Abstract
Adult stem cells often divide asymmetrically to produce one self-renewed stem cell and one differentiating cell, thus maintaining both populations. The asymmetric outcome of stem cell divisions can be specified by an oriented spindle and local self-renewal signals from the stem cell niche. Here we show that developmentally programmed asymmetric behavior and inheritance of mother and daughter centrosomes underlies the stereotyped spindle orientation and asymmetric outcome of stem cell divisions in the Drosophila male germ line. The mother centrosome remains anchored near the niche while the daughter centrosome migrates to the opposite side of the cell before spindle formation.
Adult stem cells maintain populations of highly differentiated but short-lived cells throughout the life of the organism. To maintain the critical balance between stem cell and differentiating cell populations, stem cells have a potential to divide asymmetrically, producing one stem and one differentiating cell (1). The asymmetric outcome of stem cell divisions can be specified by regulated spindle orientation, such that the two daughter cells are placed in different microenvironments that either specify stem cell identity (stem cell niche) or allow differentiation (2, 3).
Drosophila male germline stem cells (GSCs) are maintained through attachment to somatic hub cells, which constitute the stem cell niche. Hub cells secrete the signaling ligand Upd, which activates the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway in the neighboring germ cells to specify stem cell identity (4, 5). Drosophila male GSCs normally divide asymmetrically, producing one stem cell, which remains attached to the hub, and one gonialblast, which initiates differentiation. This stereotyped asymmetric outcome is controlled by the orientation of the mitotic spindle in GSCs: The spindle lies perpendicular to the hub so that one daughter cell inherits the attachment to the hub, whereas the other is displaced away (6).
The stereotyped orientation of the mitotic spindle is set up by the positioning of centrosomes during interphase (Fig. 1A) (6). GSCs remain oriented toward the niche throughout the cell cycle. In G1 phase, the single centrosome is located near the interface with the hub. When the duplicated centrosomes separate in G2 phase, one stays next to the hub, whereas the other migrates to the opposite side of the cell. Centrosomes in the GSCs separate unusually early in interphase, rather than at the G2-prophase transition, so it is common to see GSCs with fully separated centrosomes without a spindle (of >500 GSCs in the 0- to 3-day-old males counted, ~40% of the GSCs had two centrosomes that were separated to opposite sides of the cell, but no spindle was yet assembled) (6).
Fig. 1.
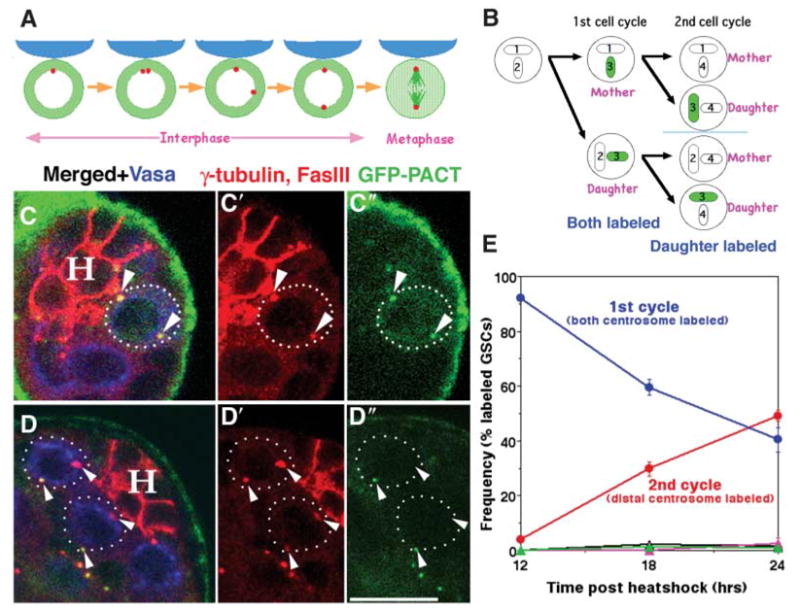
