Abstract
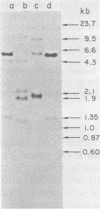
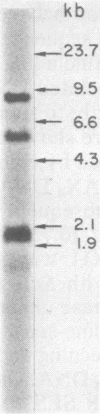
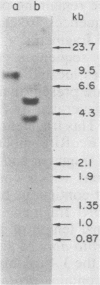
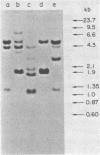
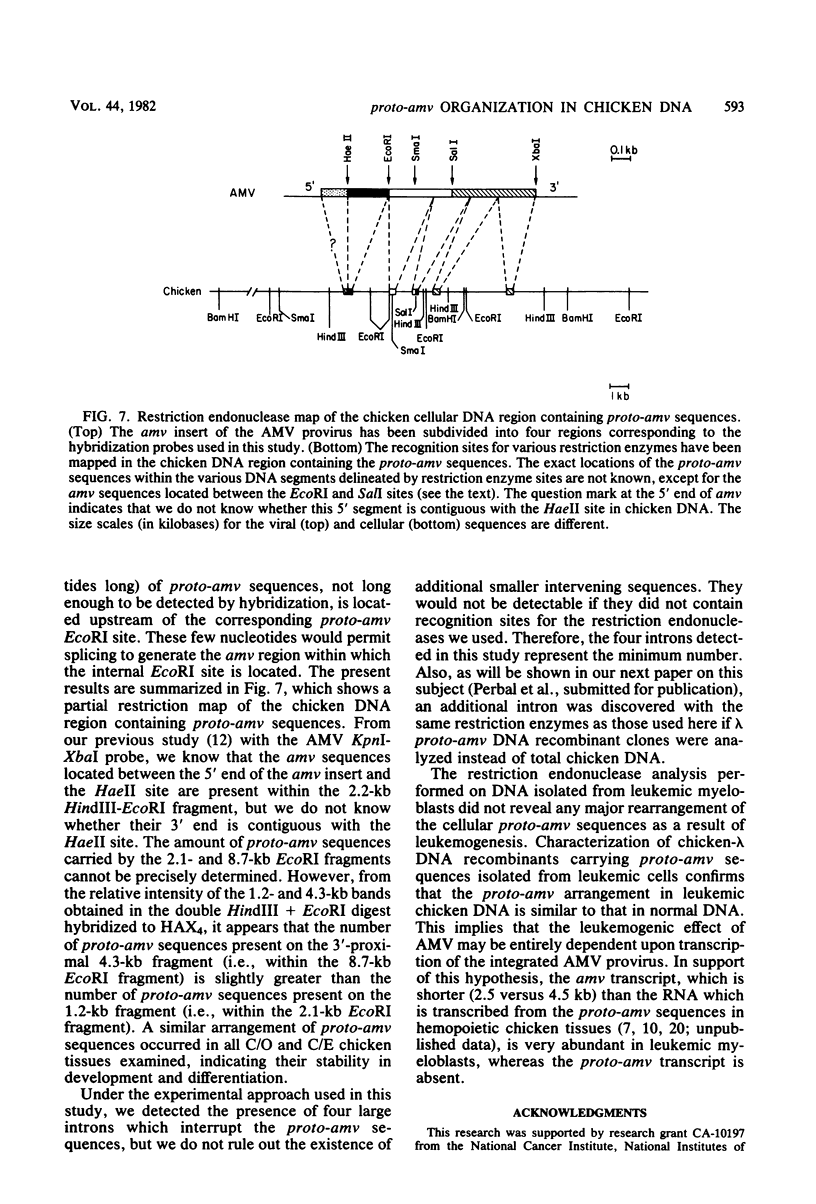
Hybridization probes consisting of cloned DNA recombinants which represent different regions of the leukemogenic sequence (amv) from avian myeloblastosis virus were used to carry out a more detailed restriction endonuclease analysis of the homologous sequences (proto-amv) present in normal and leukemic chicken DNA. The results show that four large introns interrupt the normal cellular proto-amv sequences and that there is no major rearrangement of these sequences in leukemic myeloblasts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluda M. A., Drohan W. N. Distribution of deoxyribonucleic acid