Abstract
Conversion of the carbamazepine metabolite, 3-hydroxycarbamazepine (3-OHCBZ), to the catechol, 2,3-dihydroxycarbamazepine (2,3-diOHCBZ), followed by subsequent oxidation to a reactive o-quinone species has been proposed as a possible bioactivation pathway in the pathogenesis of carbamazepine-induced hypersensitivity. Initial in vitro phenotyping studies implicated CYP3A4 as a primary catalyst of 2,3-diOHCBZ formation: 2-hydroxylation of 3-OHCBZ correlated significantly (r2≥0.929, P<0.001) with CYP3A4/5 activities in a panel of human liver microsomes (n=14) and was markedly impaired by CYP3A inhibitors (>80%), but not by inhibitors of other cytochrome P450 enzymes (≤20%). However, in the presence of troleandomycin, the rate of 2,3-diOHCBZ formation correlated significantly with CYP2C19 activity (r2=0.893, P<0.001) in the panel of human liver microsomes. Studies with a panel of cDNA-expressed enzymes revealed that CYP2C19 and CYP3A4 were high (S50=30 μM) and low (S50=203 μM) affinity enzymes, respectively, for 2,3-diOHCBZ formation and suggested that CYP3A4, but not CYP2C19, might be inactivated by a metabolite formed from 3-OHCBZ. Subsequent experiments demonstrated that preincubation of 3-OHCBZ with human liver microsomes or recombinant CYP3A4 led to decreased CYP3A4 activity, which was both preincubation time- and concentration-dependent, but not inhibited by inclusion of glutathione or N-acetylcysteine. CYP3A4, CYP3A5, CYP3A7, CYP2C19 and CYP1A2 converted [14C]3-OHCBZ into protein-reactive metabolites, but CYP3A4 was the most catalytically active enzyme. The results of this study suggest that CYP3A4-dependent secondary oxidation of 3-OHCBZ represents a potential carbamazepine bioactivation pathway via formation of reactive metabolites capable of inactivating CYP3A4, potentially generating a neo-antigen that may play a role in the etiology of carbamazepine-induced idiosyncratic toxicity.
Introduction
Introduced in the early 1960’s, the tricyclic compound, carbamazepine (CBZ; Tegretol), has proven to be a highly effective agent for the treatment of epileptic seizures, trigeminal neuralgia and psychiatric disorders. However, its therapeutic use has also been associated with a variety of serious idiosyncratic adverse reactions, including skin rashes, aplastic anemia, hepatitis and a severe, generalized drug-induced hypersensitivity reaction, which has been estimated to occur in 1 of 5000 patients (Shear and Spielberg, 1988; Tennis and Stern, 1997). Among patients experiencing CBZ-induced hypersensitivity reactions, significant cross-sensitivity has been reported with other aromatic anticonvulsants, such as phenytoin and phenobarbital (Shear and Spielberg, 1988), which suggests that the mechanisms of these adverse reactions may be related. The mechanism underlying anticonvulsant-induced hypersensitivity is not well understood, but it is generally believed that biotransformation of aromatic anticonvulsants into chemically reactive metabolites is the critical event that initiates downstream pathogenic events such as formation of protein adducts and subsequent immune responses (Park et al., 1987). Serum antibodies that recognize cytochrome P450 (P450)-related antigens; specifically epitopes present in rat CYP2C and 3A enzymes (Leeder et al., 1996) have been found in patients who have experienced a hypersensitivity reaction to aromatic anticonvulsants. Interestingly, these antibodies do not appear to recognize recombinant human CYP2C and 3A proteins; however, sera from two patients who had experienced anticonvulsant hypersensitivity reactions demonstrated pronounced immunoreactivity with an unidentified, 53 kDa protein present in liver microsomes from a patient who died of phenytoin-induced hepatitis.
Although the identity of the antigen to which the immune response is directed has remained elusive, bioactivation of CBZ has been demonstrated in vitro by the formation of both cytotoxic (Pirmohamed et al., 1993) and protein-reactive metabolites (Pirmohamed et al., 1992; Lillibridge et al., 1996; Wolkenstein et al., 1998) in human liver microsomes. Using [14C]-CBZ and recombinant human P450s, Wolkenstein et al. (1988) further demonstrated that CYPs1A1, 1A2, 2C8 and 3A4 were capable of generating protein-reactive metabolites from CBZ, however, when the relative abundance of the enzymes was considered, 65% and 31% of covalent adduct formation was attributed to CYP3A4 and CYP1A2, respectively. Masubuchi et al. (2001) investigated the possibility that cytochrome P450 enzymes might be targets of the reactive intermediates and demonstrated time-dependent inhibition of CYP1A2 activity, but not of CYP 2C8, 2C9 or 3A4 activities in human liver microsomes preincubated with CBZ. Recently, Kang et al. (2008) demonstrated that CYP3A4 converted [3H]-CBZ into a metabolite capable of covalently binding to CYP3A4, presumably within the active site. However, the CBZ-modified enzyme retained its testosterone 6β-hydroxylase activity. Unfortunately, these studies were not able to reveal the identity(s) of the protein-reactive metabolite(s).
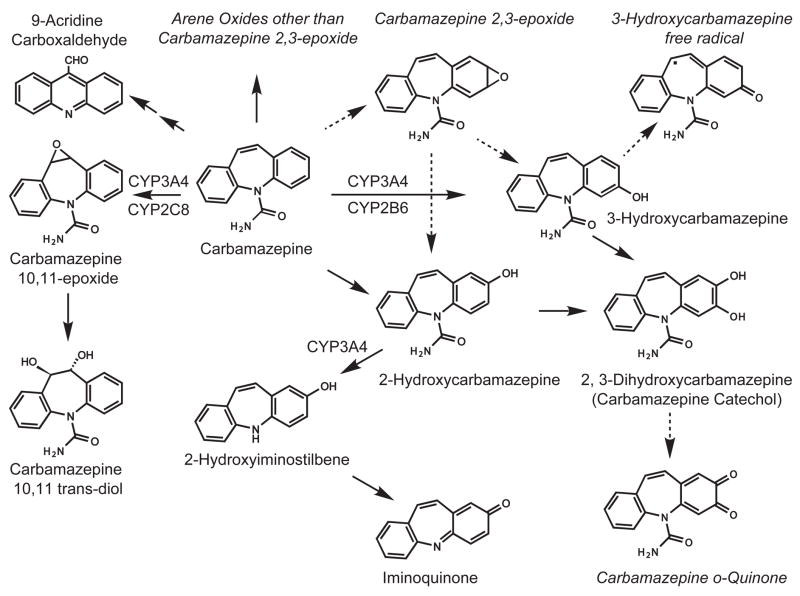
Several reactive metabolites have been proposed as the reactive species responsible for the idiosyncratic toxicity associated with CBZ, including an arene oxide (Spielberg et al., 1981), 9-acridine carboxaldehyde (Furst et al., 1995), CBZ 10,11-epoxide (Bu et al., 2005), an iminoquinone metabolite (CBZ-IQ) derived from 2-hydroxycarbamazepine (2-OHCBZ) (Ju and Utrecht, 1999) and an o-quinone metabolite (CBZ-quinone) (Lillibridge et al., 1996), which is proposed to form via oxidation of the catechol, 2,3-dihydroxy-carbamazepine (2,3-diOHCBZ) (Fig. 1). The latter two proposed species (CBZ-IQ and the o-quinone metabolite) are particularly attractive candidates for the reactive intermediate, since quinones are relatively soft electrophiles that react readily with soft nucleophiles and have been implicated in the production of CBZ-derived protein adducts (Pirmohamed et al., 1992; Lillibridge et al., 1996). We recently identified CYP3A4 as the primary catalyst responsible for converting 2-OHCBZ to CBZ-IQ and observed formation of glutathione- and N-acetylcysteine-conjugates when 2-hydroxyiminostilbene (2-OHIS) or CBZ-IQ were used as substrates, but failed to observe time-dependent loss of CYP3A activity with 2-OHCBZ, 2-OHIS or CBZ-IQ (Pearce et al., 2005). In the present study, we examined the role that human P450 enzymes play in the biotransformation of 3-OHCBZ to the catechol, 2,3-diOHCBZ, the proposed precursor to CBZ-quinone. During the course of the investigation, it was determined that CYP3A4 made a substantial contribution to 2,3-OHCBZ formation and that recombinant CYP3A4-mediated catechol formation was not proportional to time, which suggested that CYP3A4 might be inactivated by a metabolite generated from 3-OHCBZ. Hence, additional studies were undertaken to determine whether 3-OHCBZ biotransformation led to time- and concentration-dependent loss of CYP3A activity and formation of microsomal protein adducts.
Figure 1.
Metabolic pathways and proposed reactive metabolites of CBZ. Italicized species are inferred from products.
Materials and Methods
Chemicals
CBZ, α-naphthoflavone, ketoconazole, omeprazole, quinidine, sulfaphenazole, testosterone, 6β-hydroxytestosterone, TAO, GSH, glucose-6-phospate, glucose-6-phosphate dehydrogenase, isocitrate, isocitrate dehydrogenase, NAC, NADP, NADPH, and EDTA were purchased from Sigma Chemical Co. (St. Louis, MO). All other reagents were of analytical grade. Bicinchoninic acid (BCA) protein determination kits were purchased from Pierce, Inc. (Rockford, Ill). Syntheses of 3-OHCBZ and 3-hydroxy[carbonyl-14C]CBZ ([14C]3-OHCBZ) were performed as described by Lu and Uetrecht (in the companion manuscript). [3H]CBZ and [14C]3-OHCBZ were obtained commercially from GE Healthcare Biosciences Corp (Piscataway, NJ). The synthesis of 2-OHCBZ has been described previously (Pearce et al., 2002).
Biological reagents
Human liver microsomal preparations were obtained from BD GENTEST Corp. (Woburn, MA; n=14 individuals) and from XenoTech, L.L.C. (Lenexa, KS; pool). Microsomes from baculovirus-infected insect cells (SUPERSOMES™) expressing human P450 enzymes (1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, and 3A7) or control vector were purchased from BD GENTEST Corp. All recombinant enzymes were co-expressed with human NADPH-cytochrome P450 reductase; some enzymes (CYP2B6, CYP2C19, CYP2E1, CYP3A4 and CYP3A7) were also co-expressed with human cytochrome b5. The manufacturers provided protein concentrations, P450 contents and P450 enzyme activities. Microsomes from dexamethasone (Dex)-treated mice were prepared by differential ultracentrifugation of homogenates from 1.15 M KCl-perfused livers (Bornheim and Correia, 1990), and stored as pellets overlaid with PBS/10% glycerol at −80°C until use. Vials of microsomes were stored at −70ºC until use. Typically, microsomes were rapidly thawed in room temperature water and placed on ice prior to use. However, in protein binding experiments, microsomes were allowed to thaw on ice prior to use.
In vitro incubations with 3-OHCBZ
In vitro enzyme assays were performed in 96-well microtiter plates. Standard incubation reactions (100-μl) contained human liver microsomes (50 μg of microsomal protein) or insect cell microsomes containing bacculovirus-expressed cytochrome P450 enzymes (5 pmol) co-expressed with P450 reductase, potassium phosphate buffer (50 mM, pH 7.4), MgCl2 (3 mM), EDTA (1 mM), and 3-OHCBZ (5 to 500 μM) dissolved in methanol (≤ 1% v/v final concentration) at the final concentrations listed. Reactions were initiated by the addition of an NADPH-generating system, consisting of NADP (1 mM), glucose-6-phosphate (5 mM), and glucose-6-phosphate dehydrogenase (1 U/ml), incubated at 37±0.1°C in a Thermo Forma (Marietta, OH) Benchtop Orbital Shaker, and terminated after 0–60 min (standard conditions: human liver microsomes, 30 min; recombinant P450s, 60 min for screen; CYP3A4 and CYP2C19 kinetics, 10 and 15 min, respectively) by the addition of 100 μl of ice-cold methanol containing the internal standard, CBZ (1 μM final concentration). Protein was precipitated by centrifugation at 10,000 gmax for 10 min. An aliquot (50–75 μl) of the supernatant was analyzed by HPLC/MS via direct injection. Under these conditions, initial experiments conducted with 3-OHCBZ (50 μM) and pooled human liver microsomes suggested that the rates of formation for the etabolite identified as 2,3-diOHCBZ were proportional to protein concentration (up to 0.5 mg/ml) and incubation time (up to 60 min). Additional experiments conducted with 3-OHCBZ at a substrate concentration of 5 μM (to establish experimental conditions for kinetic studies) demonstrated that the rate of 2,3-diOHCBZ formation was proportional to protein concentration and incubation time in pooled human liver microsomes (up to 0.5 mg/ml; up to 30 min) and microsomes containing recombinant CYP3A4 and CYP2C19 (up to 5 pmol P450; up to 10 and 15 min, respectively). With the exception of experiments designed to examine preincubation-dependent inhibition of CYP3A4/5 activity and protein binding, biotransformation of 3-OHCBZ did not exceed 10% in in vitro incubations.