GFP-labeled daughter centrosomes migrate away from the niche. (A) Stereotyped positioning of centrosomes in male GSC during inter-phase sets up the orientation of the mitotic spindle [adapted from (6)]. Red, centrosome; blue, hub; green, tubulin. (B) Pulse-chase strategy to label the daughter centrosome. Each centrosome (circle) contains two centrioles (ovals numbered in order of generation). Transient expression of GFP-PACT labels newly assembled centrioles (3). In the first cell cycle after the GFP-PACT pulse, both the mother (1+3) and daughter (2+3) centrosomes are GFP-positive. In the second cell cycle, the mother centrosomes (1+4 or 2+4) are GFP-negative, whereas the daughter centrosomes (3+4) are GFP-positive. (C and D) Testis tips with labeled GSCs (dotted outline) in the G2 phase of the first cell cycle after the GFP-PACT pulse [(C), 12 hours after heat shock] or the second cell cycle [(D), 24 hours after heat shock]. Green, GFP-PACT; red, γ-tubulin (centrosomes are shown with arrowheads) and Fas III (hub, H); blue, Vasa (germ cells). Scale bar, 10 μm. (E) Frequency of label outcomes over time after heat shock. Blue, GSCs in the first cell cycle (two labeled, oriented centrosomes); red, GSCs in the second cell cycle (one labeled and one unlabeled centrosome) with the labeled (daughter) centrosome distal from the hub. Rarely observed outcomes were pink, the first cell cycle, with neither centrosome next to the hub; black, the second cell cycle, with the mother centrosome distal to the hub; green, the second cell cycle, with neither centrosome next to the hub. Only those GSCs that had two centrosomes, with one or both centrosomes labeled with GFP-PACT, were counted.
Differences between the mother and daughter centrosomes underlie the stereotyped behavior of the centrosomes in Drosophila male GSCs. The mother centrosome normally remains anchored to the hub-GSC interface and is inherited by the GSC, whereas the daughter centrosome moves away from the hub and is inherited by the cell that commits to differentiation. Mother and daughter centrosomes were differentially labeled by transient expression of green fluorescent protein–pericentrin/AKAP450 C-terminus (GFP-PACT) from the Drosophila pericentrin-like protein (7) under heat shock–Gal4 control (8). The PACT domain, which is necessary and sufficient for centriolar localization, is incorporated into centrioles only during centrosome duplication and does not exchange with the cytoplasmic pool (7). Both the mother and daughter centrosomes are labeled by GFP-PACT in the first cell cycle after heat shock (Fig. 1B). In the second cell cycle, the daughter centrosome retains GFP-PACT, whereas the mother centrosome is not labeled, thus distinguishing the mother and daughter centrosomes (Fig. 1B). After a short burst of GFP-PACT expression induced by a 2.5-hour heat shock, 20 to 30% of the GSCs had GFP-labeled centrosomes, indicating the duplication of centrosomes during the window of GFP-PACT expression. By 12 hours after heat shock, >90% of the labeled GSCs had two GFP-positive centrosomes, indicating that they had progressed to the G2 phase of the first cell cycle after GFP-PACT incorporation (Fig. 1, C and E).
By 18 to 24 hours after heat shock, the number of GSCs with two GFP-positive centrosomes had decreased, whereas the number of GSCs with one GFP-positive and one GFP-negative centrosome had increased, suggesting progression into the second cell cycle. Generally, the centrosome distal to the hub was labeled, whereas the centrosome proximal to the hub was GFP-negative (>95% of GSCs in the second cell cycle) (Fig. 1, D and E), indicating that the daughter centrosomes migrate away from the hub-GSC interface during asymmetric GSC divisions.