complementary to the ribonucleic acid of avian myeloblastosis virus in tissues of normal and tumor-bearing chickens. J Virol. 1972 Nov;10(5):1002–1009. doi: 10.1128/jvi.10.5.1002-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann D. G., Souza L. M., Baluda M. A. Vertebrate DNAs contain nucleotide sequences related to the transforming gene of avian myeloblastosis virus. J Virol. 1981 Nov;40(2):450–455. doi: 10.1128/jvi.40.2.450-455.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. F., O'Malley B. W., Robertson M. A., Staden R., Tanaka Y., Brownlee G. G. Nucleotide sequence homology at 12 intron--exon junctions in the chick ovalbumin gene. Nature. 1978 Oct 12;275(5680):510–513. doi: 10.1038/275510a0. [DOI] [PubMed] [Google Scholar]
- Chen J. H. Expression of endogenous avian myeloblastosis virus information in different chicken cells. J Virol. 1980 Oct;36(1):162–170. doi: 10.1128/jvi.36.1.162-170.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G., Even J., Sherr C. J., Wong-Staal F. onc sequences (v-fes) of Snyder-Theilen feline sarcoma virus are derived from noncontiguous regions of a cat cellular gene (c-fes). Nature. 1981 Mar 12;290(5802):154–157. doi: 10.1038/290154a0. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Fanshier L., Bishop J. M., Moscovici C., Moscovici M. G. The genome and the intracellular RNAs of avian myeloblastosis virus. Cell. 1981 Jan;23(1):279–290. doi: 10.1016/0092-8674(81)90292-0. [DOI] [PubMed] [Google Scholar]
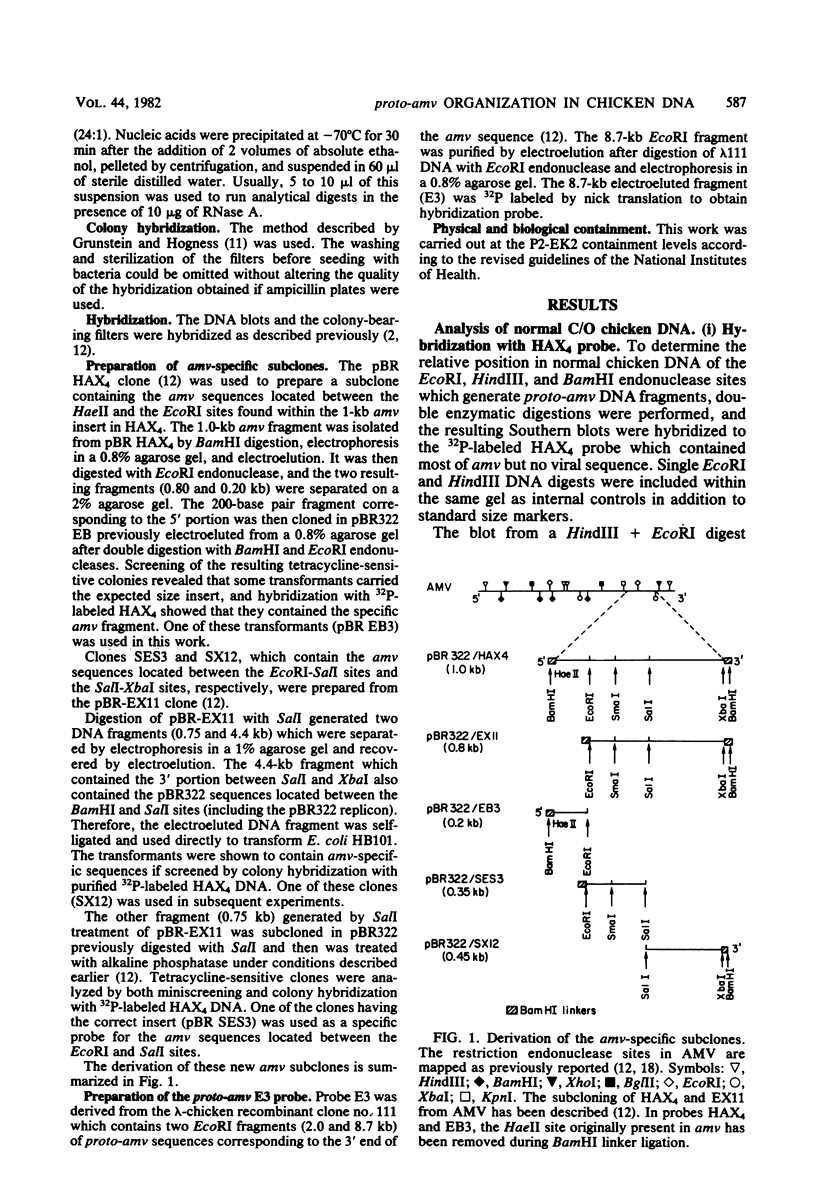
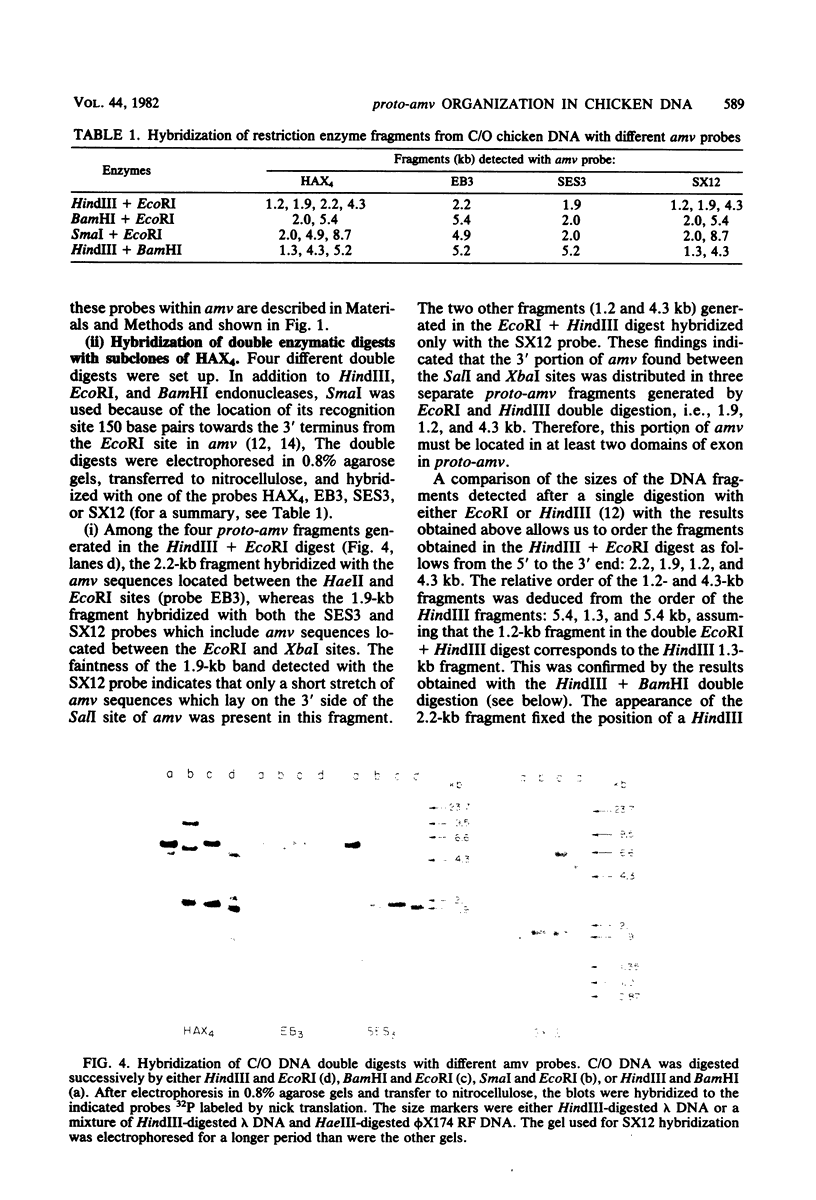
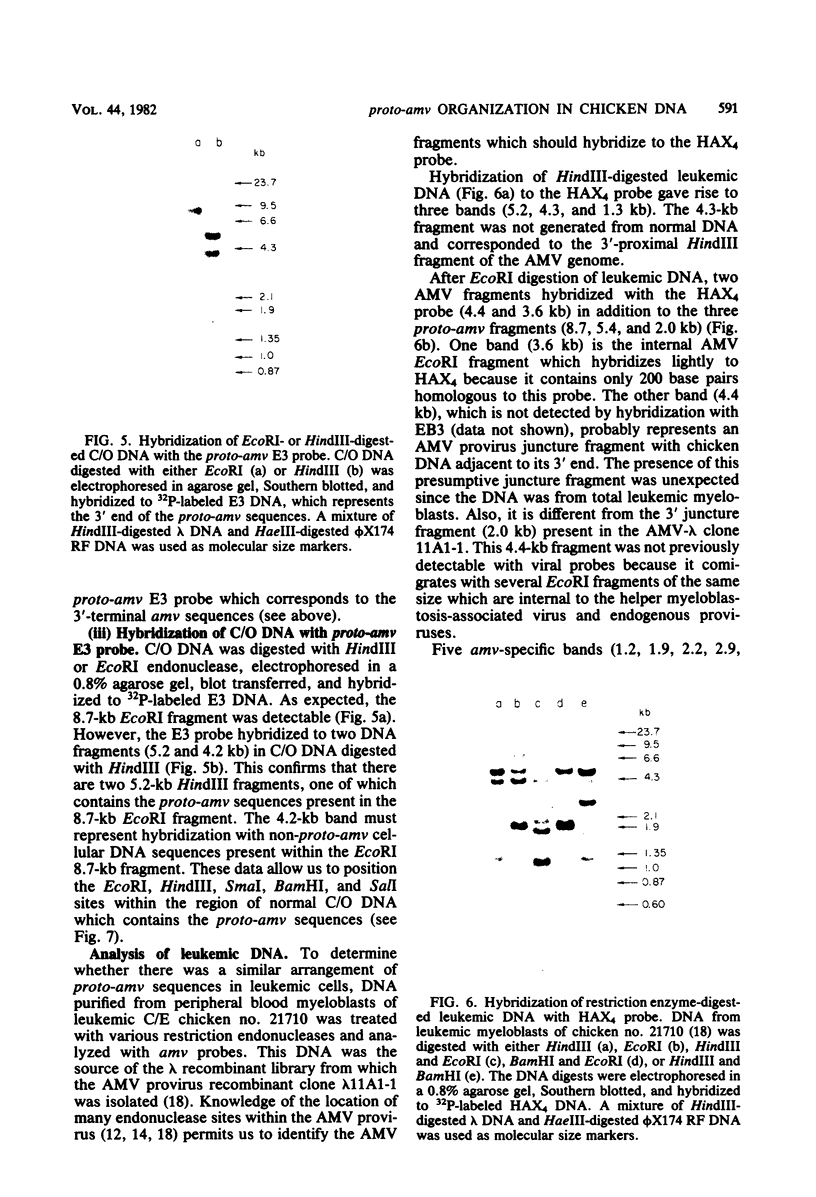
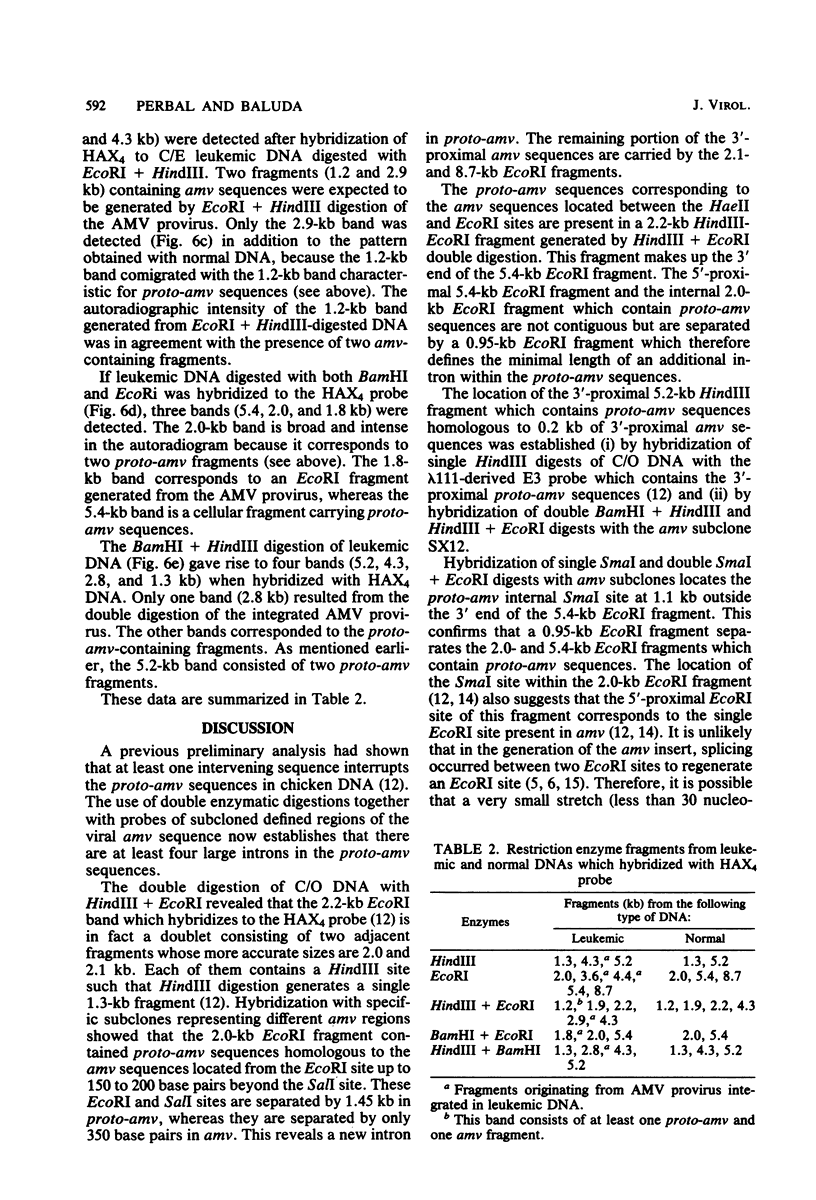