Inhibition experiments
Conversion of 3-OHCBZ (100 μM) to 2,3-diOHCBZ by human liver microsomes was evaluated in the presence or absence (i.e., control) of known P450 isoform-selective inhibitors. The following chemical inhibitors were examined at the indicated concentrations: α-naphthoflavone (CYP1A2, 1 μM), sulfaphenazole (CYP2C9, 10 μM), omeprazole (CYP2C19, 10 μM), quinidine (CYP2D6, 1 μM), ketoconazole (CYP3A4/5, 1 μM) and TAO (CYP3A4/5, 100 μM). Inhibitors were dissolved in methanol and diluted in the incubation mixtures to a final solvent concentration of 1% (v/v). Control incubations contained an equal volume of methanol. Incubations containing the mechanism-based inhibitor troleandomycin were pre-incubated with human liver microsomes and NADPH-generating system for 20 min before the reaction was started with substrate.
Preincubation-dependent inhibition of CYP3A4/5 activity
Human liver microsomes (0.5 mg protein/ml) and recombinant CYP3A4 (50 pmol/ml) were preincubated with 3-OHCBZ (10, 30, 100 or 300 μM) at 37±1ºC for up to 60 min in the presence of an NADPH-generating system and the presence or absence of either GSH (10 or 500 μM) or NAC (500 μM). Controls contained microsomes preincubated in the presence or absence of 3-OHCBZ (100 μM), but lacked NADPH. Preincubation mixtures were then diluted 10-fold with potassium phosphate buffer (50 mM, pH 7.4), MgCl2 (3mM), EDTA (1 mM) and testosterone (250 mM). Reactions were initiated by the addition of NADPH-generating system. Reactions were terminated with ice-cold methanol after 10–20 min. Protein was sedimented by centrifugation and an aliquot of the supernatant was analyzed by reverse phase HPLC via direct injection, as described by Usmani et al (2003). CYP3A4/5 activity was measured as testosterone 6β-hydroxylase activity.
Irreversible binding of CBZ and 3-OHCBZ to microsomal proteins
[14C]3-OH CBZ or [3H]CBZ (0.2 μCi; 0.5 mM) was incubated with liver microsomes from Dex-treated mice at a final concentration of 400 pmol P450 (0.14 mg protein) at 37ºC in potassium phosphate buffer (0.1 M, pH 7.4), in the presence or absence of an NADPH-regenerating system [consisting of isocitrate (5 mM) and isocitrate dehydrogenase (1 unit)1] and EDTA (1 mM) in a final incubation volume of 0.2 ml. After a 3 min-preincubation at 37°C, reactions were initiated by the addition of fresh NADPH (1.5 mM) (not added to controls). Freshly prepared GSH (1 or 4 mM) was added to some incubations to determine the level of reactive metabolite binding external to the P450 active site. After 30 min, the reaction was terminated with ice-cold methanol/5% H2SO4 (10 volumes). Rat liver microsomes (10 mg protein) were added as a protein carrier, and the protein was precipitated on ice for 15 min. The protein precipitate was collected by sedimentation at 650g for 10 min in a Beckman GS-6R centrifuge, resuspended in ice-cold methanol/5% H2SO4 (10 volumes) and resedimented. This step was repeated and followed by the addition of unlabelled CBZ or 3-OHCBZ solution in ethanol (20 mM stock; 20 μl) to the protein precipitate to reduce non-specifically bound radio-labeled compound. Following resuspension in ice-cold methanol/5% H2SO4 (10 volumes) and resedimentation, the protein precipitate was sequentially “washed” free of adventitious substrates/metabolites by resuspension/resedimentation, once with acetone, three times with ethanol/ethyl ether (3:1, v:v) and once with 80% methanol. The final precipitate was dried at room temperature and dissolved in 1N NaOH (1ml) at 60°C. A 0.2 ml aliquot was neutralized with an equal volume of 1N HCl and subjected to liquid scintillation counting. A 50 μl-aliquot of the incubation mixture (≈ 100 pmol of P450) was subjected to SDS-PAGE analyses on 10% Tris-polyacrylamide gels, the gels vacuum dried and screened with a Storm 860 Phosphor-Imager (Molecular Dynamics, Sunnyvale, CA). Protein concentration of the resuspended precipitate was determined with a BCA kit. Parallel controls for non-specific binding were prepared as described for Dex-treated mouse liver microsomes and for each recombinant P450 preparation examined (see below), except that the incubations contained no NADPH, but were otherwise processed exactly as the corresponding +NADPH-incubated enzyme preparation. Following correction for protein recovery, the rates of [3H]CBZ or [14C]3-OHCBZ covalent binding were determined in incubations conducted in the presence or in the absence of NADPH.
Incubations containing recombinant human P450 enzymes were performed as described above with the following exceptions: P450 concentrations were maintained at 0.5 μM, the concentration of [14C]3-OH CBZ or [3H]CBZ was 0.5 mM (0.2 μCi) and in cases where microsomes containing cDNA-expressed P450 enzymes (CYPs 1A1, 1A2, and 2C18) contained no co-expressed human cytochrome b5, purified cytochrome b5 was added in vitro at a molar ratio of 2:1 of each P450, and the mixtures preincubated at room temperature for 15 min, before the addition of the rest of the reaction mixture components and subsequent initiation of the reaction with NADPH, as described above. The effectiveness of this cytochrome b5-supplementation protocol was first confirmed by functional comparison of recombinant CYP3A5 with co-expressed cytochrome b5 and the corresponding recombinant CYP3A5 without co-expressed cytochrome b5, but supplemented in vitro with and without cytochrome b5, using [14C]3-OHCBZ-induced binding as a probe. After termination of the reaction with methanol/H2SO4 as described above, rat liver microsomes (≈ 10 mg protein) were added as a protein carrier and the irreversible P450 protein binding of radiolabeled CBZ or 3-OHCBZ was monitored as previously described. Purified recombinant CYP3A4, functionally reconstituted as detailed (Wang et al., 1998) was similarly incubated with [14C]3-OH CBZ or [3H]CBZ and analyzed. Functional reconstitution of purified CYP3A4 requires GSH to be included for optimal activity (Gillam et al., 1993), hence incubations containing purified CYP3A4 in the absence of GSH were not performed.
HPLC/MS analysis of 3-OHCBZ biotransformation
3-OHCBZ and its metabolites were resolved on a reversed-phase Phenomenex (Torrance, CA) Luna™ C-8 (2) column (4.6 mm × 25 cm, 5 μm particle size) preceded by a Phenomenex C-8 guard column (4 mm × 3mm i.d., 5 μm particle size) using a Hewlett Packard HP1100 HPLC system equipped with a HP1100 de-gasser, binary pump, auto-sampler, column heater, diode array detector and mass spectral detector (Hewlett Packard Instruments, Santa Clara, CA). The mobile phase consisted of 0.1% aqueous acetic acid (solvent A) and methanol (solvent B) and was delivered at a constant flow of 0.6 ml/min. The solvent program was as follows: 0–5 min, 40%B; 5–15 min, a linear gradient from 40 to 50% B; 15–20 min, a linear gradient from 50 to 70% B; 20–35 min, 70% B; 35–43 min, a linear gradient from 70 to 80% B; 43–47 min, 80% B; 47–47.1 min, a linear gradient from 80 to 40%B; 47.1–55 min, 40% B. The column temperature was maintained at 40ºC. Under these conditions, 2,3-diOHCBZ, 3-OHCBZ and the internal standard (CBZ) eluted at ~24.5, 27.0, and 29.5 min, respectively. The column effluent was monitored by UV detection (290 nm) and by atmospheric pressure chemical ionization detection with the mass spectrometer operating in a selective positive ion-monitoring mode. Ion detection was optimized for detection of 3-OHCBZ. The drying gas temperature and flow were maintained at 200ºC and 5 L/min, respectively, and the nebulizer pressure was set at 20 psig. The vaporizer temperature was maintained at 400ºC. The capillary voltage was set at 3 kV and the corona current was set at 4.5 μA. Under these conditions, 2,3-diOHCBZ yielded [MH]+ ions at m/z 269; other ions of interest were monitored as [MH]+ ions. Data were collected and integrated with Hewlett Packard Chemstation V A.0401 software. In the absence of authentic standards for 2,3-diOHCBZ, the rates of metabolite formation were estimated from the relationship between known concentrations of the parent compound, 3-OHCBZ, and their AUC.
Data analysis
Coefficients of determination (r2) between the rates of 2,3-diOHCBZ formation and the activities of cytochrome P450 enzymes were determined using least-squares regression analysis. Significance was determined by Pearson’s regression analysis using a two-tailed Student’s t test (SPSS 11.0, SPSS Inc., Chicago, IL). A two-tailed, Student’s t test (SPSS) was also used to determine significant differences in irreversible binding of radiolabeled metabolites to microsomal proteins in incubations performed in the presence of NADPH from those performed in the absence of NADPH (controls) and in the rates of 2,3-diOHCBZ formation by recombinant human P450 enzymes from the rate of 2,3-diOHCBZ formation by the vector control.
Visual inspection of Eadie–Hofstee plots (rate versus rate/[S]) derived from kinetic data suggested that the kinetics of 2,3-diOHCBZ formation might not be consistent with a simple Michaelis-Menten model. Therefore, kinetic data were subsequently analyzed by non-linear regression without weighting (GraFit 5; Erithacus Software Ltd, Surrey, UK) using a sigmoidal Vmax equation equivalent to the Hill equation
where S50 is the substrate concentration showing a half-maximal velocity, n is a measure of cooperativity, and Vmax is the maximal velocity.
Contributions of individual P450 enzymes to the formation of 2,3-diOHCBZ in human liver microsomes were estimated by application of the Relative Activity Factor (RAF) approach (Venkatakrishnan et al., 2000). RAFs were determined for specific CYPs by comparing the rate of an isoform-specific index reaction at saturating concentrations in human liver microsomes (Vmax) to the rate of the same reaction catalyzed by recombinant enzymes under identical conditions. Thus,
S-Mephenytoin 4′-hydroxylation and testosterone 6β-hydroxylation were used as the isoform-specific reactions for CYP2C19 and CYP3A4, respectively, in this study.
Predicted activities for each CYP isoform (VCYPx) in human liver microsomes were estimated by multiplying the rate of the reaction catalyzed by cDNA-expressed enzymes (Vrec-CYPx) by the appropriate scaling factor, RAF.
Predicted contributions of each recombinant P450 to the clearance of 3-OHCBZ by 2-hydoxylation were calculated using experimentally derived kinetic parameters for cDNA-expressed CYPs and the following equation:
where CYPx is an individual recombinant enzyme, ClCYPx is the clearance (Vmax/Km) of the given isoform, and sum ClCYPn is the sum of the predicted clearances for each of the recombinant forms contributing to the given reaction.
