Abstract
The purpose of this study is to determine the maximum tolerated dose (MTD), dose-limiting toxicity (DLT), and intracerebral distribution of a recombinant toxin (TP-38) targeting the epidermal growth factor receptor in patients with recurrent malignant brain tumors using the intracerebral infusion technique of convection-enhanced delivery (CED). Twenty patients were enrolled and stratified for dose escalation by the presence of residual tumor from 25 to 100 ng/ml in a 40-ml infusion volume. In the last eight patients, coinfusion of 123I-albumin was performed to monitor distribution within the brain. The MTD was not reached in this study. Dose escalation was stopped at 100 ng/ml due to inconsistent drug delivery as evidenced by imaging the coinfused 123I-albumin. Two DLTs were seen, and both were neurologic. Median survival after TP-38 was 28 weeks (95% confidence interval, 26.5–102.8). Of 15 patients treated with residual disease, two (13.3%) demonstrated radiographic responses, including one patient with glioblastoma multiforme who had a nearly complete response and remains alive >260 weeks after therapy. Coinfusion of 123I-albumin demonstrated that high concentrations of the infusate could be delivered >4 cm from the catheter tip. However, only 3 of 16 (19%) catheters produced intraparenchymal infusate distribution, while the majority leaked infusate into the cerebrospinal fluid spaces. Intracerebral CED of TP-38 was well tolerated and produced some durable radiographic responses at doses ≤100 ng/ml. CED has significant potential for enhancing delivery of therapeutic macromolecules throughout the human brain. However, the potential efficacy of drugs delivered by this technique may be severely constrained by ineffective infusion in many patients.
Keywords: brain neoplasms, convection, drug delivery systems, epidermal growth factor receptor, immunotoxins
The prognosis for patients with malignant primary or metastatic brain tumors remains poor.1 Failure of conventional therapies can be attributed, at least in part, to their lack of specificity for neoplastic tissue, which results in dose-limiting systemic or neurologic toxicity.2 Protein cytotoxins produced by bacteria and plants represent a novel class of therapeutic agents that can be conjugated to specific ligands and delivered regionally to target tumors more specifically.3–6 These targeted toxins have produced dramatic clinical responses in therapeutically refractory patients with leukemia7–9 and lymphoma.10,11 The efficacy of these agents is likely related to their potency, specificity, immunity to resistance mechanisms, and lack of dependence on cell-cycle kinetics. Results in solid tumors, however, have been less encouraging, possibly because these large molecules have difficulty penetrating solid tumors.12–15 The surface expression of targeted antigens may also be lower and the heterogeneity of antigen expression may be greater in solid neoplasms; as a result, a single antibody is less likely to target all neoplastic cells.
Delivery of high-molecular-weight agents, such as targeted toxins, to tumors within the intracerebral compartment is even more challenging because these tumors are further isolated from the systemic circulation by the restrictive blood-brain barrier (BBB).13,14,16–19 Regional intracerebral drug delivery, however, bypasses the BBB and has the potential to deliver high concentrations of the therapeutic agent directly to the site of the tumor, thereby reducing the risk of systemic toxicity. The innovative regional drug delivery technique of convection-enhanced delivery (CED) uses a fluid pressure gradient to infuse therapeutic molecules directly into the interstitial spaces of the brain that are infiltrated with tumor.20–23 The potential of this simple approach has been clearly demonstrated in preclinical studies by others, especially Lonser and Oldfield’s group, and more recently by ourselves.19,20,22–37 The results of early clinical studies21,38–47 have also shown some potential efficacy, but they have not attempted to assess the adequacy of drug distribution in humans.
TP-38 is a 43.5-kDa recombinant chimeric protein containing a genetically engineered form of the cytotoxic Pseudomonas exotoxin (PE). By replacing the native binding domain of PE with transforming growth factor-α (TGF-α), the TP-38 construct has been specifically targeted to the epidermal growth factor receptor (EGFR). EGFR is frequently overexpressed in malignant gliomas and metastatic tumors of the brain,48 but is expressed at only very low levels within normal human brain.49,50 In this phase I study we sought to determine the maximum tolerated dose (MTD), dose-limiting toxicity (DLT), and intracerebral distribution of TP-38 using the intracerebral infusion technique of CED. We show that CED of TP-38 is well tolerated at effective doses and provides some encouraging radiographic responses. However, we also show that the potential efficacy of agents delivered by CED may be severely constrained by inconsistent and ineffective infusion in many patients.