Labeling the mother rather than the daughter centrosomes confirmed that the male GSCs in the niche preferentially retain mother centrosomes over time. Centrioles assembled during early embryogenesis were labeled using the NGT40 Gal4 driver to drive the expression of GFP-PACT in blastoderm-stage embryos, shutting off after germband extension (9). In the first cell cycle after the depletion of the cytoplasmic pool of GFP-PACT in the GSCs, both the mother and daughter centrosomes should be labeled. In subsequent cell cycles, only the mother centrosomes should be labeled (Fig. 2A).
Fig. 2.
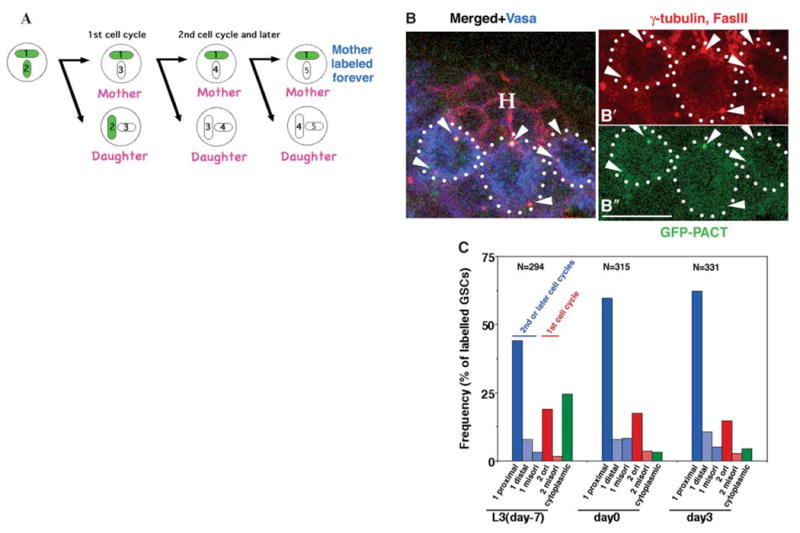
Male GSCs in the niche maintain centrioles assembled many cell generations earlier. (A) Strategy to label the mother centrosome: GFP-PACT expressed during early embryogenesis using the NGT40 driver marks the centrioles (1 and 2) retained in the mother centrosomes after the depletion of cytoplasmic GFP-PACT. (B) Testis tip from a newly eclosed male with GSCs containing GFP-labeled mother centrosomes proximal to the hub-GSC interface. Red, γ-tubulin (centrosomes are shown with arrowheads) and Fas III (hub, H); green, GFP-PACT; blue, Vasa (germ cells). Scale bar, 10 μm. (C) Frequency of label outcomes over time from NGT40/UAS-GFP-PACT flies. UAS, upstream activating sequence. Blue columns, GSCs in the second or later cell cycle after GFP-PACT depletion (only one centrosome was labeled with GFP). 1 proximal, labeled centrosome located proximal to the hub-GSC interface; 1 distal, labeled centrosome located distal from the hub-GSC interface; 1 misori, neither of the two centrosomes located near the hub-GSC interface. Red columns, GSCs in the first cell cycle (both centrosomes were labeled by GFP). 2 ori, one centrosome close to the hub-GSC interface; 2 misori, neither centrosome close to the hub-GSC interface. Green column, GSCs that still had cytoplasmic GFP-PACT. Only 50 to 60% of the total male GSCs were labeled by NGT40/GFP-PACT. The remaining 40 to 50% could contain mother centrioles assembled in early embryogenesis before GFP-PACT expression. Only those GSCs that had two centrosomes, with one or both centrosomes labeled with GFP-PACT, were counted. N, number of GSCs scored per time point.
In most GSCs in the second or later cell cycle after the depletion of cytoplasmic GFP-PACT, the labeled centrosome was positioned next to the hub-GSC interface, and the unlabeled centrosome had moved away from the hub (Fig. 2, B and C). The frequency of GSCs that had the proximal, but not distal, centrosome labeled remained constant over time for 10 days (L3 larvae to day-3 adults), suggesting that the mother centrosomes are reliably retained by the GSCs, even through multiple rounds of GSC divisions. Some GSCs maintained cytoplasmic GFP-PACT, especially in L3 larvae, suggesting that the GFP-PACT had not yet been diluted out (Fig. 2C, green column). We also observed some GSCs with two labeled centrosomes, suggesting that they are in the first cell cycle after the depletion of cytoplasmic GFP-PACT (Fig. 2C, red columns).