Results
Identification of catechol product, 2,3-diOHCBZ
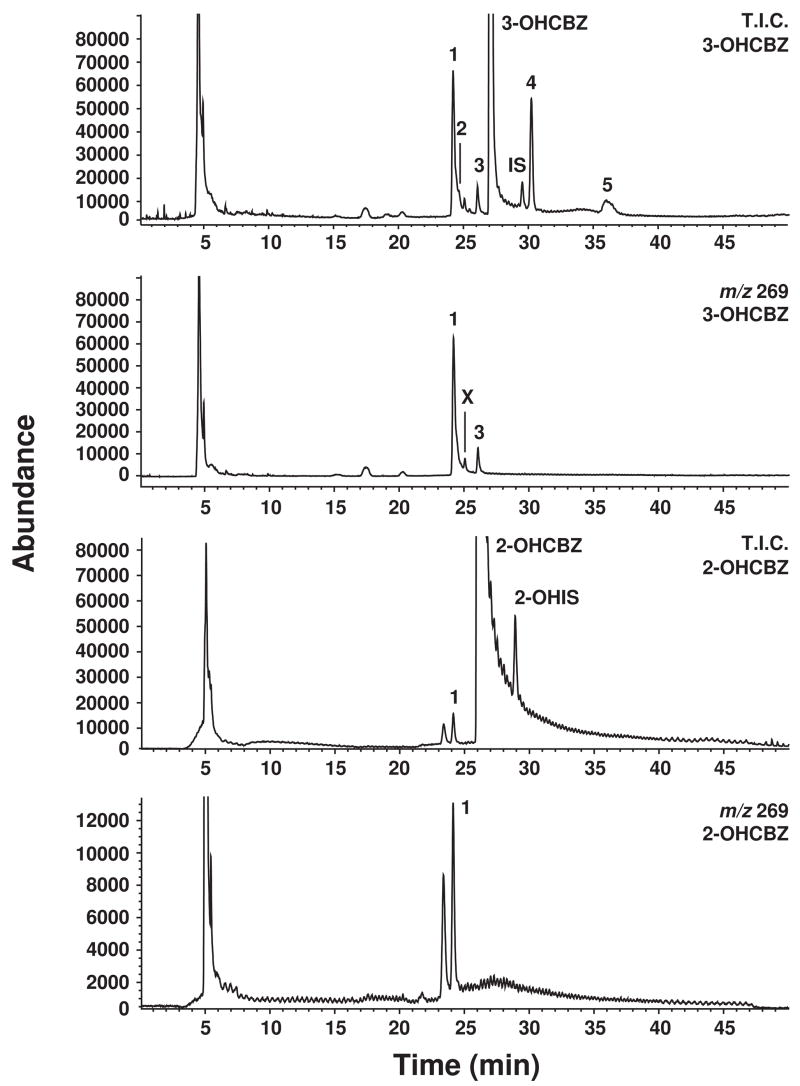
In the absence of an authentic standard for 2,3-diOHCBZ, its identification in reaction mixtures was inferred from the results of experiments conducted with 2-OHCBZ and 3-OHCBZ as substrates. Human liver microsomes converted 3-OHCBZ to at least five primary metabolites, two of which had mass and fragmentation patterns consistent with those of a dihydroxycarbamazepine metabolite by HPLC-MS. Utilizing the same HPLC/MS conditions, three metabolites with mass spectral properties consistent with those of a dihydroxycarbamazepine metabolite were detected in NADPH-fortified human liver microsomal incubations containing 2-OHCBZ as the substrate, but only one of these metabolites had the same retention time as one of the dihydroxycarbamazepine metabolites formed from 3-OHCBZ (Fig. 2). Only one dihydroxycarbamazepine metabolite, namely 2,3-diOHCBZ, has been identified as being common to the CBZ metabolic pathways that proceed through 2-OHCBZ and 3-OHCBZ (Lertratanangkoon and Horning, 1982). Therefore, we conclude that the dihydoxycarbamazepine metabolite observed in our experiments is the catechol, 2,3-diOHCBZ, although this conclusion remains to be confirmed empirically. No metabolite formation was observed from 3-OHCBZ in the absence of NADPH or human liver microsomes.
Figure 2.
Representative HPLC/MS chromatograms obtained by APCI at selected ion currents of [MH]+ ions of 3-OHCBZ or 2-OHCBZ and their respective metabolites formed by human liver microsomes. T.I.C., total ion count; 1, proposed 2,3-diOHCBZ metabolite (24.5 min); 2–5, unidentified metabolites of 3-OHCBZ (29.5 min); X, unidentified contaminant: IS, (CBZ, 29.5 min); 2-OHIS, 2hydroxyiminostilbene (28.5 min).
Although 2,3-diOHCBZ can be produced from either 2-OHCBZ or 3-OHCBZ, it appears that the metabolic pathway from CBZ to 3-OHCBZ to 2,3-diOHCBZ is responsible for the majority of 2,3-diOHCBZ produced in human liver microsomes. The basis for this statement is derived from two observations. First, the rate of 2,3-diOHCBZ formation in human liver microsomes was at least five times greater when 3-OHCBZ (10 μM) served as substrate than when 2-OHCBZ (10 μM) was the substrate (data not shown). Second, the ratio of 3-OHCBZ to 2-OHCBZ formed from CBZ (100 μM) varied from 26:1 to 80:1 in a subset of the panel of human liver microsomes used in this study (Pearce et al., 2002). This suggests that conversion of CBZ to 2,3-diOHCBZ is at least two orders of magnitude greater when it proceeds through 3-OHCBZ rather than through 2-OHCBZ. Hence a principal aim of this study was to characterize the P450 enzymes responsible for the formation of 2,3-diOHCBZ from 3-OHCBZ, but not from 2-OHCBZ.
Formation of 2,3-diOHCBZ by cDNA-expressed human P450 enzymes
Microsomes prepared from bacculovirus-infected insect cells expressing vector alone (control) or one of fourteen cDNA-expressed human P450 enzymes (CYPs 1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, and 3A7) were screened for their ability to convert 3-OHCBZ (100 μM) to 2,3-diOHCBZ. With the exception of CYPs 2A6, 2C8 and 2E1 and the vector control, each of the recombinant enzymes converted 3-OHCBZ to 2,3-diOHCBZ (Fig. 3). CYP2C19 and CYP3A4 catalyzed the highest rates of 2,3-diOHCBZ formation, forming the metabolite at rates (1.62 ± 0.04 and 1.08 ± 0.03 pmol/pmol P450/min, respectively) that were at least six fold higher than those of the next most active enzyme, namely, CYP1A1.
Figure 3.
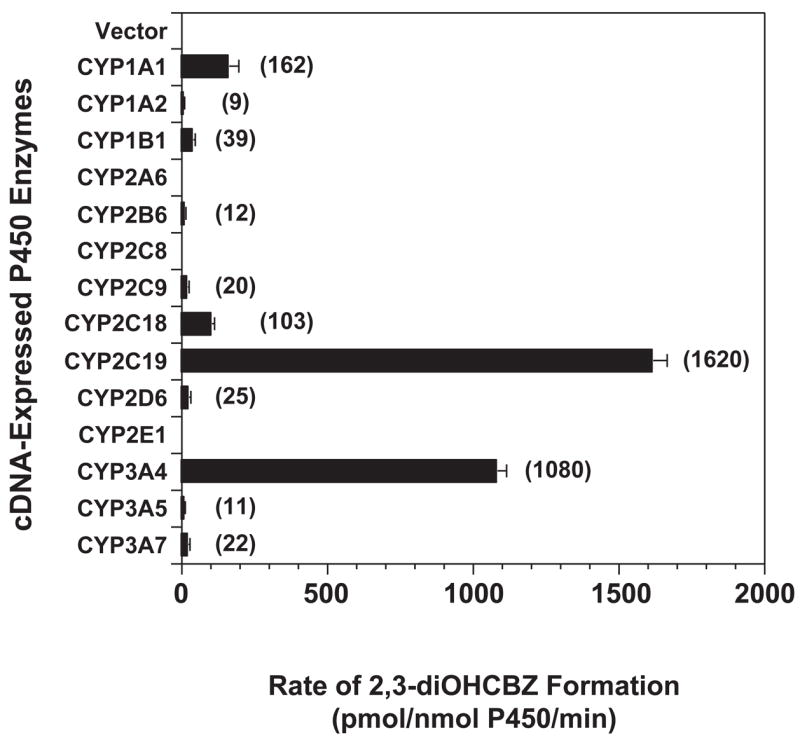
2,3-diOHCBZ formation by human recombinant cytochrome P450 enzymes. 3-OHCBZ (100 μM) was incubated with heterologously expressed human P450 enzymes as described under “Materials and Methods”. Each bar represents the mean ± S.D. of six determinations. No apparent formation of 2,3-diOHCBZ was catalyzed by microsomes containing the vector control or CYPs 2A6, 2C8 and 2E1. Formation of 2,3-diOHCBZ in microsomes containing the other cDNA-expressed human P450 enzymes examined was found to be statistically different from the limit of detection for 3-OHCBZ (a surrogate standard for 2,3-diOHCBZ; LOD = ~200 fmol) as determined by Student’s t test (p ≦ 0.05).
Concentration-dependence of 2,3-diOHCBZ formation by cDNA-expressed CYPs and human liver microsomes
Based on the experimental results described above, kinetic studies were performed with recombinant CYP2C19 and CYP3A4, and with human liver microsomes. Conversion of 3-OHCBZ (5–500 μM) to 2,3-diOHCBZ did not appear to conform to a typical Michaelis-Menten kinetic profile in any of the three systems; the kinetics exhibited a slight sigmoidicity, characterized by hooked Eadie-Hofstee plots (Fig. 4). The solubility of 3-OHCBZ (limited to ~50 mM in methanol) and consideration for solvent concentration (≤1%) in incubation mixtures precluded substrate concentrations exceeding 500 μM, a concentration below that necessary to reach enzyme saturation for human liver microsomes and CYP3A4, but one that did approach the concentration necessary to saturate CYP2C19. Kinetic parameters for 2,3-diOHCBZ formation were estimated using the Hill equation, and are presented in Table 1. The apparent S50 value for the formation of 2,3-diOHCBZ by recombinant CYP3A4 (~203 μM) was similar to the mean apparent S50 value obtained for pooled human liver microsomes whereas the apparent S50 for 2,3-diOHCBZ formation (~30 μM) by CYP2C19 was nearly an order of magnitude lower than that observed in pooled human liver microsomes (S50 = 257 μM). Following normalization of the data with respect to the mean abundance of each enzyme in human liver microsomes (Rodrigues, 1999), it was estimated that ~78% and ~22% of the in vitro intrinsic clearance (Clint; defined as Vmax/Km or Vmax/S50n) for 3-OHCBZ conversion to 2,3-diOHCBZ was attributable to CYP3A4 and CYP2C19, respectively.
Figure 4.
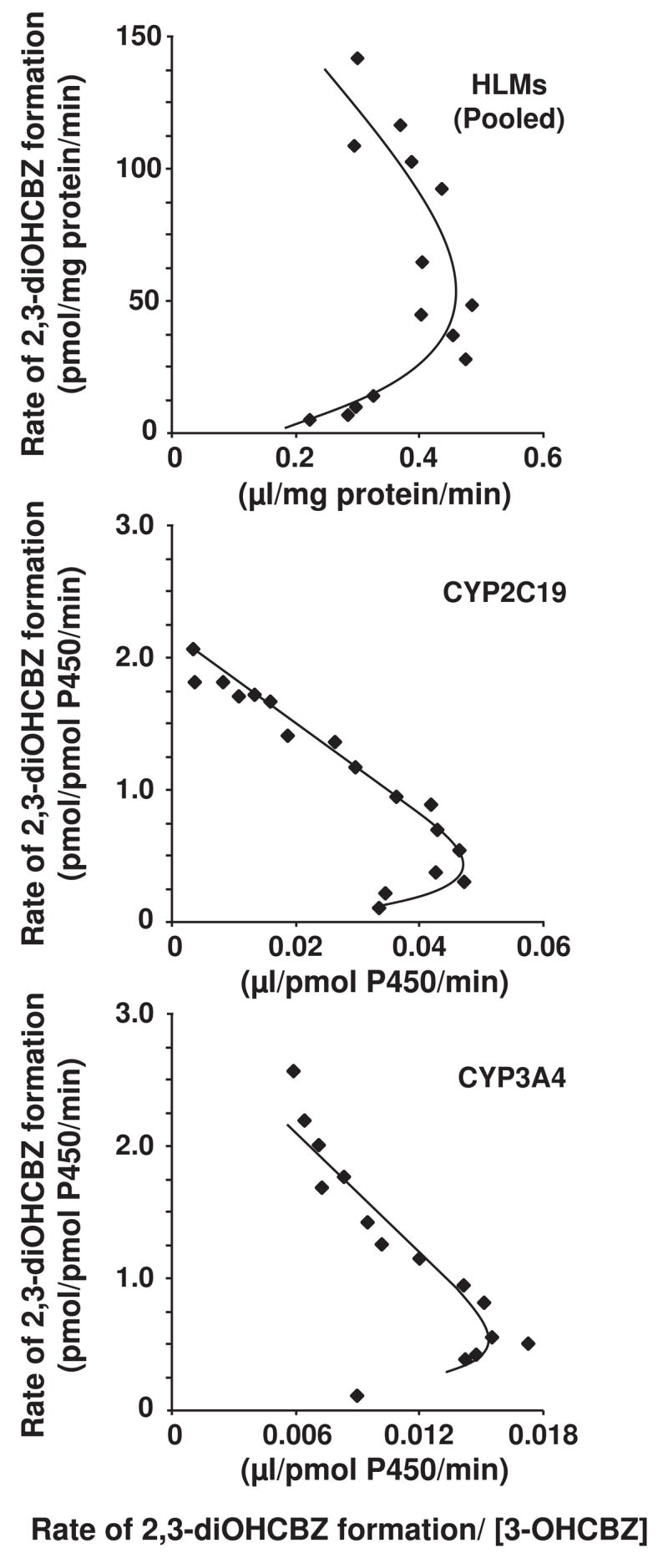
Effect of substrate concentration on the rate of 2,3-diOHCBZ formation by pooled human liver microsomes and recombinant CYP2C19 and CYP3A4 (Eadie-Hofstee plots). 3-OHCBZ (5–500 μM) was incubated with pooled human liver microsomes (0.05 mg microsomal protein) or microsomes from insect cells containing bacculovirus-expressed CYP2C19 or CYP3A4 (5 pmol) in 100-μl reaction mixtures at 37±0.1°C, and terminated with 100-μl of methanol. Incubations containing human liver micrsomes were terminated after 30 min, whereas incubations containing CYP3A4 and CYP2C19 were terminated after 10 and 15 min, respectively. Following precipitation of microsomal protein, an aliquot (75 μl) of the supernatant was analyzed by HPLC/MS via direct injection, respectively, as described under Materials and Methods.