Materials and Methods
TP-38
TP-38 is a recombinant protein consisting of a genetic fusion of TGF-α and a modified PE, PE-38, formed by the deletion of domain Ia/Ib. This deletion results in a toxin (containing amino acids 253–364 and 381–604) with intact adenosine diphosphate-ribosylating activity but negligible cytotoxicity because of its inability to bind cells. Replacing the native binding domain with TGF-α selectively targets the toxin to cells expressing the EGFR. Prior to delivery, TP-38 was diluted with 0.2% human albumin (Plasbumin-25; Bayer Corporation, Elkhart, IN, USA) in 0.9% saline.
Patient Selection, Study Design, and Toxicity Monitoring
Twenty adult patients with a KPS score ≥60 with recurrent or progressive malignant primary or metastatic brain tumors were enrolled at Duke University (19 patients) or the University of California, San Francisco (1 patient). Patients were excluded if they had a tumor ≥5 cm in maximum diameter, midline brain shift ≥0.5 cm, evidence of cerebral uncal herniation, or diffuse subependymal disease. An interval of at least 4 weeks between prior radiation or chemotherapy and enrollment was required. The Duke University and University of California at San Francisco institutional review boards (0344-01) and the U.S. Food and Drug Administration (BB-IND-9184) approved the protocol. Informed consent was obtained from the patients after the nature of all procedures was explained.
Two barium-impregnated catheters (PS Medical cerebrospinal fluid [CSF]-ventricular catheter no. 41207; Medtronic, Inc., Minneapolis, MN, USA) with an outer diameter of 2.1 mm were placed in each patient with stereotactic guidance. These standard ventricular catheters had perforations extending proximally from the catheter tip for 17 mm. The tip of each catheter was positioned to target either residual contrast-enhancing tumor or deep white matter adjacent to areas of previously resected tumor. All patients underwent radiographic imaging to confirm catheter placement prior to infusion. Corticosteroids were administered every 6 h and continued for at least 72 h after the completion of the TP-38 infusion. TP-38 was infused over 50 h at a flow rate from each catheter of 0.4 ml/h for a total volume of 40 ml. Intracranial pressure (ICP) was monitored throughout the infusion.
Toxicity and Response Evaluation
Patients were treated on a dose-escalation protocol to determine the MTD and DLT. Patients were stratified by the presence or absence of residual disease for dose escalation but not for toxicity assessment. Three escalating concentrations of TP-38 were selected for study as follows: 25 ng/ml, 50 ng/ml, and 100 ng/ml. The volume of infusate and flow rate were kept constant at each concentration. At least three patients were entered at each dose level in each stratum. If one of the three patients developed a DLT, as described below, an additional three patients were entered at that dose level. The MTD was defined as the highest dose level at which fewer than two subjects developed DLT. DLT was defined by the NCI Common Toxicity Criteria as a grade 3 or greater hematologic or grade 4 or greater nonhematologic toxicity, although seizures that were not different in character or increased in frequency were not considered DLTs. In addition, an ICP of ≥30 mmHg for greater than 30 min, a decrease in the Glasgow Coma Scale (GCS) score of ≥2 points, and development of a new focal neurologic deficit that did not resolve within 2 weeks with medical management were also considered DLTs.
Patients underwent physical and neurologic examinations, and MR imaging upon completion of the TP-38 infusion and at 4, 8, 16, 24, 36, and 48 weeks postinfusion. Radiographic responses were defined as previously outlined51 on consecutive contrast-enhanced MR or CT scans at least 4 weeks apart, combined with clinical neurologic stability or improvement and no increase in steroid dose. All adverse events were recorded, whether or not they were thought to be drug related. Corticosteroids must have been at a stable dose for at least 1 week prior to entry. Thereafter, they were allowed to be increased if necessary, but episodes of acute cerebral edema requiring emergent intervention were considered DLTs.
Imaging
For the first 48 h of each infusion in the last eight patients treated at Duke University, 123I-labeled albumin was coinfused with TP-38 in the same infusion volume. The radiolabeled albumin was used for imaging the distribution of the infusion instead of the unlabeled albumin routinely used as a carrier protein for these infusions. This was done because the protein doses of TP-38 used were too low to be radiolabeled with sufficient radioactivity to be imaged directly and because the radiolabeling procedure has the potential to damage the EGFR binding site of the toxin. The goal of this approach was to determine which infusions provided effective distribution of the infusate intraparenchymally and which leaked into the subarachnoid space or ventricle. The albumin was purified to homogeneity by ion-exchange high-pressure liquid chromatography and radiolabeled with 123I (MDS Nordion International, Vancouver, BC, Canada) using a modified iodogen method with a target dosage of 80 mCi on 10 mg of albumin. 123I-labeled albumin was chosen because its size, shape, and molecular weight (66.5 kDa) are similar to those of TP-38 (44 kDa) and albumin forms an otherwise essential component of cytotoxin drug formulations. In addition, recent work by Murad et al.52 has shown in well-controlled animal studies that labeled albumin does precisely track the distribution of a similar targeted toxin. Although the use of albumin as a surrogate for imaging drug distribution does not allow evaluation of the potential influence of drug-binding kinetics on distribution, it should be noted that TP-38 was used at a concentration in this study predicted to exceed that needed to saturate all potential binding sites.