The mother centrosomes in GSCs appeared to maintain robust interphase microtubule arrays. Ultrastructural analysis of the GSCs revealed that the centrosome proximal to the hub was commonly associated with many microtubules throughout the cell cycle. Nineteen centrosomes in GSCs were scored in serial sections of the apical tips of five wild-type testes. Eleven centrosomes were localized close to the adherens junctions between the hub and the GSCs. Nine of these proximal centrosomes appeared to be in interphase cells, based on nuclear morphology and microtubule arrangement. Typically, these interphase centrosomes proximal to the hub were associated with numerous microtubules (Fig. 3A and fig. S1A). In some samples, microtubules appeared to extend from the centrosome toward the adherens junctions (Fig. 3A′, arrowheads). The other two proximal centrosomes appeared to be in cells in mitotic prophase (fig. S1B), based on their robust microtubule arrays containing bundled microtubules running parallel to or piercing the nuclear surface.
Fig. 3.
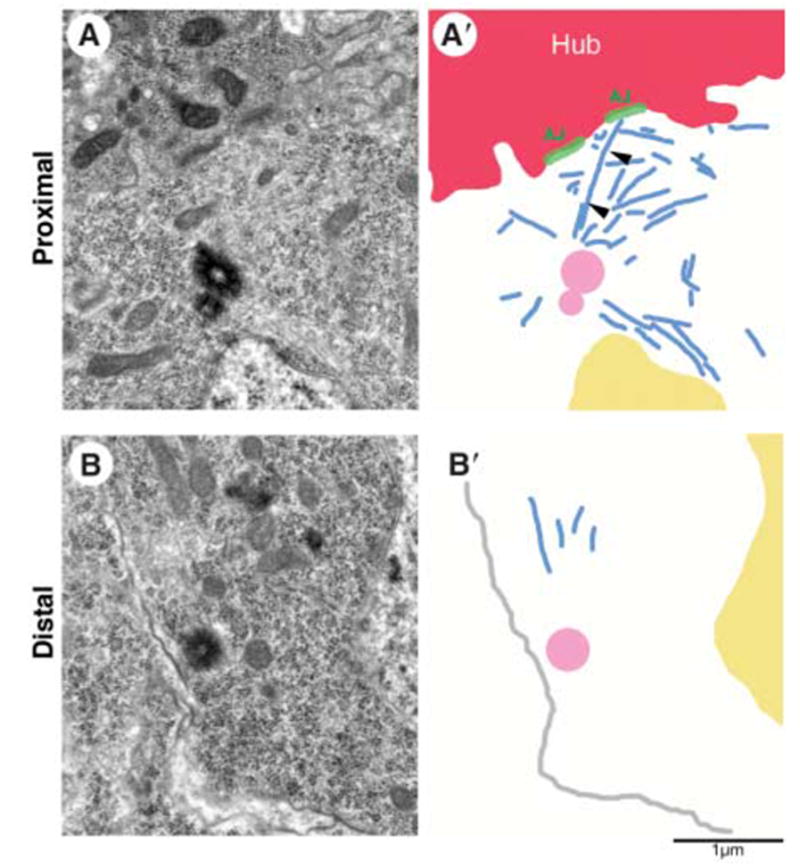
Centrosomes next to the hub harbor robust microtubule arrays. (A) Electron micrograph and (A′) summary diagram of a proximal centrosome in a GSC. Arrowheads in (A′) show a microtubule that runs from the centrosome to the adherens junction. (B) Electron micrograph and (B′) summary diagram of a distal centrosome in a GSC. Red, hub; blue, microtubules; green, adherens junctions; yellow, nucleus; pink, centriole; gray in (B′), plasma membrane. Scale bar, 1 μm.