Table 1.
Estimated kinetic parameters for the formation of 2,3-DiOHCBZ by cDNA-expressed human P450 enzymes and human liver microsomes.
| Enzyme | Mean Specific P450Contenta | Per Cent of Total Hepatic P450a | 2,3-DiOHCBZ formation
|
|||||
|---|---|---|---|---|---|---|---|---|
| S50b,c | nb | Vmaxb | Clint | Abundance Normalized Clintg | Percentage Normalized Clin | |||
| CYP2C19 | 19 | 4 | 30 ± 3 | 1.13 ± 0.09 | 2.11 ± 0.09 d | 0.045 f | 0.90 | 22 |
| CYP3A4 | 108 | 20 | 203 ± 85 | 0.87 ± 0.10 | 2.93 ± 0.51 d | 0.029 f | 3.13 | 78 |
|
| ||||||||
| Pooled HLM | N.A. | N.A. | 257 ± 76 | 1.22 ± 0.15 | 190 ± 32 e | 0.218g | N.D. | N.D. |
Mean specific content of P450 or per cent of total P450 in human liver microsomes (Rodrigues, 1999),
Cooperativity coefficient (value ± S.D),
μM,
pmol/pmol P450/min,
pmol/mg protein/min,
μl/pmol P450/min, and
μl/mg protein/min. HLM, human liver microsomes. N.A., not available. N.D., not determined.
Intersubject variability and correlation experiments
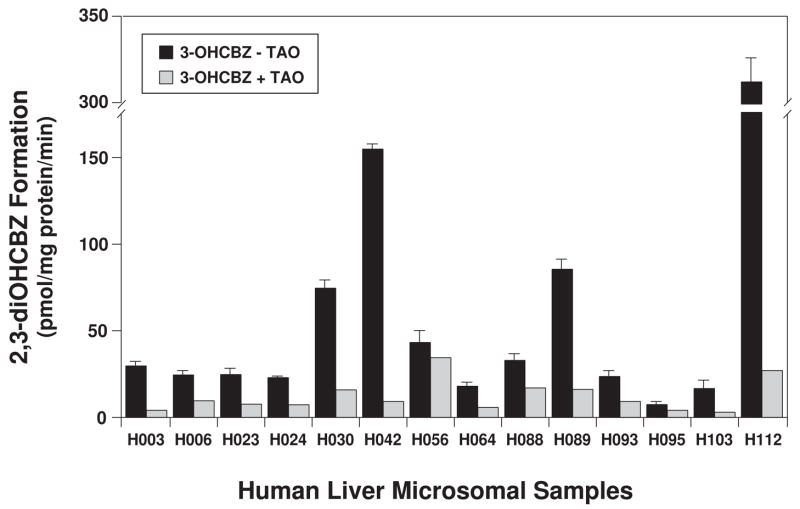
Based on the preceding results, human liver microsomes prepared from 14 donors were examined for their ability to convert 3-OHCBZ to 2,3-diOHCBZ at two substrate concentrations (10 and 100 μM; Fig 5). All of the microsomal samples catalyzed the formation of 2,3-diOHCBZ from 3-OHCBZ. At a substrate concentration of 10 μM, the rate of 2,3-diOHCBZ formation varied ~15-fold among the microsomal samples [range (rates ± S.D.): 2.70 ± 0.42 to 33.9 ± 3.6 pmol/mg protein/min], whereas at the higher substrate concentration, 2,3-diOHCBZ formation varied > 45-fold (6.46 ± 1.75 to 312 ± 28 pmol/mg protein/min). The sample-to-sample variation in the rates of 2,3-diOHCBZ formation correlated significantly with CYP3A4/5 activity (r2 ≥ 0.929, P < 0.001) and with CYP2B6 activity (r2 ≥ 0.593, P < 0.01) at both substrate concentrations examined. Rates of 2,3-diOHCBZ formation were also significantly correlated with CYP2C19 activity (r2 = 0.423, P = 0.012), but only at the 10 μM substrate concentration. The correlations between 2,3-diOHCBZ formation and activities selective for other CYP isoforms were not statistically significant. Complete results are available in Appendix 1.
Figure 5.
Effect of troleandomycin (TAO) on the 2-hydroxylation of 3-OHCBZ by a panel of human liver microsomes. Human liver microsomes (0.05 mg microsomal protein) were incubated with 3-OHCBZ (100 μM) in 100-μl reaction mixtures at 37±0.1°C, terminated after 30 min with 100-μl of methanol and analyzed by HPLC/MS via direct injection, as described under Materials and Methods. Incubations containing the mechanism-based inhibitor TAO (100 μM) were pre-incubated with human liver microsomes and NADPH-generating system for 20 min before the reaction was started with substrate. Bars representing uninhibited rates of 2,3-diOHCBZ formation are the mean ± S.D. of six determinations, whereas bars representing rates of 2,3-diOHCBZ formation in the presence of TAO are the mean of duplicate determinations.
Appendix 1.
Regression analysis (r2) of the relationship between the rates of 3-OHCBZ conversion to 2,3-diOHCBZ with the sample-to-sample variation in cytochrome P450 activity in human liver microsomes.
| Enzymatic Reaction (Enzyme) | 2,3-DiOHCBZ Formation
|
|||||
|---|---|---|---|---|---|---|
|
10 μM 3-OHCBZa |
100 μM 3-OHCBZa |
100 μM 3-OHCBZa+ TAOb |
||||
| r2 | P | r2 | P | r2 | P | |
| Phenacetin O-deethylation (CYP1A2) | 0.013 | 0.698 | 0.008 | 0.759 | 0.391 | 0.017 |
| Coumarin 7-hydroxylation (CYP2A6) | 0.006 | 0.789 | 0.002 | 0.867 | >0.001 | 0.972 |
| S-Mephenytoin N-hydroxylation (CYP2B6) | 0.593 | 0.001 | 0.687 | <0.001 | 0.104 | 0.259 |
| Paclitaxel 6α-hydroxylation (CYP2C8) | 0.171 | 0.141 | 0.174 | 0.138 | 0.014 | 0.683 |
| Diclofenac 4′-hydroxylation (CYP2C9) | 0.099 | 0.272 | 0.047 | 0.457 | 0.102 | 0.265 |
| S-Mephenytoin 4′-hydroxylation (CYP2C19) | 0.424 | 0.012 | 0.227 | 0.085 | 0.893 | <0.001 |
| Bufurolol 1′-hydroxylation (CYP2D6) | 0.098 | 0.273 | 0.069 | 0.365 | 0.082 | 0.321 |
| Chlorzoxazone 6-hydroxylation (CYP2E1) | 0.240 | 0.075 | 0.283 | 0.051 | 0.141 | 0.185 |
| Testosterone 6β-hydroxylation (CYP3A4/5) | 0.929 | <0.001 | 0.949 | <0.001 | 0.260 | 0.059 |
| Lauric acid 12-hydroxylation (CYP4A9/11) | 0.097 | 0.277 | 0.030 | 0.554 | 0.317 | 0.036 |
r2, Regression coefficient.
Concentration of 3-OHCBZ present in microsomal incubations.
Microsomes pre-incubated for 20 min with TAO (100 μM).
Values in bold indicate significant correlations between P450 activity and residual activity for CBZ metabolites at P<0.05.
Inhibition of 2,3-diOHCBZ formation
Selective chemical inhibitors were chosen to assess further the contribution of CYP3A4 and other selected P450 enzymes (CYP1A1/2, CYP2C9, CYP2C19 and CYP2D6) in the conversion of 3-OHCBZ (100 μM) to 2,3-diOHCBZ. The CYP3A inhibitors ketoconazole and TAO markedly inhibited the formation of 2,3-diOHCBZ (~80%) in pooled human liver microsomes (Fig. 6). The other chemicals examined caused little or no inhibition of 2,3-diOHCBZ formation (<15%), although α-naphthoflavone, an inhibitor of CYP1A2 with the ability to stimulate CYP3A activity (Shou et al., 1994), caused a 2- to 3-fold increase in the formation of 2,3-diOHCBZ. Omeprazole and quinidine, which are substrates for CYP3A4 as well as inhibitors of CYP2C19 and CYP2D6, respectively, caused slight increases (< 1.5-fold) in the rate of 3-OHCBZ conversion to 2,3-diOHCBZ.
Figure 6.
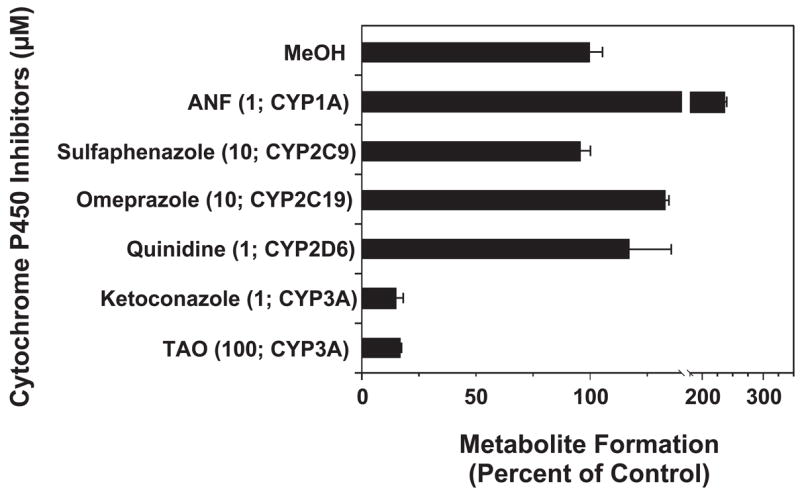
Effects of various P450 isoform-selective inhibitors on the formation of 2,3-diOHCBZ by human liver microsomes. Pooled human liver microsomes were incubated with 3-OHCBZ (100 μM) in the presence or absence of various chemicals, as described under “Materials and Methods”. Final inhibitor concentrations and the major P450 isoform inhibited are indicated in the brackets. Bars represent rates of 2,3-diOHCBZ formation (mean ± S.D. of duplicate determinations) and are expressed as a per cent of the control rate (96.8 ± 4.8 pmol/mg protein/min).
Under conditions that virtually eliminate CYP3A activity in human liver microsomes, i.e., a concentration of 100 μM TAO and a pre-incubation period of 20 min prior to addition of substrate, TAO markedly inhibited, but did not eliminate formation of 2,3-diOHCBZ. This finding suggested that the residual formation of 2,3-diOHCBZ (~20%) might be catalyzed by CYP2B6, CYP2C19 or other additional P450 enzymes. To investigate this observation further, the panel of 14 human liver microsomal preparations was incubated with 3-OHCBZ (100 μM) following a 20-min preincubation with or without TAO (100 μM), and the results are shown in Fig. 5. In the presence of TAO, the residual rate of 2,3-diOHCBZ formation correlated significantly with the activities of CYP2C19 (r2 = 0.893, P < 0.001), CYP1A2 (r2 = 0.390, P = 0.017) and CYP4A (r2 = 0.316, P = 0.036), but not with the activities of any other P450 enzymes (Appendix 1). The correlation between 2,3-diOHCBZ formation and the latter two P450 activities may be fortuitous since CYP2C19 activity was significantly correlated with CYP1A2 (r2 = 0.410, P = 0.014) and CYP4A (r2 = 0.350, P = 0.026) activities in the panel of human liver microsomes.