Single-photon emission computed tomography (SPECT) scans of the head were obtained at 24 h and at the completion of the infusion with a three-head SPECT scanner (Trionix Research Labs, Twinsburg, OH, USA) fitted with two Triad LESR (low-energy super-high resolution) fanbeam collimators and a Precise (Precise Corp., Caryville, TN, USA) pinhole collimator. The volume of distribution was subsequently determined by a threshold pixel method that has proved to be accurate at our institution for calculating the volume of small spheres ranging in size from 1.3 cm3 to 5.3 cm3 in a brain phantom model with a resolution of 3 mm. Isodose contours were calculated using a three-dimensional discrete Fourier transform convolution. Fiduciary markers were used to co-register these SPECT images with MR with a spatial margin of error of less than 1 mm.
Results
Patient Characteristics
Demographic and treatment information for all participants is given in Table 1. All patients had recurrent disease in the brain except for the patient with the spindle cell metastasis (patient 7). In the strata without residual disease, three patients received TP-38 at the initial dose level of 25 ng/ml (1 μg total dose), and two received 50 ng/ml (2 μg). In the strata with residual disease at the time of TP-38 treatment, three patients received TP-38 at the initial dose level of 25 ng/ml (1 μg total dose); six received 50 ng/ml (2 μg); and six received 100 ng/ml (4 μg). Eighteen of the 20 patients received no further therapy after TP-38 until progressive disease was identified pathologically, and two patients received systemic chemotherapy after TP-38 before evidence of tumor recurrence. One of these two patients (patient 7) received chemotherapy after voluntarily withdrawing from the protocol. The other patient (patient 19) had never been treated with chemotherapy and so elected to do so after TP-38 therapy but before tumor recurrence.
Table 1.
Patient demographic summary
| No. | Age | KPS | Histologic Diagnosis | Therapy Prior to TP-38 | TP-38 (ng/ml) | Therapy after TP-38 |
|---|---|---|---|---|---|---|
| Residual tumor | ||||||
| 2 | 59 | 80 | GBM | OR, EBRT, carmustine, cisplatin, etoposide, carmustine wafers/temozolomide, irinotecan, cyclophosphamide, tamoxifen | 25 | None |
| 3 | 40 | 80 | GBM | OR, EBRT, carmustine/mercaptopurine × 1, EBRT, lomustine × 6, temozolomide × 6 | 25 | None |
| 4 | 55 | 60 | GBM | OR × 2, 131I-monoclonal antibody, EBRT, temozolomide × 2, lomustine × 4, etoposide, irinotecan/temozolomide | 25 | Thalidomide |
| 5 | 55 | 60 | GBM | OR, EBRT, procarbazine/lomustine/vincristine × 6, temozolomide/tamoxifen × 4, OR | 50 | None |
| 6 | 51 | 90 | GBM | OR × 2 EBRT, lomustine × 6 | 50 | Temozolomide |
| 8 | 55 | 90 | GBM | OR, EBRT, lomustine × 2, radiosurgery, temozolomide × 5, OR | 50 | None |
| 9 | 58 | 90 | GSC | OR, OR, EBRT, carmustine/irinotecan × 1, temozolomide × 4, tamoxifen × 4 | 50 | Etoposide |
| 10 | 45 | 90 | GBM | OR, EBRT, OR, 131I-monoclonal antibody, EBRT, temozolomide × 4, lomustine × 4, etoposide × 2, temozolomide × 1, lomustine × 4 | 50 | None |
| 13 | 52 | 90 | GBM | OR, EBRT, lomustine × 2, temozolomide × 2, irofulven × 3 | 50 | Irinotecan |
| 15 | 62 | 70 | GBM | OR, 125I brachytherapy, EBRT, Cu2+ chelation, temozolomide × 9, irinotecan × 8 | 100 | None |
| 16 | 49 | 90 | GBM | OR, EBRT, temozolomide | 100 | None |
| 17 | 50 | 90 | AO | OR, EBRT, procarbazine/lomustine/vincristine × 6, OR | 100 | Temozolomide/ irinotecan × 5 |
| 18 | 48 | 90 | GBM | OR, EBRT | 100 | None |
| 19 | 31 | 90 | GBM | OR, EBRT | 100 | Irinotecan |
| 20 | 21 | 90 | GBM | OR × 2, EBRT | 100 | None |
| No residual tumor | ||||||
| 1 | 59 | 90 | GBM | OR, EBRT, lomustine × 2, etoposide/tamoxifen × 2, irinotecan × 2, lomustine × 1, temozolomide × 3, thalidomide, radiosurgery, topotecan | 25 | None |
| 7 | 23 | 90 | Metastatic spindle cell | None for brain metastasis | 25 | Temozolomide/ imatinib mesylate |
| 11 | 56 | 90 | GBM | OR, EBRT, carmustine × 2, OR | 25 | Temozolomide/ O6-benzylguanine |
| 12 | 55 | 90 | GBM | OR, EBRT, lomustine × 2, temozolomide × 2, OR | 50 | None |
| 14 | 68 | 100 | GBM | OR, radiosurgery, EBRT, temozolomide × 1 | 50 | OSI-774 |
Abbreviations: GBM, glioblastoma multiforme; OR, operative resection; EBRT, external beam radiation therapy; GSC, gliosarcoma; AO, anaplastic oligodendroglioma.