In contrast, of the five distal centrosomes in the apparently interphase cells that were scored, four had few associated microtubules (Fig. 3B and fig. S1C). The remaining three distal centrosomes appeared to be in cells in mitotic prophase, based on microtubule arrays containing bundled microtubules (fig. S1D). Thus, the mother centrosomes may maintain interphase microtubule arrays that anchor them to the hub-GSC interface, whereas the daughter centrosomes may initially have few associated microtubules and be free to move, establishing a robust microtubule array only later in the cell cycle.
Consistent with the idea that astral microtubules anchor the mother centrosomes to the hub-GSC interface, mother- versus daughter-centrosome positioning was randomized in GSCs that were homozygous mutant for centrosomin (cnn) (8), an integral centrosomal protein required to anchor astral microtubules to centrosomes (10–12). Analysis of mother and daughter centrosomes after transient expression of GFP-PACT revealed that, for cnn homozygous mutant GSCs where one of the two centrosomes was positioned next to the hub, it was essentially random whether the mother or the daughter centrosome stayed next to the hub (Fig. 4). In addition, in >25% of total labeled GSCs, neither of the two centrosomes was next to the hub, which is consistent with our previous observations (Fig. 4) (6).
Fig. 4.
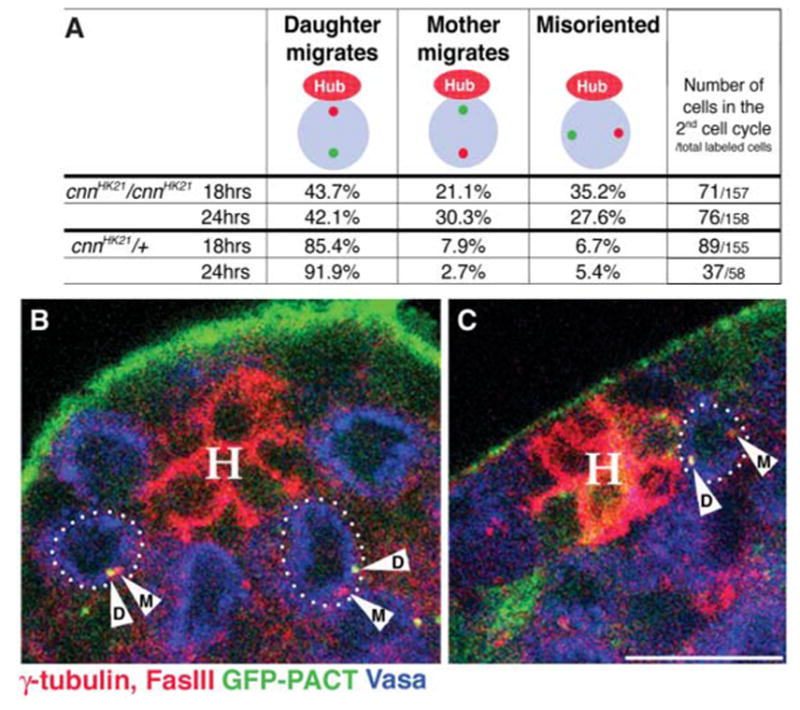
cnn is required for nonrandom segregation of mother and daughter centrosomes. (A) Summary of the centrosome-positioning pattern in cnnHK21 homozygous mutant and control cnnHK21/+ GSCs. Daughter centrosomes were labeled by a pulse of GFP-PACT as in Fig. 1B. Only counts of cells in the second cell cycle are shown. (B and C) Testis tips from cnn males with GSCs in the second cell cycle with misoriented centrosomes (B) or with the mother rather than the daughter centrosome segregated to the opposite side of the GSC (C). Red, γ-tubulin [centrosomes are shown with arrowheads (M, mother; D, daughter)] and Fas III (hub, H); green, GFP-PACT; blue, Vasa (germ cells). Scale bar, 10 μm.
