Abstract
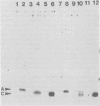
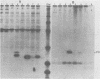
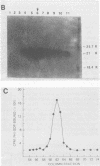
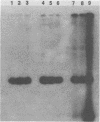
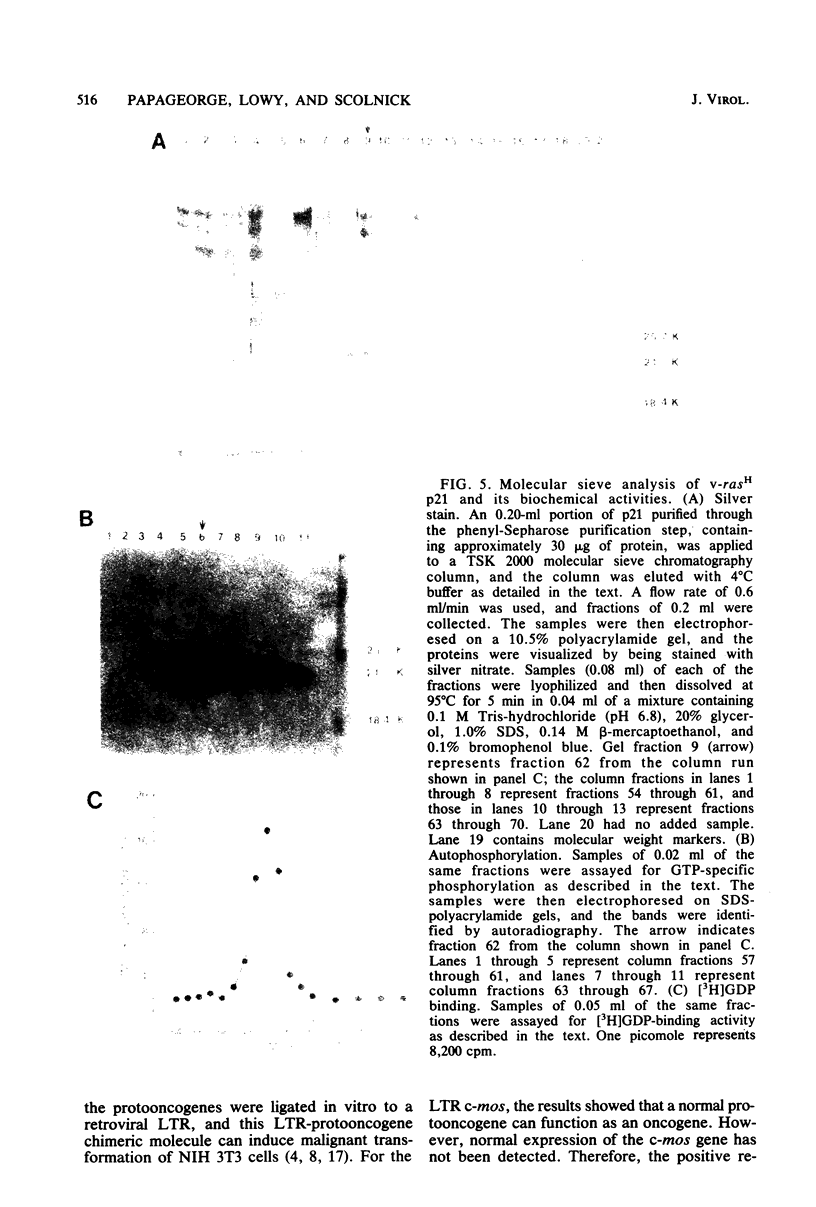
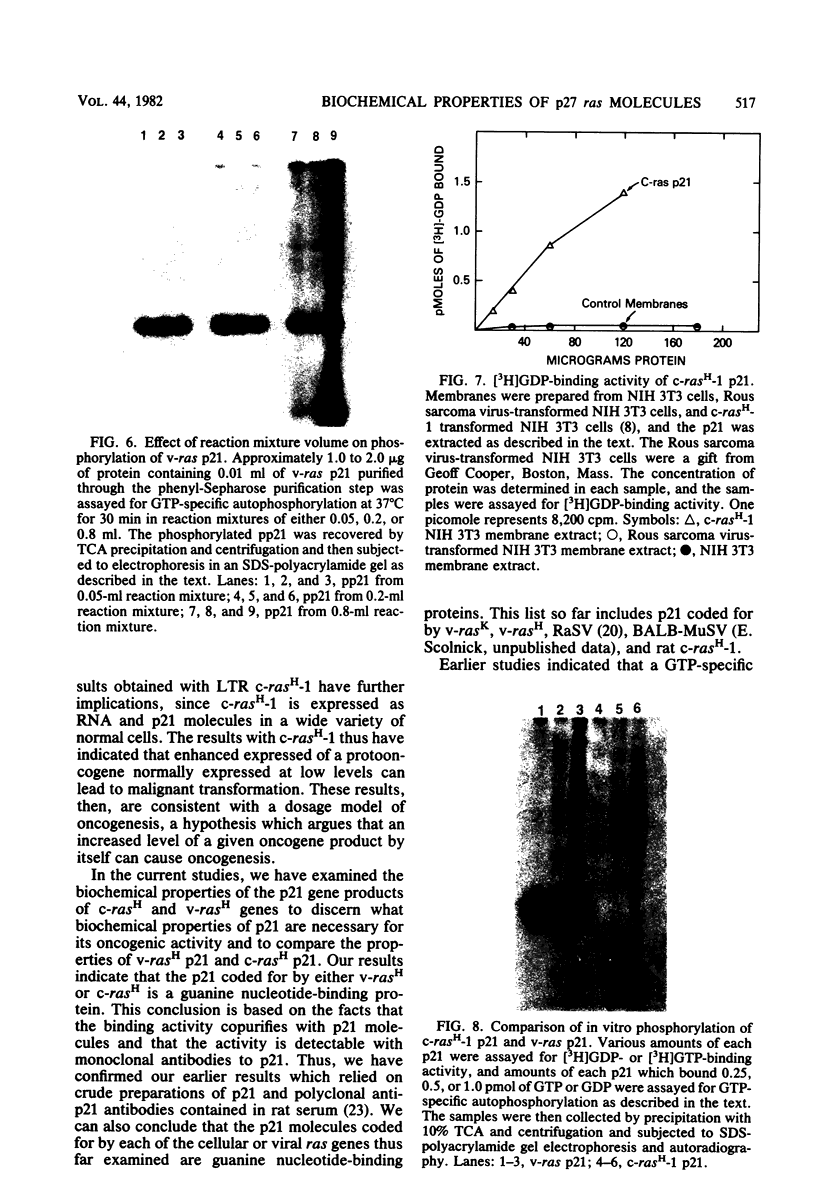
In earlier studies, we molecularly cloned a normal cellular gene, c-rasH-1, homologous to the v-ras oncogene of Harvey murine sarcoma virus (v-rasH). By ligating a type c retroviral promotor to c-rasH-1, we could transform NIH 3T3 cells with the c-rasH-1 gene. The transformed cells contained high levels of a p21 protein coded for by the c-rasH-1 gene. In the current studies, we have purified extensively both v-rasH p21 and c-rasH p21 and compared the in vivo and in vitro biochemical properties of both these p21 molecules. The p21 proteins coded for by v-rasH and c-rasH-1 shared certain properties: each protein was synthesized as a precursor protein which subsequently became bound to the inner surface of the plasma membrane; each protein was associated with guanine nucleotide-binding activity, a property which copurified with p21 molecules on a high-pressure liquid chromatography molecular sizing column. In some other properties, the v-rasH and c-rasH p21 proteins differed. In vivo, approximately 20 to 30% of v-rasH p21 molecules were in the form of phosphothreonine-containing pp21 molecules, whereas in vivo only a minute fraction of c-rasH-1 p21 contained phosphate, and this phosphate was found on a serine residue. v-rasH pp21 molecules with an authentic phosphothreonine peptide could be synthesized in vitro in an autophosphorylation reaction in which the gamma phosphate of GTP was transferred to v-rasH p21. No autophosphorylating activity was associated with purified c-rasH-1 p21 in vitro. The results indicate a major qualitative difference between the p21 proteins coded for by v-rasH and c-rasH-1. The p21 coded for by a mouse-derived oncogenic virus, BALB murine sarcoma virus, resembled the p21 coded for by c-rasH-1 in that it bound guanine nucleotides but did not label appreciably with 32Pi. The forms of p21 coded for by other members of the ras gene family were compared, and the results indicate that the guanine nucleotide-binding activity is common to p21 molecules coded for by all known members of the ras gene family.