Toxicity
All toxicities encountered have been neurologic, and no hepatic, renal, or hematologic toxicities have occurred. Five patients, all with preexisting seizure disorder, had a seizure during the TP-38 infusion, but all seizures resolved following anticonvulsant medication adjustments. Other adverse events are shown in Table 2.
Table 2.
Summary of adverse eventsa
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Summary by dose | ||||
| 25 ng/ml | 2 | |||
| 50 ng/ml | 1 | 9 | 1 (8)b,c | |
| 100 ng/ml | 1 | 5 | 1 | 1 (15)b |
| Summary by type | ||||
| Neuropathy—motor (hemiparesis) | 2 | 1 (8)b,c | ||
| Headache | 1 | 5 | 1 | |
| Ocular/visual | 2 | |||
| Speech | 3 | |||
| Seizured | 1 | |||
| Constitutional | 1 | 1 | 1 (15)b | |
Events that were related to catheter placement (before TP-38 delivery) are not included. All adverse events resolved with the exception of the grade 3 and 4 events shown. For events occurring more than once, only the highest grade event for each patient is recorded. Some patients had more than one event.
Dose-limiting toxicities; patient numbers are shown in parentheses.
May have been related to recurrent tumor.
Overall, 5 of 20 patients (25%) had seizures prior to TP-38 therapy. These were not included as adverse events unless there was an increase in frequency.
DLTs were identified in two patients (Table 3). One patient (patient 8) with residual disease, treated at the 50-ng/ml dose level, developed a grade 3 hemiparesis. Although this was considered to be a DLT, it may also have been related to tumor recurrence, which was diagnosed 3 weeks later. Three more patients with residual disease were treated at the 50-ng/ml dose level as a precaution, but no DLTs were identified in these subsequent three patients. In addition, during this interval, two patients without residual disease were also treated at this dose level without incident. However, one patient (patient 15), also with residual disease, treated at the 100-ng/ml dose level developed grade 4 constitutional symptoms (fatigue) possibly related to TP-38. This patient had a substantial leak of the infusate intraventricularly, which may have been the cause of this toxicity. Three additional patients with residual disease were treated at the 100-ng/ml dose level and experienced no DLTs. In addition to these events, one patient developed a subdural hygroma, and one patient had an intra-cerebral hemorrhage after catheter placement but before TP-38 therapy.
Table 3.
Dose-limiting toxicities
| Dose Level | No. Patients | Grade 3 or 4 Hematologic Toxicity | Grade 4 Nonhematologic Toxicity | Increased ICP | Decrease in GCS or New Focal Neurologic Deficit | Percent DLT |
|---|---|---|---|---|---|---|
| Residual tumor | ||||||
| 25 ng/ml | 3 | 0 | 0 | 0 | 0 | 0% |
| 50 ng/ml | 6 | 0 | 0 | 0 | 1a | 16.7% |
| 100 ng/ml | 6 | 0 | 1b | 0 | 0 | 16.7% |
| No residual tumor | ||||||
| 25 ng/ml | 3 | 0 | 0 | 0 | 0 | 0% |
| 50 ng/ml | 2 | 0 | 0 | 0 | 0 | 0% |
| 100 ng/ml | 0 | 0 | 0 | 0 | 0 | 0% |
Abbreviations: ICP, intracranial pressure; GCS, Glasgow Coma Scale score; DLT, dose-limiting toxicity.
Hemiparesis that may have been related to recurrent tumor.
Constitutional.
