Abstract
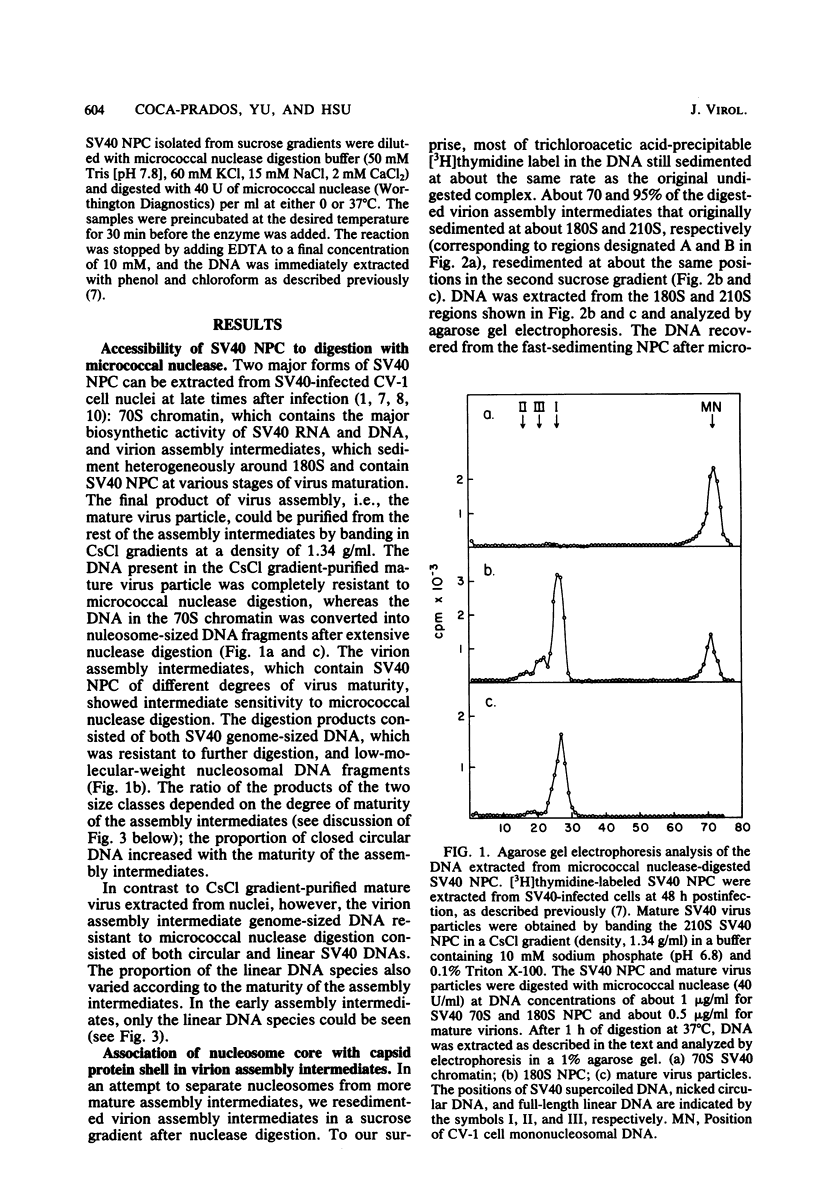
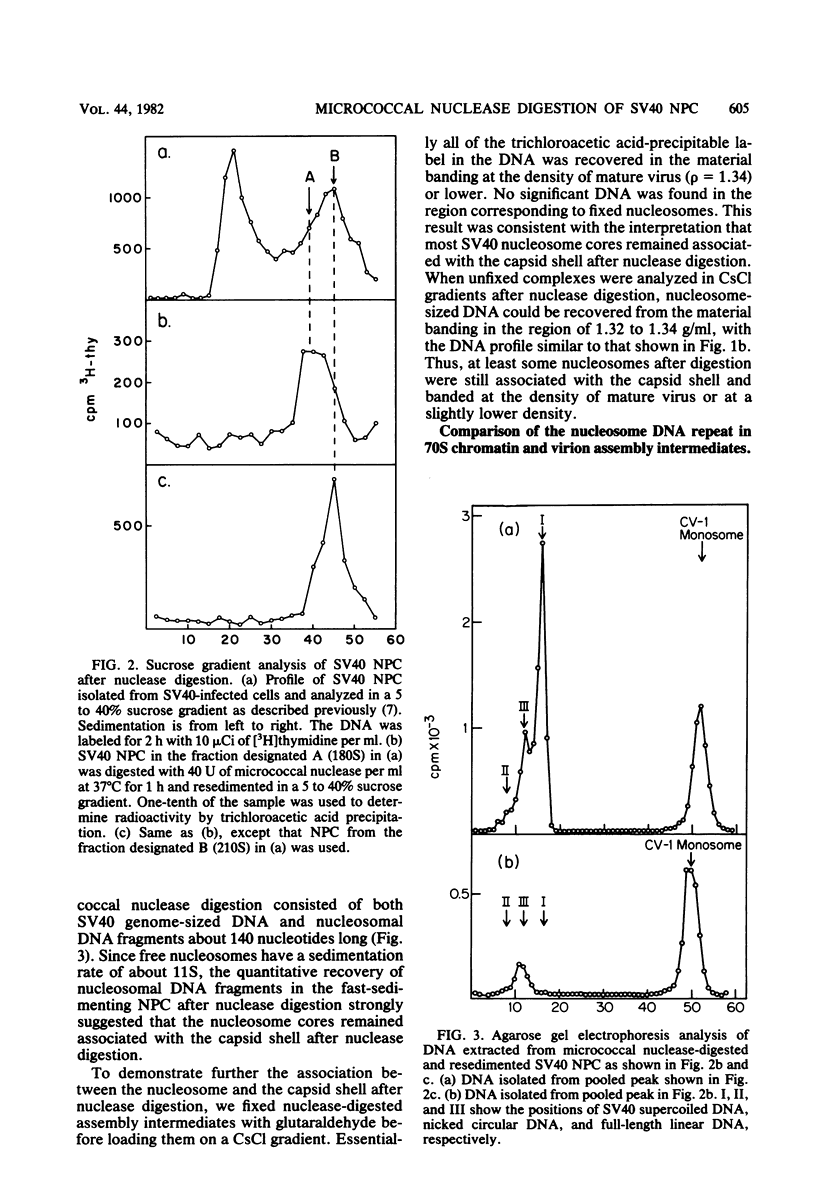
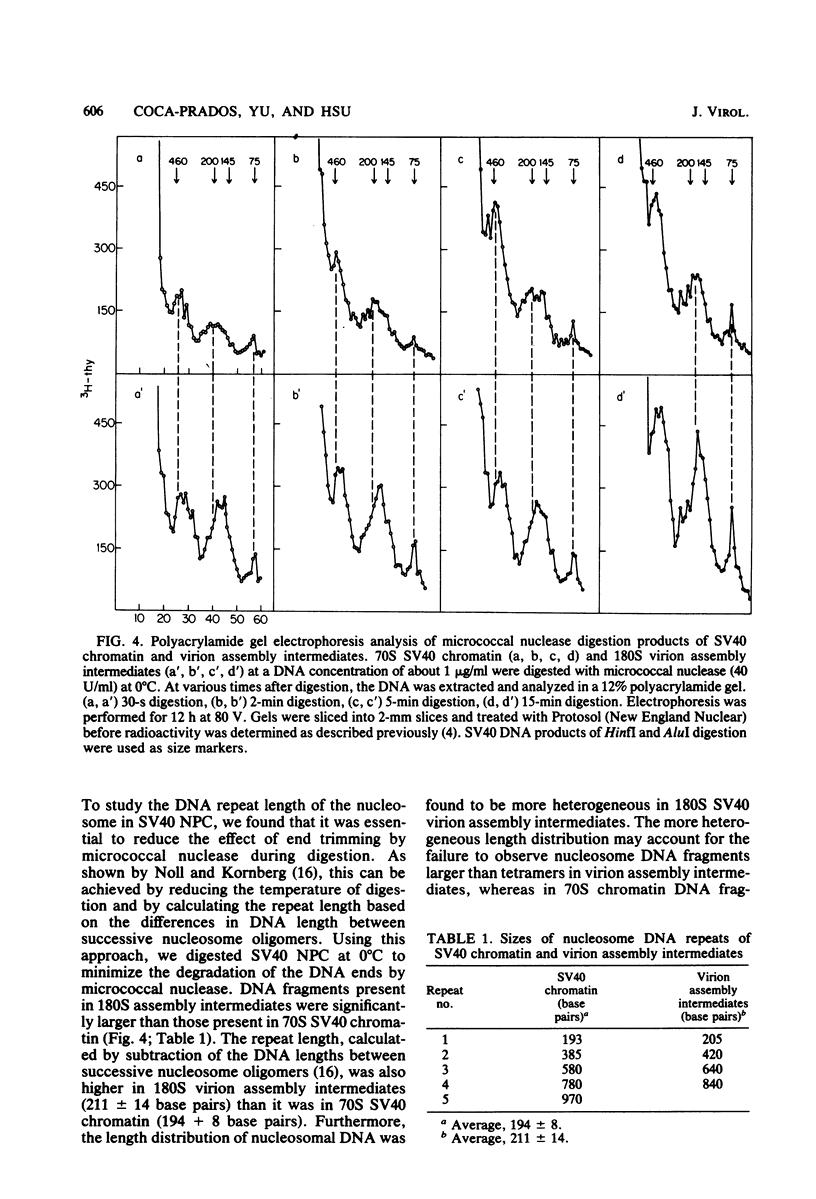
The structures of DNAs present in various intracellular forms of simian virus 40 (SV40) nucleoprotein complexes were analyzed by micrococcal nuclease digestion. The results showed that the 70S SV40 chromatin was completely sensitive to nuclease digestion, whereas CsCl gradient-purified mature virion was completely resistant. Virion assembly intermediates with different degrees of virion maturation showed intermediate resistance, and three products were found: nucleosomal DNA fragments, representing the fraction of intermediates that were sensitive to nuclease; linear SV40 genome-sized DNA, representing the more mature intermediates that contained one or limited defects in the capsid shell; and supercoiled SV40, which was derived from mature virions. These digestion products, however, remained associated with capsid shells after nuclease digestion. These results were consistent with the model in which maturation of the SV40 virion is achieved through the organization of capsid proteins that accumulate around SV40 chromatin. Mild digestion of SV40 nucleoprotein complexes with micrococcal nuclease revealed the difference in nucleosome repeat length between SV40 chromatin and virion assembly intermediates. A novel DNA fragment of about 75 nucleotides was observed early in nuclease digestion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner I., Kuhn C., Fanning E. Identification and characterization of fast-sedimenting SV40 nucleoprotein complexes. Virology. 1979 Jul 15;96(1):54–63. doi: 10.1016/0042-6822(79)90172-7. [DOI] [PubMed] [Google Scholar]