- Perbal B., Baluda M. A. Avian myeloblastosis virus transforming gene is related to unique chicken DNA regions separated by at least one intervening sequence. J Virol. 1982 Jan;41(1):250–257. doi: 10.1128/jvi.41.1.250-257.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rushlow K. E., Lautenberger J. A., Papas T. S., Baluda M. A., Perbal B., Chirikjian J. G., Reddy E. P. Nucleotide sequence of the transforming gene of avian myeloblastosis virus. Science. 1982 Jun 25;216(4553):1421–1423. doi: 10.1126/science.6283631. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. BKV splice sequences based on analysis of preferred donor and acceptor sites. Nucleic Acids Res. 1979 Jul 25;6(10):3387–3398. doi: 10.1093/nar/6.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Zelenetz A. D., Cooper G. M. Molecular cloning and characterization of the chicken gene homologous to the transforming gene of Rous sarcoma virus. Cell. 1981 May;24(2):531–541. doi: 10.1016/0092-8674(81)90344-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Briskin M. J., Hillyard R. L., Baluda M. A. Identification of the avian myeloblastosis virus genome. II. Restriction endonuclease analysis of DNA from lambda proviral recombinants and leukemic myeoblast clones. J Virol. 1980 Nov;36(2):325–336. doi: 10.1128/jvi.36.2.325-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Strommer J. N., Hillyard R. L., Komaromy M. C., Baluda M. A. Cellular sequences are present in the presumptive avian myeloblastosis virus genome. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5177–5181. doi: 10.1073/pnas.77.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]