Response, Progression, and Survival
Overall, 2 of 15 (13.3%) patients with residual tumor at the time of therapy have demonstrated radiographic responses. One patient (patient 2), also described above, who was treated for a multirecurrent bifrontal glioblastoma multiforme (GBM) at the 25-ng/ml dose level, had a nearly complete response that was sustained for 198 weeks, but ultimately developed tumor recurrence (Fig. 1).46 Another patient (patient 18) treated at the 100-ng/ml dose, had a partial response with >50% shrinkage of tumor diameter 24 weeks after TP-38 therapy but then died 34 weeks after TP-38 therapy of unrelated infectious complications (Fig. 2). In addition, a third patient (patient 12), without residual disease at the time of treatment, developed a large area of contrast enhancement 9 weeks after treatment that was never biopsied but has subsequently regressed without additional treatment (Fig. 3). It remains unclear whether this enhancing area represented a tumor recurrence with spontaneous remission or an asymptomatic inflammatory reaction to infusion or the drug.53 This patient with GBM remains alive and without evidence of tumor progression >211 weeks after initial diagnosis.
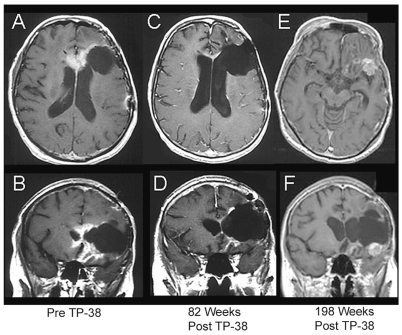
Fig. 1.
Axial and coronal MR images of a 59-year-old female with a recurrent glioblastoma multiforme (GBM) (patient 2) showing a sustained radiographic response to TP-38. This patient had failed surgery, external beam radiation therapy, carmustine, cisplatin, etoposide, temozolomide with carmustine wafers, irinotecan, cyclophosphamide, and tamoxifen prior to receiving TP-38 therapy. Serial images from left to right show her scans before TP-38 therapy (A, B); 82 weeks after TP-38 with a nearly complete response (C, D); and, most recently, 198 weeks after TP-38 with biopsy-proven, recurrent GBM (E, F). She remains working full time with a KPS of 90.
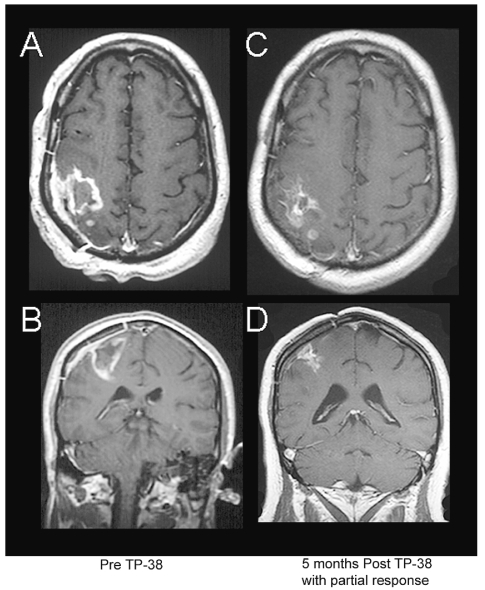
Fig. 2.
Axial and coronal MR images of a 48-year-old male with recurrent glioblastoma multiforme (patient 18) showing a partial radiographic response to TP-38. This patient had failed prior surgery and external beam radiation therapy. Images show his scans before TP-38 therapy (A, B) and 5 months after therapy with a partial response (C, D). He subsequently died from an unrelated sepsis. Up to that time, however, he was working full time with a KPS of 90.
Fig. 3.
Axial and coronal MR images of a 55-year-old female with recurrent glioblastoma multiforme (patient 12) showing delayed enhancement. Serial images from left to right show her scans postoperatively and before TP-38 therapy (A, B); 6 weeks after therapy (C, D); 11 months after therapy with a new area of contrast enhancement (E, F); 24 months after therapy with partial spontaneous resolution of this area of enhancement without treatment (G, H); 30 months after therapy with further resolution of the contrast-enhancing area (I, J); and most recently, 43 months after TP-38 therapy with nearly complete resolution of all enhancing areas (K, L).
All but two patients in this study have died from progressive disease. One patient (patient 12) with GBM remains alive and without progression >211 weeks after TP-38 therapy, and another patient (patient 2) with GBM went 198 weeks without progressive disease after a nearly complete response to TP-38 and remains alive >260 weeks from TP-38 therapy.46 Overall median time to progression (TTP) was 14.9 weeks (95% confidence interval [CI], 4.1–45.1; range, <1–210.57 weeks) in this mostly heavily pretreated group. Median TTP was slightly longer in patients without residual disease at the time of treatment (17.1 weeks; 95% CI, 9.6–129.3) when compared to those with residual disease (14.1 weeks; 95% CI, 3.9–42.1), but this difference was not statistically significant (p = 0.32). The study, however, was not powered to detect such differences.