- Bellard M., Oudet P., Germond J. E., Chambon P. Subunit structure of simian-virus-40 minichromosome. Eur J Biochem. 1976 Nov 15;70(2):543–553. doi: 10.1111/j.1432-1033.1976.tb11046.x. [DOI] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Characterization of a DNA-protein complex and capsomere subunits derived from polyoma virus by treatment with ethyleneglycol-bis-N,N'-tetraacetic acid and dithiothreitol. J Virol. 1978 Jul;27(1):193–204. doi: 10.1128/jvi.27.1.193-204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coca-Prados M., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. II. Biochemical and electron microscopic analysis of simian virus 40 virion assembly. J Virol. 1979 Jul;31(1):199–208. doi: 10.1128/jvi.31.1.199-208.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coca-Prados M., Vidali G., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. III. Study of histone modifications. J Virol. 1980 Nov;36(2):353–360. doi: 10.1128/jvi.36.2.353-360.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H. A., Shelton E. R., Wassarman P. M., DePamphilis M. L. Structure of simian virus 40 chromosomes. Appendix. J Mol Biol. 1978 Nov 15;125(4):511–514. doi: 10.1016/0022-2836(78)90313-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Coca-Prados M., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. I. Methods of isolation and characterization in CV-1 cells. J Virol. 1979 Feb;29(2):612–623. doi: 10.1128/jvi.29.2.612-623.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Seidman M. M., Levine A. J. The detection and characterization of multiple forms of SV40 nucleoprotein complexes. Virology. 1978 Oct 15;90(2):305–316. doi: 10.1016/0042-6822(78)90315-x. [DOI] [PubMed] [Google Scholar]
- Hartmann J. P., Scott W. A. Distribution of DNase I-sensitive sites in simian virus 40 nucleoprotein complexes from disrupted virus particles. J Virol. 1981 Mar;37(3):908–915. doi: 10.1128/jvi.37.3.908-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits E. B., Aloni Y. Isolation and characterization of various forms of simian virus 40 DNA-protein complexes. Virology. 1980 Apr 15;102(1):107–118. doi: 10.1016/0042-6822(80)90074-4. [DOI] [PubMed] [Google Scholar]