Contribution of CYP2C19 and CYP3A4 to 2,3-diOHCBZ formation in human liver microsomes
Cumulatively, the data from experiments with recombinant P450s, correlation studies and inhibition studies consistently identified CYP2C19 and CYP3A4 as the primary catalysts of 2,3-diOHCBZ formation from 3-OHCBZ. Therefore, the relative contributions of these two P450s to the 2-hydroxylation of 3-OHCBZ (10 μM) were investigated in the panel of 14 human liver microsomal preparations using the RAF approach, as described in Materials and Methods. Using this approach, calculated rates of 2,3-diOHCBZ formation by CYP2C19 and CYP3A4 in human liver microsomes were comparable to rates determined empirically (r2 = 0.865). In most cases, calculated rates reflected the rates observed experimentally in the human liver microsomal samples (Table 2). However in subjects H064 and H103, for example, the calculated rate of 2,3-diOHCBZ formation was markedly different from that determined experimentally (up to 2.4-fold different). Thus, it is possible that the estimated contributions of CYP2C19 and CYP3A4 to 2,3-diOHCBZ formation presented in Table 2 may not represent the true contribution of these enzymes to formation of 2,3-diOHCBZ in all individuals. One explanation is that, enzymes in addition to CYP2C19 and CYP3A4 contribute to 2,3-diOHCBZ. Nevertheless, the derived kinetic parameters for the recombinant enzymes provide an adequate estimate of each isoform’s contribution to the overall intrinsic clearance of 2,3-diOHCBZ formation. The estimated per cent contribution of CYP2C19 to the intrinsic clearance of 2,3-diOHCBZ formation ranged from 0.8 to 60.1%, whereas the contribution by CYP3A4 accounted for most of the remaining activity (39.9 to 99.1%). The per cent contribution of each of the enzymes to 2,3-diOHCBZ formation appears to be highly variable among individuals and dependent on the P450 expression profile of each individual. In most of the microsomal preparations, CYP3A4 appears to be the dominant enzyme catalyzing 2,3-diOHCBZ formation at low substrate concentrations. However, in human liver microsomes prepared from donor H056, CYP2C19 appears to be the principal catalyst of this reaction..
Table 2.
RAF normalized rates of 3-hydroxycarbamazepine (10 μM) conversion to 2,3-diOHCBZ by cDNA-expressed human P450 enzymes.
| Human Liver Microsomal Sample | RAF
|
3-OHCBZ (10 μM) conversion to 2,3-diOHCBZ
|
Clearance
|
|||||
|---|---|---|---|---|---|---|---|---|
| CYP2C19 | CYP3A4 | CYP2C19a | CYP3A4a | Total | Measured | CYP2C19 | CYP3A4 | |
| H003 | 2.6 | 33.9 | 1.2 | 6.8 | 8.0 | 5.6 | 0.12 | 0.98 |
| H006 | 2.1 | 16.7 | 1.0 | 3.3 | 4.3 | 5.7 | 0.10 | 0.48 |
| H023 | 3.6 | 19.8 | 1.7 | 3.9 | 5.6 | 4.8 | 0.16 | 0.57 |
| H024 | 2.1 | 22.8 | 1.0 | 4.5 | 5.5 | 5.1 | 0.09 | 0.66 |
| H030 | 19.1 | 48.9 | 9.0 | 9.7 | 18.7 | 16.5 | 0.86 | 1.42 |
| H042 | 0.4 | 83.3 | 0.2 | 16.6 | 16.8 | 20.3 | 0.02 | 2.42 |
| H056 | 28.6 | 29.4 | 13.5 | 5.9 | 19.4 | 14.7 | 1.29 | 0.85 |
| H064 | 1.0 | 10.0 | 0.5 | 2.0 | 2.5 | 5.9 | 0.05 | 0.29 |
| H088 | 10.1 | 27.2 | 4.8 | 5.4 | 10.2 | 9.6 | 0.46 | 0.79 |
| H089 | 11.3 | 66.7 | 5.3 | 13.3 | 14.6 | 17.1 | 0.51 | 1.93 |
| H093 | 2.3 | 9.4 | 1.1 | 1.9 | 3.0 | 4.7 | 0.10 | 0.27 |
| H095 | 1.8 | 4.2 | 0.8 | 0.9 | 1.7 | 2.7 | 0.08 | 0.12 |
| H103 | 1.3 | 15.6 | 0.6 | 3.1 | 3.7 | 7.4 | 0.06 | 0.45 |
| H112 | 22.6 | 138.9 | 10.7 | 27.6 | 38.3 | 39.6 | 1.02 | 4.03 |
Estimated rates calculated by multiplying the rate of the reaction catalyzed by cDNA-expressed enzymes (Vrec-CYPx) by the appropriate scaling factor, RAF.
Estimated per cent contribution of each isoform. Values in parentheses are the estimated per cent contribution of CYP2C19 and CYP3A4 calculated under the assumption that formation of 2,3-diOHCBZ is principally catalyzed by these two isoforms at a concentration of 10 μM 3-OHCBZ.
Preincubation-dependent inhibition of CYP3A4/5
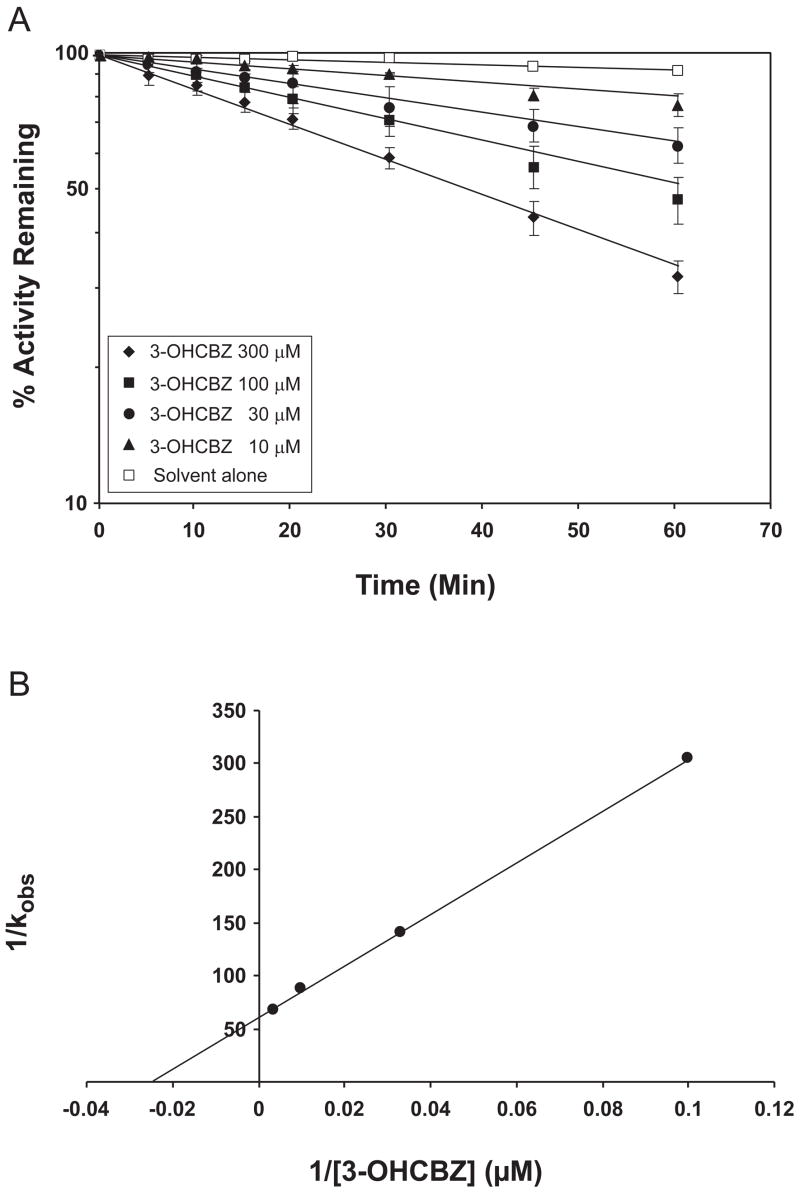
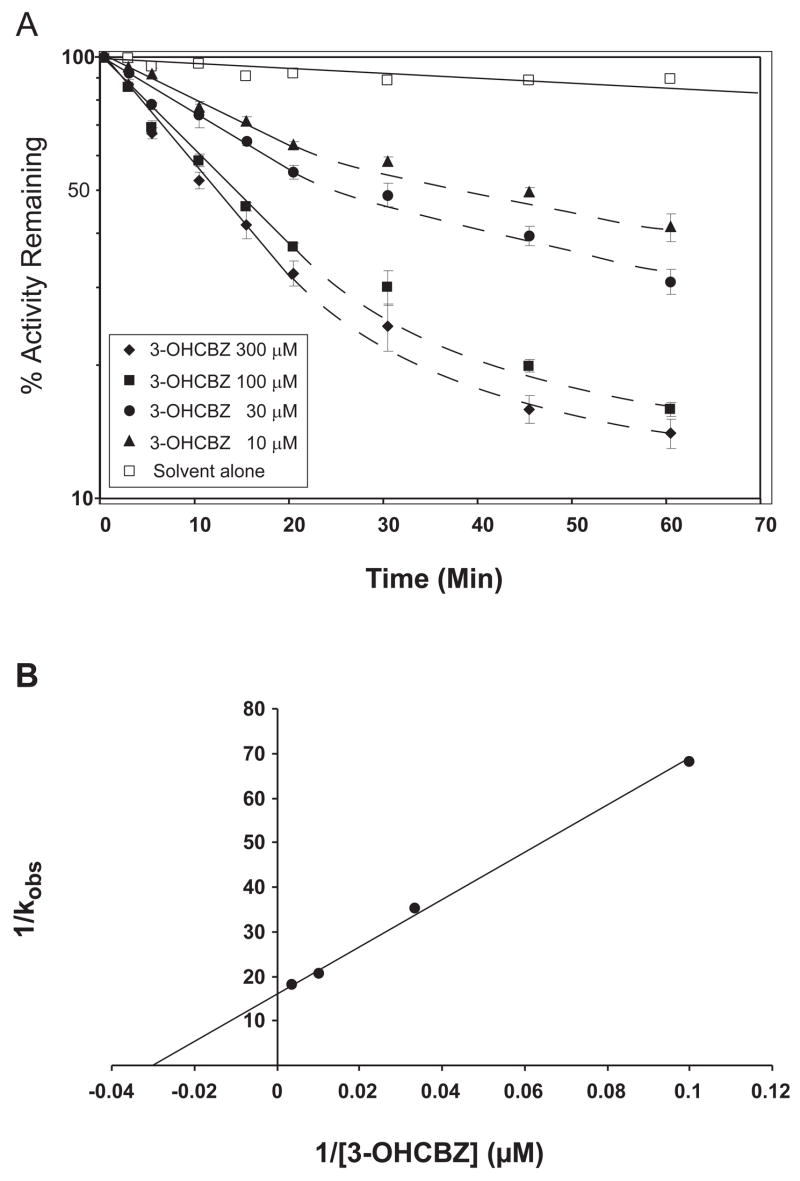
In preliminary experiments designed to optimize conditions for 2,3-diOHCBZ formation by recombinant CYP2C19 and CYP3A4, we observed that formation of 2,3-diOHCBZ was linear with time (up to 60 min) with CYP2C19, whereas with CYP3A4, the rate of metabolite formation was proportional with time only up to 15 min, with a decline in the rate thereafter (Fig. 7). As these results suggested that CYP3A4, but not CYP2C19, may be inactivated by a metabolite generated from 3-OHCBZ, studies were conducted to examine preincubation time-dependent inhibition of CYP3A4 by 3-OHCBZ (10, 30, 100 or 300 μM) in pooled human liver microsomes and in cDNA-expressed CYP3A4. In order to minimize any competitive enzyme inhibition that might be present, human liver microsomes were preincubated with 3-OHCBZ in the presence of NADPH-generating system for 0–60 min, followed by a 10-fold dilution of the incubation volume prior to the determination of CYP3A activity, as measured by testosterone 6β-hydroxylase activity. Using this approach, preincubation of 3-OHCBZ with human liver microsomes or cDNA-expressed CYP3A4 led to decreased CYP3A4 activity, which was both preincubation time- and concentration-dependent (Figs. 8 and 9). Loss of CYP3A activity due to 3-OHCBZ preincubation was also determined to be NADPH-dependent. Kinetic constants of inactivation were determined from the fit of the rate constants of inactivation (slope of enzyme of log-transformed decay plots) versus 3-OHCBZ concentrations to a Kitz-Wilson plot (double reciprocal). The concentration of inactivator required for half-maximal inactivation (Ki) of cDNA expressed CYP3A4 was 38.7 μM and the maximal rate constant of inactivation at saturation (kinact) was 0.065 min−1. Ki and kinact values for 3-OHCBZ-mediated inhibition of CYP3A activity in pooled human liver microsomes were estimated to be 41.0 μM and 0.017 min−1, respectively. Neither GSH (10 μM, 500 μM or 5 mM) nor NAC (500 μM) reduced the rate of testosterone 6β-hydroxylase inactivation by 3-OHCBZ (100 μM) when added to incubation mixtures containing pooled human liver microsomes.