Overall median survival after TP-38 therapy for all patients was 28 weeks (95% CI, 26.5–102.8; range, 1.1 to >260 weeks). For patients with residual disease at the time of TP-38 therapy, median survival was only 20.1 weeks (95% CI, 16.7–110.0), whereas for those without radiographic evidence of residual disease, median survival was 33.0 weeks (95% CI, 0–170.4), but again this difference was not statistically significant (p = 0.60). However, the study was not powered to detect such differences.
Drug Distribution Imaging
SPECT imaging of coinfused 123I-labeled albumin during and immediately after infusion demonstrated that the intracerebral delivery technique of CED is capable of producing extensive and relatively homogeneous distribution of high-molecular-weight molecules within the human brain in some patients (Fig. 4). For example, in patient 12, after 24 h of infusion (9.6 ml), the volumes of distribution from each catheter at the 10% isodose level relative to infused dose were found to be 54 ml and 60 ml, respectively. Similarly, at the end of the 50-h infusion, the volumes of distribution at a 10% isodose level were found to be 174 ml and 292 ml, respectively. Thus, this novel technique for bypassing the BBB allowed for distribution of at least 10% of the injected concentration of this macromolecule within a nearly spherical radius of 3.5 cm and 4.1 cm (patient 12).
Fig. 4.
Co-registered images showing optimal distribution of 123I-albumin after convection-enhanced delivery. (A) Single-photon emission computed tomography (SPECT) image of 123I-albumin distribution co-registered with axial MR image for anterior right frontal catheter positioned below the resection cavity (patient 12). (B) SPECT image of 123I-albumin distribution co-registered with axial MR image for posterior right frontal catheter positioned behind the resection cavity. (C, D) Corresponding SPECT-derived isodose contours co-registered with axial T1-weighted MR images located at the tip of each catheter in the right frontal lobe of the brain. Isodose lines show the percent concentration of 123I-albumin relative to the infused concentration (0.25 mg/ml) such that at the 50% isodose line the concentration of albumin is 0.125 mg/ml and at the 10% isodose line the concentration of albumin is 0.025 mg/ml. (E, F) Oblique reconstructions of complete SPECT image showing areas covered and not covered by the infusion.
Although in some cases CED did provide dramatic distributions of the infusate, very concerning was the number of catheters for which imaging identified significant leaks of infusate into the subarachnoid or intraventricular CSF spaces (Table 4; Fig. 5). We imaged infusions from eight patients with a total of 16 catheters. In all but three patients (19%) infusate distributions were significantly influenced by such leaks and failed to produce any significant intraparenchymal distribution. Typically, these leaks were thought to be due to the catheter trajectory perforating a deep pial or ependymal border that allowed a path of least resistance for the infusate away from the interstitial space. In one specific example, the catheter trajectory traversed the deep portions of the Sylvian fissure (Fig. 6). Based on the imaging studies, which failed to show adequate delivery of the drug in the majority of patients, the study was halted at a dose of 100 ng/ml without reaching an MTD.
Table 4.
Catheter infusion findingsa
| Infusion Findings | No. Infusions | Percentage of Infusions |
|---|---|---|
| Leak into subarachnoid space | 7/16 | 44% |
| Leak into ventricle | 2/16 | 12% |
| Pooling in resection cavity or necrotic area | 4/16 | 25% |
| Successful intraparenchymal infusion | 3/16 | 19% |
A subset of eight patients was infused with 123I-labeled albumin to monitor drug distribution.
Fig. 5.
Co-registered images showing ineffective distribution of 123I-albumin after convection-enhanced delivery: selected axial MR image (A) and coronal images (B, C) from three different patients showing single-photon emission computed tomography image of 123I-albumin distribution co-registered with MR images demonstrating leakage of infusate into the subarachnoid cerebrospinal fluid spaces over the superior hemispheric surface of frontoparietal lobe of the brain (A–C) and along the tentorial surface of the occipital lobe (C).
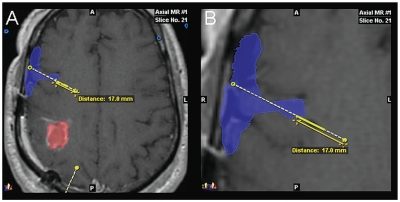
Fig. 6.
Co-registered images showing distribution of 123I-albumin into the Sylvian fissure after convection-enhanced delivery from a catheter with proximal ports: outline (A) and magnified view (B) of single-photon emission computed tomography image of 123I-albumin distribution (blue) shown co-registered with axial MR image (patient 18). The tumor is outlined in red. A second catheter trajectory is shown in yellow at the bottom of A. The most proximal port on this catheter is located 17.0 mm from the tip. In this instance, the infusate appears to have exited the catheter through the most proximal port, which was communicating with the subarachnoid cerebrospinal fluid (CSF) space within the deep portion of the Sylvian fissure between the frontal and temporal lobes of the brain. This allowed the infusate to leak completely into the CSF spaces without any penetration of the parenchyma.
