(76)90236-9. [DOI] [PubMed] [Google Scholar]
- Janin J. Surface and inside volumes in globular proteins. Nature. 1979 Feb 8;277(5696):491–492. doi: 10.1038/277491a0. [DOI] [PubMed] [Google Scholar]
- Kaptein J. S., Nayak D. P. Complete nucleotide sequence of the polymerase 3 gene of human influenza virus A/WSN/33. J Virol. 1982 Apr;42(1):55–63. doi: 10.1128/jvi.42.1.55-63.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., Bouloy M. Are the 5' ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell. 1979 Oct;18(2):329–334. doi: 10.1016/0092-8674(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Ueda M., Palese P. Temperature-sensitive mutants of influenza WSN virus defective in virus-specific RNA synthesis. J Virol. 1975 Oct;16(4):790–796. doi: 10.1128/jvi.16.4.790-796.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J., Choppin P. W. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4170–4174. doi: 10.1073/pnas.78.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell. 1980 Sep;21(2):475–485. doi: 10.1016/0092-8674(80)90484-5. [DOI] [PubMed] [Google Scholar]
- Massicot J. G., Van Wyke K., Chanock R. M., Murphy B. R. Evidence for intrasegmental complementation between two influenza A viruses having ts mutations on their P1 genes. Virology. 1982 Mar;117(2):496–500. doi: 10.1016/0042-6822(82)90488-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nayak D. P. Defective interfering influenza viruses. Annu Rev Microbiol. 1980;34:619–644. doi: 10.1146/annurev.mi.34.100180.003155. [DOI] [PubMed] [Google Scholar]
- Nayak D. P., Sivasubramanian N., Davis A. R., Cortini R., Sung J. Complete sequence analyses show that two defective interfering influenza viral RNAs contain a single internal deletion of a polymerase gene. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2216–2220. doi: 10.1073/pnas.79.7.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A. K., Rubin R. A., Kim S. H., Modrich P. DNA sequences of structural genes for Eco RI DNA restriction and modification enzymes. J Biol Chem. 1981 Mar 10;256(5):2131–2139. [PubMed] [Google Scholar]
- Palese P., Ritchey M. B. Live attenuated influenza virus vaccines. Strains with temperature-sensitive defects in P3 protein and nucleoprotein. Virology. 1977 May 1;78(1):183–191. doi: 10.1016/0042-6822(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. Mapping of the influenza virus genome. II. Identification of the P1, P2, and P3 genes. Virology. 1977 Jan;76(1):114–121. doi: 10.1016/0042-6822(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. P1 and P3 proteins of influenza virus are required for complementary RNA synthesis. J Virol. 1977 Mar;21(3):1187–1195. doi: 10.1128/jvi.21.3.1187-1195.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Krug R. M. Transfer of 5'-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W. The inhibition of influenza virus RNA synthesis by actinomycin D and cycloheximide. Virology. 1973 Jan;51(1):120–128. doi: 10.1016/0042-6822(73)90372-3. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Queen C. L., Korn L. J. Computer analysis of nucleic acids and proteins. Methods Enzymol. 1980;65(1):595–609. doi: 10.1016/s0076-6879(80)65062-9. [DOI] [PubMed] [Google Scholar]
- Robertson J. S. 5' and 3' terminal nucleotide sequences of the RNA genome segments of influenza virus. Nucleic Acids Res. 1979 Aug 24;6(12):3745–3757. doi: 10.1093/nar/6.12.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C. Influenza virus genetics. Adv Genet. 1979;20:1–36. doi: 10.1016/s0065-2660(08)60544-1. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. The genome of the influenza virus. Curr Top Microbiol Immunol. 1978;80:139–169. doi: 10.1007/978-3-642-66956-9_5. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Griffin B. E. Polyoma virus DNA: complete nucleotide sequence of the gene which codes for polyoma virus capsid protein VP1 and overlaps the VP2/VP3 genes. J Virol. 1980 Feb;33(2):619–630. doi: 10.1128/jvi.33.2.619-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Broni B. A., Krug R. M. Role of two of the influenza virus core P proteins in recognizing cap 1 structures (m7GpppNm) on RNAs and in initiating viral RNA transcription. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7355–7359. doi: 10.1073/pnas.78.12.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rompuy L., Min Jou W., Huylebroeck D., Devos R., Fiers W. Complete nucleotide sequence of the nucleoprotein gene from the human influenza strain A/PR/8/34 (HON1). Eur J Biochem. 1981 May 15;116(2):347–353. doi: 10.1111/j.1432-1033.1981.tb05341.x. [DOI] [PubMed] [Google Scholar]
- Verhoeyen M., Fang R., Jou W. M., Devos R., Huylebroeck D., Saman E., Fiers W. Antigenic drift between the haemagglutinin of the Hong Kong influenza strains A/Aichi/2/68 and A/Victoria/3/75. Nature. 1980 Aug 21;286(5775):771–776. doi: 10.1038/286771a0. [DOI] [PubMed] [Google Scholar]
- Winter G., Fields S., Brownlee G. G. Nucleotide sequence of the haemagglutinin gene of a human influenza virus H1 subtype. Nature. 1981 Jul 2;292(5818):72–75. doi: 10.1038/292072a0. [DOI] [PubMed] [Google Scholar]
- Winter G., Fields S. Cloning of influenza cDNA ino M13: the sequence of the RNA segment encoding the A/PR/8/34 matrix protein. Nucleic Acids Res. 1980 May 10;8(9):1965–1974. doi: 10.1093/nar/8.9.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Fields S. Nucleotide sequence of human influenza A/PR/8/34 segment 2. Nucleic Acids Res. 1982 Mar 25;10(6):2135–2143. doi: 10.1093/nar/10.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Fields S. The structure of the gene encoding the nucleoprotein of human influenza virus A/PR/8/34. Virology. 1981 Oct 30;114(2):423–428. doi: 10.1016/0042-6822(81)90223-3. [DOI] [PubMed] [Google Scholar]
- van Heuverswyn H., van de Voorde A., Fiers W. Nucleotide sequence of the simian virus 40 Hind II + III restriction fragment J and the total amino acid sequence of the major structural protein VP1. Eur J Biochem. 1978 Nov 15;91(2):415–430. doi: 10.1111/j.1432-1033.1978.tb12694.x. [DOI] [PubMed] [Google Scholar]