Figure 7.
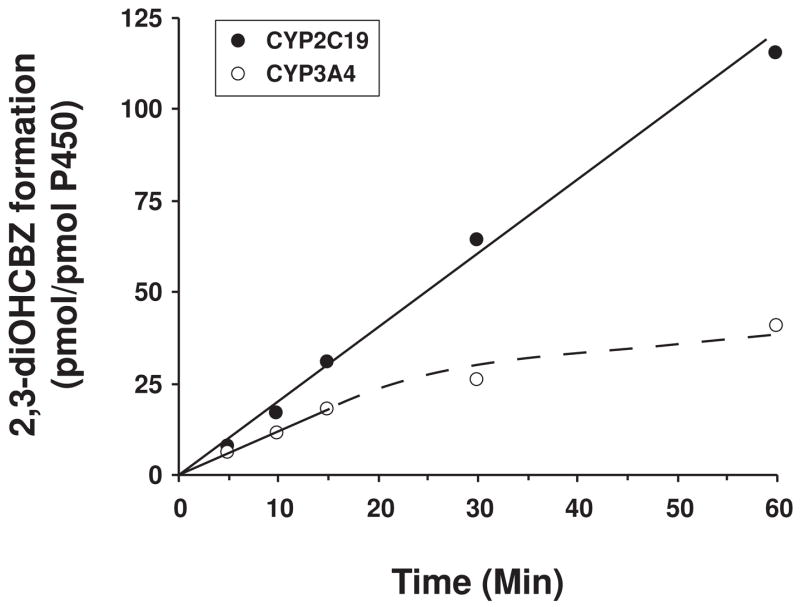
Effect of time on the formation of 2,3-diOHCBZ by human recombinant CYP2C19 and CYP3A4. 3-OHCBZ (100 μM) was incubated with insect cell microsomes containing baculovirus-expressed CYP2C19 or CYP3A4 (5 pmol/100-μl incubation) at 37±0.1°C for 0–60 min and analyzed by HPLC/MS as described under “Materials and Methods”. Each data point represents the mean of duplicate determinations.
Figure 8.
Time- and concentration-dependent inactivation of testosterone 6β-hydroxylase activity in human liver microsomes. A. Human liver microsomes (0.5 mg protein/ml) were pre-incubated with 3-OHCBZ (0, 10, 30, 100 or 300 μM) at 37±1ºC for up to 60 min and assayed for residual testosterone 6β-hydroxylase activity, as described under “Materials and Methods”. Each point shown represents the mean and standard deviation from three separate experiments performed in duplicate. B. Double reciprocal plot of the rates of inactivation as a function of 3-OHCBZ concentration. The kinact (0.017 min−1) and Ki (41.0 μM) were obtained from the y-intercept and the negative reciprocal of the x-intercept, respectively.
Figure 9.
Time- and concentration-dependent inactivation of testosterone 6β-hydroxylase activity by recombinant CYP3A4. A. Insect cell microsomes containing baculovirus-expressed CYP3A4 (50 pmol/ml) were pre-incubated with 3-OHCBZ (0, 10, 30, 100 or 300 μM) at 37±1ºC for up to 60 min and assayed for residual testosterone 6β-hydroxylase activity, as described under “Materials and Methods”. Each point shown represents the mean and standard deviation from three separate experiments performed in duplicate. B. Double reciprocal plot of the rates of inactivation as a function of 3-OHCBZ concentration. The kinact (0.065 min−1) and Ki (38.7 μM) were obtained from the y-intercept and the negative reciprocal of the x-intercept, respectively.
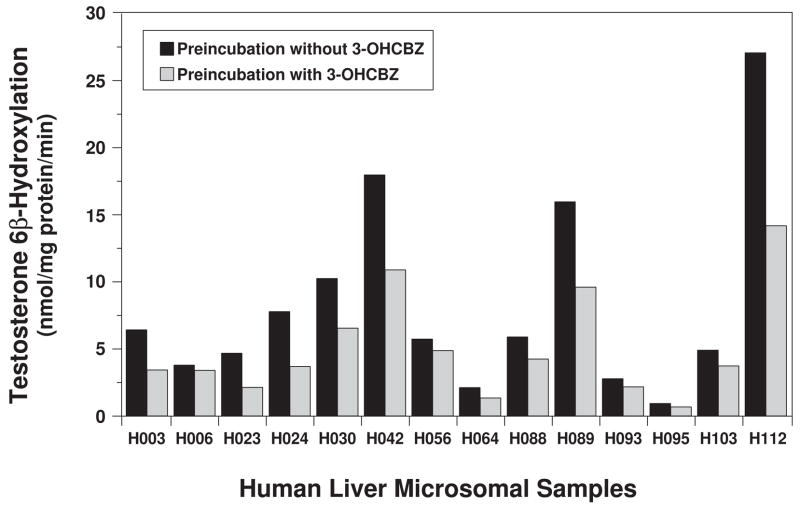
The observation that CYP3A4 could be inactivated by 3-OHCBZ metabolism suggested the possibility that this inactivation might be related to formation of 2,3-diOHCBZ in these incubations. To investigate this possibility, testosterone 6β-hydroxylase activity was determined in the panel of 14 human liver microsomal preparations following a 30 min pre-incubation with 3-OHCBZ (100 μM) and compared with the rates of 2,3-diOHCBZ formation in these samples. 3-OHCBZ-mediated inhibition of CYP3A activity ranged from 10.5 to 55.7% among the microsomal samples (Fig. 10), and the rate of 3-OHCBZ- mediated inactivation of CYP3A activity was correlated significantly with the rate of 2,3-diOHCBZ formation (r2 = 0.880, P < 0.001).
Figure 10.
Effect of pre-incubation with 3-OHCBZ on testosterone 6β-hydroxylation by a panel of human liver microsomes. Human liver microsomes (0.05 mg microsomal protein) were incubated in the presence or absence of 3-OHCBZ (100 μM) in 100-μl reaction mixtures at 37±0.1°C for 30 min, followed by determination of testosterone 6β-hydroxylase activity, as described under “Materials and Methods”.
Irreversible binding of CBZ and 3-OHCBZ to microsomal proteins
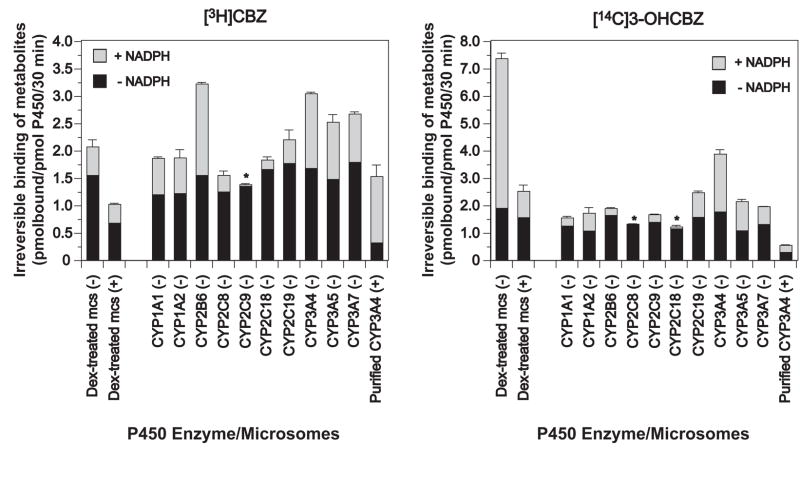
Incubation of CYP3A-enriched liver microsomes (from dexamethasone-treated mice) with [3H]CBZ resulted in 0.51 ± 0.13 pmol/pmol P450/30 min (N = 3–4 individual experiments) of irreversible microsomal protein binding, that was quenched 37% by inclusion of GSH (1 mM). As demonstrated in Fig. 11 and Fig. 12, parallel incubation with [14C]3-OHCBZ resulted in irreversible microsomal protein binding (5.32 ± 0.19 pmol/pmol P450/30 min) that was attenuated >80% by GSH inclusion. These findings suggested that mouse liver microsomal P450s were capable of bioactivating CBZ and 3-OHCBZ to reactive electrophilic metabolites capable of binding covalently to nucleophilic sites on microsomal proteins. As shown in Fig. 11, the irreversible binding of metabolites generated from [14C]3-OHCBZ appeared to be concentrated in a single band with a molecular weight of ~55 kDa, a molecular mass consistent with those of P450 enzymes. An image of the full gel is included as supplementary information. In addition, the ability of GSH to quench microsomal covalent binding suggests that a large proportion of the reactive metabolites formed was capable of escaping the P450 active site prior to covalent bond formation with microsomal proteins.
Figure 11.
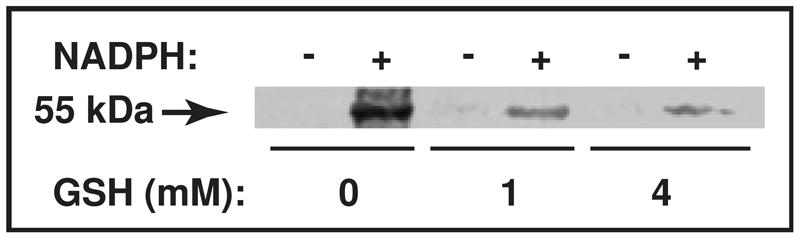
SDS-PAGE/PhosphorImager analysis of [14C]3-OHCBZ covalent binding to mouse liver microsomal protein. Liver microsomes from Dex-treated mice were incubated as described (Materials and Methods) with freshly prepared GSH (0, 1 or 4 mM). A 50 μl-aliquot of the incubation mixture (≈ 100 pmol P450) was removed at 30 min, mixed with the SDS-PAGE loading buffer, heated at 95°C for 5 min and subjected to SDS-PAGE analyses. The gel was fixed, the signal amplified with Amersham Amplify™ fluorographic reagent, and then subjected to fluorography by PhosphorImager analyses. Under these conditions, covalent binding of [14C]3-OHCBZ to mouse liver microsomes in incubations containing NADPH was 5.32 ± 0.19 (in the absence of GSH), 0.91 ± 0.23 (with 1 mM GSH), and 0.38 ± 0.10 (with 4 mM GSH) pmol/pmol P450/30 min, respectively, and appeared to be concentrated in a single band with a molecular weight of ~55 kDa. An image of the full gel is included as supplementary information.
Figure 12.
Irreversible binding of metabolites generated from [3H]CBZ or [14C]3-OHCBZ to mouse liver microsomal or human recombinant P450 proteins. CYP3A enriched liver microsomes from dexamethasone-treated (Dex-treated) mice or microsomes containing cDNA-expressed human P450 enzymes co-expressed with P450 reductase and cytochrome b5 (BD Gentest Supersomes™) were incubated with [3H]CBZ or [14C]3-OHCBZ (0.2 μCi; 0.5 mM) in the presence or absence of NADPH (+ NADPH and − NADPH, respectively), as described in Materials and Methods. Incubations conducted in the absence of NADPH were performed in duplicate, whereas incubations conducted in the presence of NADPH were performed at least in triplicate (N = 3 or 4). Some incubations also contained freshly prepared GSH (1 mM or 4mM in incubations containing liver microsomes from Dex-treated mice or purified recombinant CYP3A4 functionally reconstituted, respectively) and are denoted with a (+); whereas incubations conducted in the absence of GSH are denoted with a (−). Functional reconstitution of purified CYP3A4 requires that GSH be included for optimal activity (Gillam et al, 1993), hence incubations containing purified CYP3A4 in the absence of GSH were not performed. After 30 min, reactions were terminated with ice-cold methanol/5% H2SO4, microsomal proteins precipitated, sedimented and sequentially “washed” to reduce non-specifically bound radiolabelled substrates or metabolites, as described in Materials and Methods. Each of the rates of irreversible binding determined in incubations performed in the presence of NADPH were found to be significantly different from the corresponding rates determined in incubations performed in the absence of NADPH (controls) as detemined using a two-tailed, paired Student’s t test (p ≤ 0.05). The incubations denoted with an asterisk (*) were found to be not statistically different.