Our results indicate that the two centrosomes in Drosophila male GSCs have different characters and fates. The mother centrosome stays next to the junction with the niche and is inherited by the cell that self-renews stem cell fate. Thus, GSCs can maintain an old centriole assembled many cell generations earlier. In contrast, the daughter centrosome migrates away from the niche and is inherited by the cell that will initiate differentiation. We postulate that the mother centrosomes in male GSCs may remain anchored to the GSC-niche interface throughout the cell cycle by attachment to astral microtubules connected to the adherens junction, whereas the daughter centrosomes may initially have few associated microtubules and thus can move away from the niche. Microtubule-dependent differential segregation of mother and daughter spindle-pole bodies (equivalent to centrosomes in higher organisms) is observed in budding yeast (13). In cultured vertebrate cells, the centrioles mature slowly over the cell cycle, and the mother centrosomes (containing a mature centriole) attach astral microtubules more effectively and are more stationary than daughter centrosomes in interphase (14). The unusually early separation of centrosomes in interphase male GSCs may provide a way to move the daughter centrosome out of range of the stabilizing influence of the adherens junction complex before it becomes competent to hold a robust microtubule array.
Developmentally programmed anchoring of the mother centrosome may provide a key mechanism to ensure the stereotyped orientation of the mitotic spindle and thus the reliably asymmetric outcome of the male GSC divisions. Although it is tempting to speculate that determinants associated with the mother or daughter centrosome may play a role in specifying stem cell or differentiating-cell fates, such determinants are yet to be identified. Rather, the asymmetric inheritance of mother and daughter centrosomes in male GSCs may be a consequence of the cytoskeletal mechanisms that are imposed as part of the stem cell program to anchor one centrosome next to the niche throughout the interphase, ensuring a properly oriented spindle.
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/315/5811/518/DC1
Materials and Methods
Fig. S1
References
References and Notes
- 1.Morrison SJ, Kimble J. Nature. 2006;441:1068. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 2.Spradling A, Drummond-Barbosa D, Kai T. Nature. 2001;414:98. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 3.Watt FM, Hogan BLM. Science. 2000;287:1427. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 4.Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Science. 2001;294:2542. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- 5.Tulina N, Matunis E. Science. 2001;294:2546. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita YM, Jones DL, Fuller MT. Science. 2003;301:1547. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Campos M, Basto R, Baker J, Kernan M, Raff JW. J Cell Biol. 2004;165:673. doi: 10.1083/jcb.200402130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Materials and methods are available as supporting material. Science. Online. [Google Scholar]
- 9.Tracey WD, Jr, Ning X, Klingler M, Kramer SG, Gergen JP. Genetics. 2000;154:273. doi: 10.1093/genetics/154.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Megraw TL, Kao LR, Kaufman TC. Curr Biol. 2001;11:116. doi: 10.1016/s0960-9822(01)00017-3. [DOI] [PubMed] [Google Scholar]
- 11.Vaizel-Ohayon D, Schejter ED. Curr Biol. 1999;9:889. doi: 10.1016/s0960-9822(99)80393-5. [DOI] [PubMed] [Google Scholar]
- 12.Megraw TL, Li K, Kao LR, Kaufman TC. Development. 1999;126:2829. doi: 10.1242/dev.126.13.2829. [DOI] [PubMed] [Google Scholar]
- 13.Pereira G, Tanaka TU, Nasmyth K, Schiebel E. EMBO J. 2001;20:6359. doi: 10.1093/emboj/20.22.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. J Cell Biol. 2000;149:317. doi: 10.1083/jcb.149.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.We thank the Bloomington Stock Center, the Developmental Studies Hybridoma Bank, and R. Lehmann for reagents; the Stanford Cell Science Imaging Facility for assistance in microscopy; Fuller lab members for comments; the Jose Carreras International Leukemia Foundation for a fellowship to Y.M.Y.; and NIH for grant P01 DK53074 to M.T.F.