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. R., Devare S. G., Tronick S. R., Ellis R. W., Aaronson S. A., Scolnick E. M. Generation of BALB-MuSV and Ha-MuSC by type C virus transduction of homologous transforming genes from different species. Cell. 1981 Oct;26(1 Pt 1):129–134. doi: 10.1016/0092-8674(81)90041-6. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Enemies within: the genesis of retrovirus oncogenes. Cell. 1981 Jan;23(1):5–6. doi: 10.1016/0092-8674(81)90263-4. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Blair D. G., McClements W. L., Oskarsson M. K., Fischinger P. J., Vande Woude G. F. Biological activity of cloned Moloney sarcoma virus DNA: Terminally redundant sequences may enhance transformation efficiency. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3504–3508. doi: 10.1073/pnas.77.6.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Chang E. H., Lander M. R., Ellis R. W., Scolnick E. M., Lowy D. R. Amplification and rearrangement of onc genes in mammalian species. Nature. 1982 Mar 25;296(5855):361–363. doi: 10.1038/296361a0. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Varmus H. E., Bishop J. M., Essex M., Hardy W. D., Jr, Martin G. S., Rosenberg N. E., Scolnick E. M., Weinberg R. A., Vogt P. K. Proposal for naming host cell-derived inserts in retrovirus genomes. J Virol. 1981 Dec;40(3):953–957. doi: 10.1128/jvi.40.3.953-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeo D., Gonda M. A., Young H. A., Chang E. H., Lowy D. R., Scolnick E. M., Ellis R. W. Analysis of two divergent rat genomic clones homologous to the transforming gene of Harvey murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3328–3332. doi: 10.1073/pnas.78.6.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager G. L., Chang E. H., Chan H. W., Garon C. F., Israel M. A., Martin M. A., Scolnick E. M., Lowy D. R. Molecular cloning of the Harvey sarcoma virus closed circular DNA intermediates: initial structural and biological characterization. J Virol. 1979 Sep;31(3):795–809. doi: 10.1128/jvi.31.3.795-809.