Of the 10 individual recombinant P450 enzymes examined, CYPs 2B6, 3A4 and 3A5 activated [3H]CBZ to reactive/electrophilic metabolite(s) to the greatest extent, yielding the highest level of irreversible [3H]CBZ-protein binding, whereas CYPs 3A7, 1A1 and 1A2 yielded an intermediate level of protein-reactive metabolites. In contrast, CYPs 2C8 and 2C19 were relatively less reactive, yielding considerably lower levels of protein-reactive metabolites, and CYPs 2C9 and 2C18 catalyzed little or no reactive metabolite formation. When [14C]3-OHCBZ was used as the substrate, CYP3A4 was clearly the most active enzyme catalyzing 3-OHCBZ bioactivation, whereas CYPs 3A5, 3A7, 2C19 and 1A2 had intermediate catalytic activity, CYPs 1A1, 2C9 and 2B6 exhibited considerably lower activity and CYPs 2C8 and 2C18 had little or no capacity to bioactivate 3-OHCBZ. As shown in Fig. 12, the abilities of the recombinant P450 enzymes to bioactivate CBZ and 3-OHCBZ varied by isoform, as determined by the relative extent of irreversible [3H]CBZ/[14C]3-OHCBZ protein binding. Whereas CYP2B6 appears to be considerably more potent in activating CBZ than 3-OHCBZ (which suggests that the reactive metabolite(s) formed from CBZ by CYP2B6 involves a metabolic pathway independent of 3-OHCBZ), CYP3A5 can activate both compounds equivalently, and CYP3A4 activates 3-OHCBZ to a much greater extent than CBZ (2.11 versus 1.35 pmol bound/pmol P450/30 min from [14C]3-OHCBZ and [3H]CBZ, respectively). This latter observation suggests that 3-OHCBZ is a key intermediate in CYP3A4-dependent CBZ activation.
Discussion
As is the case with most drug-induced hypersensitivity reactions, the mechanism underlying the hypersensitivity responses to aromatic anticonvulsants is not well understood. Drug molecules (<1000 Da) are generally considered to be too small to be inherently immunogenic, hence it has been proposed that most drug-induced hypersensitivity reactions require the bioactivation of a drug into one or more reactive metabolites capable of binding covalently with cellular proteins (Park et al., 1987). These modified proteins may subsequently function as neo-antigens capable of initiating an immune response. However, the process by which most of these drug-modified macromolecules elicit an immune response is unclear.
Reactive metabolites formed from several drugs have been demonstrated to inactivate cytochrome P450s by binding to the protein in or near the active site, or alternatively, by binding to the heme moiety. This mechanism-based inhibition is characterized by NADPH-, time- and substrate concentration-dependent inactivation (Guengerich, 2001). In most instances, these damaged proteins do not elicit an immune response, but simply undergo intracellular degradation. However, under the appropriate circumstances, the formation of P450 adducts with certain drugs in susceptible individuals results in the initiation of an immune response that targets either the P450-adduct or the native P450 enzyme itself.
Antibodies which recognize drug-adducted P450s (trifluoroacetyl-CYP2E1 and tienilic acid-CYP2C9) have been detected in the sera of patients with halothane-induced (Eliasson and Kenna, 1996) and tienilic acid-induced (Lecoeur et al., 1996) hepatitis, respectively. However, most often drug-induced serum anti-P450 antibodies appear to recognize native protein sequences, such as that seen with dihydralazine (CYP1A2) (Bourdi et al., 1994), disulfiram (CYP1A2) (Eliasson et al., 1998), isoflurane (CYP2A6) (Martin et al., 2001), the immunosuppressants, cyclosporine and FK506 (CYP3A4, CYP2E1, CYP1A2 and CYP2C9) (Lytton et al., 2002), ethanol (CYP2E1 and CYP2C9) (Lytton et al., 1999), the anticonvulsants, phenytoin and CBZ (CYP3A and CYP2C forms) (Riley et al., 1993; Leeder et al., 1996) as well as halothane (CYP2E1) (Eliasson and Kenna, 1996) and tienilic acid (CYP2C9) (Lecoeur et al., 1996), rather than the drug-derived hapten.
Circulating anti-P450 antibodies present in patients treated with halothane, tienilic acid or dihydralazine have been shown to be directed at the P450s responsible for the bioactivation of these compounds. Moreover, the enzymes responsible for catalyzing the formation of protein reactive metabolites all suffered inactivation that was NADPH-, time and substrate concentration dependent. These data support the hypothesis that there is a group of drugs which are associated with idiosyncratic toxicity that are converted to metabolites that inactivate the enzyme responsible for their bioactivation and subsequently become targets of circulating antibodies after initiation of an immune response. The results of the present study indicate that the CBZ metabolite, 3-OHCBZ, can be converted into a reactive species that not only forms a CYP3A4 covalent adduct, but is also capable of inactivating CYP3A4 in a mechanism-based manner.
Previous in vitro studies have demonstrated the bioactivation of CBZ by the formation of both cytotoxic (Pirmohamed et al., 1993) and protein-reactive metabolites in human liver microsomes (Pirmohamed et al., 1992; Lillibridge et al., 1996; Wolkenstein et al., 1998; Masubuchi et al., 2001). Additional in vitro studies with recombinant human P450 enzymes reported the covalent binding of CBZ with proteins belonging to the 1A, 2C and 3A P450 subfamilies (Wolkenstein et al., 1998; Masubuchi et al., 2001, Kang et al., 2008). However, no evidence of mechanism-based inactivation of human CYP2C or CYP3A enzymes was reported. The results of the present study indicate that the CBZ metabolite, 3-OHCBZ, can be converted into a reactive species that not only forms a CYP3A4 covalent adduct, but it is also capable of inactivating CYP3A4 in a mechanism-based manner. When combined with the knowledge that sera from CBZ-hypersensitive patients often contain antibodies that recognize CYP3A proteins (Leeder et al., 1996), this suggests that CBZ may belong with the aforementioned group of drugs that initiate idiosyncratic hypersensitivity following the inactivation and covalent binding of reactive metabolites with the P450 enzyme mediating their formation.
Unfortunately, it was not possible to ascertain the identity of the metabolite(s) responsible for CYP3A4 inactivation and/or adduct formation from the studies reported here. Initially, we speculated that conversion of 3-OHCBZ to the catechol, 2,3-diOHCBZ, followed by a subsequent oxidation step could generate a reactive o-quinone species. Although trace amounts of a metabolite with a m/z ratio (267) consistent with that of CBZ-quinone (data not shown) were observed in incubations containing relatively high concentrations of 3-OHCBZ (≥100 μM), the identity of this metabolite has not been confirmed nor is there direct evidence to determine whether CBZ-quinone was involved in the CYP3A4 inactivation and/or adduct formation observed in the studies reported here. It is possible that CYP3A4 converts 3-OHCBZ into metabolites other than (or in addition to) CBZ-quinone that are sufficiently reactive to inactivate CYP3A4 directly. Evidence is presented in the accompanying paper for myeloperoxidase- and H2O2-mediated formation of one or more free radical intermediates from 3-OHCBZ (depicted in Fig. 1). Similar reactive species could be formed by CYP3A4, since it is known that P450s are capable of generating free radical species via a single electron oxidation followed by release of the intermediate, resulting in an interruption of the typical P450 two-electron oxidation cycle (Guengerich, 2001). Certainly any uncoupling of the CYP3A4 cycle, would generate reactive oxygen species, which could damage the CYP3A4 active site either directly or indirectly, possibly through accelerated oxidation of 2,3-diOHCBZ to CBZ-quinone, as has been proposed previously for a catechol metabolite of tamoxifen (Notley et al., 2002). Another possibility is that CYP3A4 could catalyze the formation of a reactive quinone methide from 3-OHCBZ, in a manner analogous to that of CBZ-iminoquinone formation from 2-OHCBZ.
Significant cross-sensitivity between CBZ and phenytoin has been observed in patients experiencing hypersensitivity reactions to aromatic anticonvulsants, which suggests that the mechanisms mediating adverse reactions to these two drugs may be similar, including the formation of similar metabolites. The biotransformation/bioactivation pathway converting CBZ to 2,3-diOHCBZ demonstrates a striking similarity to the bioactivation pathway described for phenytoin. Like CBZ, phenytoin can be converted to a catechol, 3′,4′-dihydroxyphenytoin, which has been demonstrated to form protein reactive intermediates via the subsequent oxidation of the catechol to reactive semiquinone and quinone species (Munns et al., 1997). Furthermore, the P450 enzymes responsible for catalyzing the formation of the catechol, largely CYP2C18, CYP2C19 and CYP3A4, have also been shown to be the major targets of covalent adduct formation (Cuttle et al., 2000; Komatsu et al., 2000; Kinobe et al., 2005). Interestingly, phenytoin has also been reported to form a reactive free radical intermediate (Parman et al., 1998). The results of the present study indicate that the principal P450 enzymes responsible for catalyzing 2,3-diOHCBZ formation are CYP3A4 and CYP2C19, the same enzymes largely responsible for catalyzing 3′,4′-dihydroxy-phenytoin formation and that are major targets of covalent adduct formation with phenytoin-derived reactive species. Although CYP2C19 appears to be predominantly expressed in liver and duodenum (Klose et al., 1999), the tissue distribution of CYP3A4 includes liver and a number of extrahepatic tissues, such as kidney, skin, intestine, and blood (Lamba et al., 2002), which parallels the sites of involvement observed in anticonvulsant hypersensitivity. When this information is combined with the bioactivation data for phenytoin and 3-OHCBZ and with the previous epitope mapping studies of patients’ serum antibodies (Leeder et al., 1996), it becomes enticing to speculate that an anticonvulsant adduct of CYP3A4 (or an altered degradation product of such an adduct) may function as at least one of the antigens eliciting a hypersensitivity response to aromatic anticonvulsants. Involvement of a common target and a similar mechanism of bioactivation for phenytoin and CBZ could potentially explain, at least in part, the ability of these compounds to cross-react in the development of hypersensitivity reactions. Ultimately, investigation of the cellular consequences of reactive metabolite/covalent adduct formation may lead to the elucidation of the mechanism by which drug bioactivation and immune responses interact and contribute to the pathogenesis of idiosyncratic reactions to aromatic anticonvulsants.
In summary, the results of this study have demonstrated that preincubation of 3-OHCBZ with human liver microsomes or recombinant CYP3A4 led to decreased CYP3A4 activity, which was both preincubation time- and concentration-dependent. Incubations with 3-OHCBZ and mouse liver microsomes or with human recombinant P450s, in particular CYP3A4, were shown to form covalent adducts with microsomal proteins that could be partially inhibited by inclusion of GSH. The results of this study further demonstrated that CYP3A4 and CYP2C19 are primarily responsible for P450-mediated conversion of 3-OHCBZ to 2,3-diOHCBZ in human liver microsomes; the extent to which either of these enzymes participates in the reaction is dependent on the relative expression of each of the enzymes in a given individual. Further in vitro experiments utilizing 2,3-diOHCBZ as substrate are warranted to ascertain whether a protein-reactive intermediate may be generated from the catechol and which, if any, P450s are involved in its formation and/or are the object of covalent modification.
Supplementary Material
Acknowledgments
Supported in part by Grants # R01GM58883 (J.S.L.), National Institute of General Medical Sciences, #U01H044239 (J.S.L.), National Institutes of Child Health and Human Development, and R01GM44037 (M. A. C.), National Institute of General Medical Sciences.