Discussion
This study shows that intracerebral CED of the EGFR-targeted PE conjugate, TP-38, has an acceptable safety profile at the doses tested and that prolonged clinical and radiographic responses are possible with this agent at these dose levels despite suboptimal drug delivery. Of note, the drug concentrations studied in this study are much lower than those used in similar trials employing targeted toxins for brain tumor therapy where concentrations >0.5 μg/ml have been used,21,43 yet they are several fold higher than the estimated concentration required to kill the EGFR-overexpressing A431 cell line in vitro (0.023 ng/ml) (I. Pastan, unpublished data).
CED, the intracerebral drug delivery approach employed in this trial, uses a fluid pressure gradient to directly infuse therapeutic molecules into the interstitial spaces of the brain via an indwelling catheter. As such, this approach holds tremendous promise for intracerebral drug delivery for intracerebral neoplastic, infectious, and neurodegenerative disease.24,54–56 The distribution of 123I-albumin provided by CED in this study was approximately tenfold greater than the injected volume and several thousandfold greater than the predicted distribution for diffusion from a point source of drug.23 These data suggest that CED may be capable of saturating large areas of brain with macromolecular therapeutic agents.
Despite some encouraging results, however, we clearly saw evidence in this trial that CED using the technology described here is quite frequently ineffective. This factor is concerning because CED has become a routine method of drug delivery to the brain in human trials, including a few phase III trials that are evaluating therapeutic agents for intracerebral malignancies21,38–47,57 and degenerative disease.54–56 However, drug distribution in these trials has not been monitored. This lack of fundamental information on drug distribution has the potential to confound interpretation of these clinical studies, making it difficult to distinguish between lack of effectiveness due to delivery or therapeutic activity factors.
We saw definite evidence of failed drug delivery in this study that may have hindered our ability to assess the efficacy or toxicity of TP-38. Importantly, these findings may explain the failure in trials using a similar delivery technique to treat neurodegenerative disease as well.54–56 Optimization of catheter placement and infusion parameters will clearly be needed if the true potential and toxicity of agents delivered by CED is to be assessed. This may be assisted by software algorithms that are able to predict fluid distribution after CED with some reliability.58 New catheter designs may also enhance the predictability of drug delivery.59 Because the catheters used in this study were existing ventricular catheters that had perforations extending proximally from the catheter tip for 17 mm, it may be that infusate left the catheters entirely from the more proximal catheter ports and entered unintended anatomic locations of low resistance, such as the Sylvian fissure.
The mechanisms of the antitumor activity of these targeted toxins may also not fully be understood. In all reported series using these agents, including our own, radiographic responses have been slow to manifest, often requiring many months to become apparent. This finding seems at odds with the purported action mechanism of these agents, which rapidly terminate protein synthesis once internalized. This feature raises the possibility that these agents may have additional mechanisms of action, perhaps immunologically mediated, that may play a role in the efficacy, or even toxicity,53 of these agents. Further quantitative analysis of patient samples for expression of EGFR, EGFRvIII, or other potentially relevant molecules such as PTEN may provide additional insights in future studies.
In conclusion, we show that at effective dose levels, the EGFR-targeted toxin TP-38 has an acceptable safety profile in patients with recurrent malignant brain tumors. We also show that the intraparenchymal drug delivery technique of CED can provide broad and homogeneous distribution of large molecules in the brain in some patients but that frequently misdirected drug infusions may limit drug efficacy independent of drug activity. Further study of TP-38 and optimization and validation of the delivery technique of CED is clearly warranted.
Acknowledgments
We acknowledge the clinical contributions of Denise Lally-Goss and Sharon McGehee-Norman and administrative assistance of Michelle Smith and Shenell Summers.
This research was supported by Accelerated Brain Cancer Cure (ABC2) (J.H.S.), NIH/NCRR K23 RR16065 (J.H.S.), NIH/NCI R01 CA097611 (J.H.S.), NIH/NCI CA11898 (D.D.B.), NCI SPORE 2P50-NS20023 (D.D.B., J.H.S.), NIH/NCRR MO1 RR 30 (General Clinical Research Centers Program), P50-CA097257 (M.S.B.), and IVAX, Inc. This research was also supported in part by the Intramural Research Program of the NIH, NCI, Center for Cancer Research. Experimental data were acquired using shared instrumentation funded by the National Center for Research Resources of the National Institutes of Health (S10 RR15697).