- Jakobovits E. B., Bratosin S., Aloni Y. A nucleosome-free region in SV40 minichromosomes. Nature. 1980 May 22;285(5762):263–265. doi: 10.1038/285263a0. [DOI] [PubMed] [Google Scholar]
- La Bella F., Vesco C. Late modifications of simian virus 40 chromatin during the lytic cycle occur in an immature form of virion. J Virol. 1980 Mar;33(3):1138–1150. doi: 10.1128/jvi.33.3.1138-1150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Oudet P., Wasylyk B., Chambon P. Effect of histone acetylation on structure and in vitro transcription of chromatin. Nucleic Acids Res. 1978 Oct;5(10):3523–3547. doi: 10.1093/nar/5.10.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki B. L., Neelin J. M. DNA repeat lengths of erythrocyte chromatins differing in content of histones H1 and H5. Nucleic Acids Res. 1980 Feb 11;8(3):529–542. doi: 10.1093/nar/8.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N. R. A comparison of the structure of chicken erythrocyte and chicken liver chromatin. Cell. 1976 Dec;9(4 Pt 1):627–632. doi: 10.1016/0092-8674(76)90045-3. [DOI] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Saragosti S., Moyne G., Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980 May;20(1):65–73. doi: 10.1016/0092-8674(80)90235-4. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Staphylococcal nuclease makes a single non-random cut in the simian virus 40 viral minichromosome. J Mol Biol. 1979 Aug 15;132(3):535–546. doi: 10.1016/0022-2836(79)90274-2. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Föhring B., Chowdhury K., Gruss P., Sauer G. Origin of DNA replication in papovavirus chromatin is recognized by endogenous endonuclease. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5964–5968. doi: 10.1073/pnas.75.12.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. The nucleosome repeat length increases during erythropoiesis in the chick. Nucleic Acids Res. 1978 Apr;5(4):1179–1188. doi: 10.1093/nar/5.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]