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langbeheim H., Shih T. Y., Scolnick E. M. Identification of a normal vertebrate cell protein related to the p21 src of Harvey murine sarcoma virus. Virology. 1980 Oct 30;106(2):292–300. doi: 10.1016/0042-6822(80)90252-4. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Peters R. L., Rabstein L. S., VanVleck R., Kelloff G. J., Huebner R. J. Naturally occurring sarcoma virus of the BALB/cCr mouse. J Natl Cancer Inst. 1974 Dec;53(6):1725–1729. [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Huebner R. J. In vitro isolation of stable rat sarcoma viruses. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2972–2976. doi: 10.1073/pnas.75.6.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Papageorge A. G., Shih T. Y. Guanine nucleotide-binding activity as an assay for src protein of rat-derived murine sarcoma viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5355–5359. doi: 10.1073/pnas.76.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Gruss P., Dhar R., Oroszlan S., Scolnick E. M. Identification of a precursor in the biosynthesis of the p21 transforming protein of harvey murine sarcoma virus. J Virol. 1982 Apr;42(1):253–261. doi: 10.1128/jvi.42.1.253-261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scholnick E. M. Identification of a sarcoma virus-coded phosphoprotein in nonproducer cells transformed by Kirsten or Harvey murine sarcoma virus. Virology. 1979 Jul 15;96(1):64–79. doi: 10.1016/0042-6822(79)90173-9. [DOI] [PubMed] [Google Scholar]
- Spector M., O'Neal S., Racker E. Regulation of phosphorylation of the beta-subunit of th Ehrlich ascites tumor Na+K+-ATPase by a protein kinase cascade. J Biol Chem. 1981 May 10;256(9):4219–4227. [PubMed] [Google Scholar]
- Temin H. M. The protovirus hypothesis: speculations on the significance of RNA-directed DNA synthesis for normal development and for carcinogenesis. J Natl Cancer Inst. 1971 Feb;46(2):3–7. [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]
- Young H. A., Shih T. Y., Scolnick E. M., Rasheed S., Gardner M. B. Different rat-derived transforming retroviruses code for an immunologically related intracellular phosphoprotein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3523–3527. doi: 10.1073/pnas.76.7.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]