Abbreviations
- CBZ
Carbamazepine
- CBZ-IQ
carbamazepine iminoquinone
- CBZ-quinone
carbamazepine o-quinone
- CYP
cytochrome P450
- Dex
dexamethasone
- 2
3-diOHCBZ, 2,3-dihydroxycarbamazepine
- EDTA
ethylenediaminetetraacetic acid
- GSH
reduced glutathione
- HPLC
high performance liquid chromatography
- 3-OHCBZ
3-hydroxycarbamazepine
- MH+
protonated molecular ion
- MS
mass spectrometry
- m/z ratio
mass to charge ratio
- NAC
N-acetyl cysteine
- TAO
troleandomycin
Footnotes
The NADPH-regenerating system consisting of isocitrate and isocitrate dehydrogenase (ICDH) was added to incubations along with NADPH (1.5 mM) in order to recycle any NADP+ generated in the reaction back into NADPH. The ICDH conversion of isocitrate and NADP+ to oxalosuccinate + NADPH does not require Mn+2 (or Mg+2) ions (Moyle, J, Biochem J, 63:552, 1956). However, the subsequent coupled reaction, i.e., the formation of α-ketoglutarate from oxalosuccinate does, and Mn+2 is found to accelerate NADPH formation by stimulating the forward reaction, but is not an obligatory cofactor (Moyle, 1956). The Sigma ICDH enzyme used in the experiments described in this manuscript was supplied with Mn+2 ions (manganous sulfate), and the 0.1 M phosphate buffer used in the reaction also contained traces of Mn+2 and Mg+2 ions. For these combined reasons additional Mg+2 was not included, as is necessary if NADPH is to be generated totally from NADP+.
References
- Bornheim LM, Correia MA. Selective inactivation of mouse liver cytochrome P-450IIIA by cannabidiol. Mol Pharmacol. 1990;38:319–326. [PubMed] [Google Scholar]
- Bourdi M, Tinel M, Beaune PH, Pessayre D. Interactions of dihydralazine with cytochromes P4501A: a possible explanation for the appearance of anti-cytochrome P4501A2 autoantibodies. Mol Pharmacol. 1994;45:1287–1295. [PubMed] [Google Scholar]
- Bu HZ, Kang P, Deese AJ, Zhao P, Pool WF. Human In Vitro Glutathionyl And Protein Adducts Of Carbamazepine-10,11-Epoxide A Stable And Pharmacologically Active Metabolite Of Carbamazepine. Drug Metab Dispos. 2005;30:1920–1924. doi: 10.1124/dmd.105.006866. [DOI] [PubMed] [Google Scholar]
- Cuttle L, Munns AJ, Hogg NA, Scott JR, Hooper WD, Dickinson RG, Gillam EM. Phenytoin metabolism by human cytochrome P450: involvement of P450 3A and 2C forms in secondary metabolism and drug-protein adduct formation. Drug Metab Dispos. 2000;28:945–950. [PubMed] [Google Scholar]
- Eliasson E, Kenna JG. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol Pharmacol. 1996;50:573–582. [PubMed] [Google Scholar]
- Eliasson E, Stal P, Oksanen A, Lytton S. Expression of autoantibodies to specific cytochromes P450 in a case of disulfiram hepatitis. J Hepatol. 1998;29:819–825. doi: 10.1016/s0168-8278(98)80264-x. [DOI] [PubMed] [Google Scholar]
- Furst SM, Sukhai P, McClelland RA, Uetrecht JP. Covalent binding of carbamazepine oxidative metabolites to neutrophils. Drug Metab Dispos. 1995;23:590–594. [PubMed] [Google Scholar]
- Gillam EM, Baba T, Kin BR, Ohmori S, Guengerich FP. Expression of modified human cytochrome P450 3A4 in Escherichia coli and purification and reconstitution of the enzyme. Arch Biochem Biophys. 1993;15:123–131. doi: 10.1006/abbi.1993.1401. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- Ju C, Utrecht JP. Detection of 2-hydroxyiminostilbene in the urine of patients taking carbamazepine and its oxidation to a reactive iminoquinone intermediate. J Pharmacol Exp Ther. 1999;288:51–56. [PubMed] [Google Scholar]
- Kang P, Liao M, Leeder JS, Pearce RE, Correia MA. CYP3A4-Mediated carbamazepine (CBZ) metabolism: Formation of a covalent CBZ-adduct and alteration of the enzyme kinetic profile. Drug Metab Dispos. 2008;36(3):490–9. doi: 10.1124/dmd.107.016501. Epub 2007 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinobe RT, Parkinson OT, Mitchell DJ, Gillam EM. P450 2C18 catalyzes the metabolic bioactivation of phenytoin. Chem Res Toxicol. 2005;18:1868–1875. doi: 10.1021/tx050181o. [DOI] [PubMed] [Google Scholar]
- Klose TS, Blaisdell JA, Goldstein JA. Gene structure of CYP2C8 and extrahepatic distribution of the human CYP2Cs. J Biochem Mol Toxicol. 1999;13:289–295. doi: 10.1002/(sici)1099-0461(1999)13:6<289::aid-jbt1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Komatsu T, Yamazaki H, Asahi S, Gillam EM, Guengerich FP, Nakajima M, Yokoi T. Formation of a dihydroxy metabolite of phenytoin in human liver microsomes/cytosol: roles of cytochromes P450 2C9, 2C19, and 3A4. Drug Metab Dispos. 2000;28:1361–1368. [PubMed] [Google Scholar]
- Lamba JK, Lin YS, Scheutz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–1294. doi: 10.1016/s0169-409x(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Lecoeur S, Andre C, Beaune PH. Tienilic acid-induced autoimmune hepatitis: anti-liver and-kidney microsomal type 2 autoantibodies recognize a three-site conformational epitope on cytochrome P4502C9. Mol Pharmacol. 1996;50:326–333. [PubMed] [Google Scholar]
- Leeder JS, Gaedigk A, Lu X, Cook VA. Epitope mapping studies with human anti-cytochrome P450 3A antibodies. Mol Pharmacol. 1996;49:234–243. [PubMed] [Google Scholar]
- Lertratanangkoon K, Horning MG. Metabolism of carbamazepine. Drug Metab Dispos. 1982;10:1–10. [PubMed] [Google Scholar]
- Lillibridge JH, Amore BM, Slattery JT, Kalhorn TF, Nelson SD, Finnell RH, Bennett GD. Protein-reactive metabolites of carbamazepine in mouse liver microsomes. Drug Metab Dispos. 1996;24:509–514. [PubMed] [Google Scholar]
- Lytton SD, Berg U, Nemeth A, Ingelman-Sundberg M. Autoantibodies against cytochrome P450s in sera of children treated with immunosuppressive drugs. Clin Exp Immunol. 2002;127:293–302. doi: 10.1046/j.1365-2249.2002.01754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton SD, Helander A, Zhang-Gouillon ZQ, Stokkeland K, Bordone R, Arico S, Albano E, French SW, Ingelman-Sundberg M. Autoantibodies against cytochromes P-4502E1 and P-4503A in alcoholics. Mol Pharmacol. 1999;55:223–233. doi: 10.1124/mol.55.2.223. [DOI] [PubMed] [Google Scholar]
- Martin JL, Keegan MT, Vasdev GM, Nyberg SL, Bourdi M, Pohl LR, Plevak DJ. Fatal hepatitis associated with isoflurane exposure and CYP2A6 autoantibodies. Anesthesiology. 2001;95:551–553. doi: 10.1097/00000542-200108000-00043. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Nakano T, Ose A, Horie T. Differential selectivity in carbamazepine-induced inactivation of cytochrome P450 enzymes in rat and human liver. Arch Toxicol. 2001;75:538–543. doi: 10.1007/s002040100270. [DOI] [PubMed] [Google Scholar]
- Moyle J. Some properties of purified isocitric enzyme. Biochem J. 1956;63:552–558. doi: 10.1042/bj0630552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns AJ, De Voss JJ, Hooper WD, Dickinson RG, Gillam EM. Bioactivation of phenytoin by human cytochrome P450: characterization of the mechanism and targets of covalent adduct formation. Chem Res Toxicol. 1997;10:1049–1058. doi: 10.1021/tx9700836. [DOI] [PubMed] [Google Scholar]
- Notley L, de Wolfe C, Wunsch R, Lancaster R, Gillam EM. Bioactivation of Tamoxifen by recombinant human cytochrome P450 enzymes. Chem Res Toxicol. 2001;15:614–622. doi: 10.1021/tx0100439. [DOI] [PubMed] [Google Scholar]
- Park BK, Coleman JW, Kitteringham NR. Drug disposition and drug hypersensitivity. Biochem Pharmacol. 1987;36:581–590. [PubMed] [Google Scholar]
- Parman T, Chen G, Wells PG. Free radical intermediates of phenytoin and related teratogens. Prostaglandin H synthase-catalyzed bioactivation, electron paramagnetic resonance spectrometry, and photochemical product analysis. J Biol Chem. 1998;273:25079–25088. doi: 10.1074/jbc.273.39.25079. [DOI] [PubMed] [Google Scholar]
- Pearce RE, Uetrecht J, Leeder JS. Pathways of Carbamazepine Bioactivation In Vitro II. The Role of Human Cytochrome P450 Enzymes in the Formation of 2-Hydroxyiminostilbene. Drug Metab Dispos. 2005;33:1819–1826. doi: 10.1124/dmd.105.004861. [DOI] [PubMed] [Google Scholar]
- Pearce RE, Vakkalagadda GR, Leeder JS. Pathways of carbamazepine bioactivation in vitro I. Characterization of human cytochromes P450 responsible for the formation of 2- and 3-hydroxylated metabolites. Drug Metab Dispos. 2002;30:1170–1179. doi: 10.1124/dmd.30.11.1170. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M, Kitteringham NR, Guenthner TM, Breckenridge AM, Park BK. An investigation of the formation of cytotoxic, protein-reactive and stable metabolites from carbamazepine in vitro. Biochem Pharmacol. 1992;43:1675–1682. doi: 10.1016/0006-2952(92)90696-g. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M, Templeton E, Wilson AS, Madden S, Kitteringham NR, Park BK. The effect of enzyme induction on the cytochrome P450-mediated bioactivation of carbamazepine by mouse liver microsomes. Biochem Pharmacol. 1993;46:1529–1538. doi: 10.1016/0006-2952(92)90674-8. [DOI] [PubMed] [Google Scholar]
- Riley RJ, Smith G, Wolf CR, Cook VA, Leeder JS. Human anti-endoplasmic reticulum autoantibodies produced in aromatic anticonvulsant hypersensitivity reactions recognise rodent CYP3A proteins and a similarly regulated human P450 enzyme(s) Biochem Biophys Res Commun. 1993;191:32–40. doi: 10.1006/bbrc.1993.1180. [DOI] [PubMed] [Google Scholar]
- Rodrigues AD. Integrated cytochrome P450 reaction phenotyping: attempting to bridge the gap between cDNA-expressed cytochromes P450 and native human liver microsomes. Biochem Pharmacol. 1999;57:465–480. doi: 10.1016/s0006-2952(98)00268-8. [DOI] [PubMed] [Google Scholar]
- Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J Clin Invest. 1988;82:1826–1832. doi: 10.1172/JCI113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg SP, Gordon GB, Blake DA, Mellits ED, Bross DS. Anticonvulsant toxicity in vitro: possible role of arene oxides. J Pharmacol Exp Ther. 1981;217:386–389. [PubMed] [Google Scholar]
- Tennis P, Stern RS. Risk of serious cutaneous disorders after initiation of use of phenytoin, carbamazepine, or sodium valproate: a record linkage study. Neurology. 1997;49:542–546. doi: 10.1212/wnl.49.2.542. [DOI] [PubMed] [Google Scholar]
- Usmani KA, Rose RL, Hodgson E. Inhibition and activation of the human liver microsomal and human cytochrome P450 3A4 metabolism of testosterone by deployment-related chemicals. Drug Metab Dispos. 2003;31:384–391. doi: 10.1124/dmd.31.4.384. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan K, von Moltke LL, Court MH, Harmatz JS, Crespi CL, Greenblatt DJ. Comparison between cytochrome P450 (CYP) content and relative activity approaches to scaling from cDNA-expressed CYPs to human liver microsomes: ratios of accessory proteins as sources of discrepancies between the approaches. Drug Metab Dispos. 2000;28:1493–1504. [PubMed] [Google Scholar]
- Wang H, Dick R, Yin H, Licad-Coles E, Kroetz DL, Szklarz G, Harlow G, Halpert JR, Correia MA. Structure-function relationships of human liver cytochromes P450 3A: aflatoxin B1 metabolism as a probe. Biochemistry. 1998;37(36):12536–12545. doi: 10.1021/bi980895g. [DOI] [PubMed] [Google Scholar]
- Wolkenstein P, Tan C, Lecoeur S, Wechsler J, Garcia_Martin N, Charue D, Bagot M, Beaune P. Covalent binding of carbamazepine reactive metabolites to P450 isoforms present in the skin. Chem-Biol Interact. 1998;113:39–50. doi: 10.1016/s0009-2797(98)00021-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.