References
- 1.CBTRUS, Central Brain Tumor Registry of the United States. Statistical Report: Primary Brain Tumors in the United States, 1997–2001. Chicago: CBTRUS; 2004. [Google Scholar]
- 2.Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818–822. doi: 10.1002/ana.410280614. [DOI] [PubMed] [Google Scholar]
- 3.Pai LH, Pastan I. Immunotoxins and recombinant toxins. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Biologic Therapy of Cancer. Philadelphia: J.B. Lippincott; 1995. pp. 521–533. [Google Scholar]
- 4.Phillips PC, Levow C, Catterall M, et al. Transforming growth factor-alpha-Pseudomonas exotoxin fusion protein (TGF-alpha-PE38) treatment of subcutaneous and intracranial human glioma and medulloblastoma xenografts in athymic mice. Cancer Res. 1994;54:1008–1015. [PubMed] [Google Scholar]
- 5.Pastan I. Targeted therapy of cancer with recombinant immunotoxins. Biochimica et Biophysica Acta. 1997;1333:C1–C6. doi: 10.1016/s0304-419x(97)00021-8. [DOI] [PubMed] [Google Scholar]
- 6.Pastan I, FitzGerald D. Recombinant toxins for cancer treatment. Science. 1991;254:1173–1177. doi: 10.1126/science.1683495. [DOI] [PubMed] [Google Scholar]
- 7.Kreitman RJ, Wilson WH, White JD, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 8.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 9.Frankel AE, McCubrey JA, Miller MS, et al. Diphtheria toxin fused to human interleukin-3 is toxic to blasts from patients with myeloid leukemias. Leukemia. 2000;14:576–585. doi: 10.1038/sj.leu.2401743. [DOI] [PubMed] [Google Scholar]
- 10.Frankel AE, Kreitman RJ, Sausville EA. Targeted toxins. Clin Cancer Res. 2000;6:326–334. [PubMed] [Google Scholar]
- 11.Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 12.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 13.Jain RK. Vascular and interstitial bariers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990;9:253–266. doi: 10.1007/BF00046364. [DOI] [PubMed] [Google Scholar]
- 14.Jain RK. Tumor physiology and antibody delivery. Front Radiat Ther Oncol. 1990;24:32–46. [PubMed] [Google Scholar]
- 15.Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990;50:1. [PubMed] [Google Scholar]
- 16.Jain RK. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6:559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- 17.Groothuis DR. The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro-Oncology. 2000;2:45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zalutsky MR, Moseley RP, Coakham HB, Coleman RE, Bigner DD. Pharmacokinetics and tumor localization of 131I-labeled anti-tenascin monoclonal antibody 81C6 in patients with gliomas and other intracranial malignancies. Cancer Res. 1989;49:2807–2813. [PubMed] [Google Scholar]
- 19.Grossi PM, Ochiai H, Archer GE, et al. Efficacy of intracerebral micro-infusion of trastuzumab in an athymic rat model of intracerebral metastatic breast cancer. Clin Cancer Res. 2003;9:5514–5520. [PubMed] [Google Scholar]
- 20.Laske DW, Morrison PF, Lieberman DM, et al. Chronic interstitial infusion of protein to primate brain: determination of drug distribution and clearance with single-photon emission computerized tomography imaging. J Neurosurg. 1997;87:586–594. doi: 10.3171/jns.1997.87.4.0586. [DOI] [PubMed] [Google Scholar]
- 21.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg. 1995;82:1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- 23.Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL. High-flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol. 1994;266(1 pt 2):R292–R305. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton JF, Morrison PF, Chen MY, et al. Heparin coinfusion during convection-enhanced delivery (CED) increases the distribution of the glial-derived neurotrophic factor (GDNF) ligand family in rat striatum and enhances the pharmacological activity of neurturin. Exp Neurol. 2001;168:155–161. doi: 10.1006/exnr.2000.7571. [DOI] [PubMed] [Google Scholar]
- 25.Morrison PF, Chen MY, Chadwick RS, Lonser RR, Oldfield EH. Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am J Physiol. 1999;277(4 pt 2):R1218–R1229. doi: 10.1152/ajpregu.1999.277.4.R1218. [DOI] [PubMed] [Google Scholar]
- 26.Bobo RH, Laske DW, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laske DW, Ilercil O, Akbasak A, Youle RJ, Oldfield EH. Efficacy of direct intratumoral therapy with targeted protein toxins for solid human gliomas in nude mice. J Neurosurg. 1994;80:520–526. doi: 10.3171/jns.1994.80.3.0520. [DOI] [PubMed] [Google Scholar]
- 28.Heimberger AB, Archer GE, McLendon RE, et al. Temozolomide delivered by intracerebral microinfusion is safe and efficacious against malignant gliomas in rats. Clin Cancer Res. 2000;6:4148–4153. [PubMed] [Google Scholar]
- 29.Sanftner LM, Sommer JM, Suzuki BM, et al. AAV2-mediated gene delivery to monkey putamen: evaluation of an infusion device and delivery parameters. Exp Neurol. 2005;194:476–483. doi: 10.1016/j.expneurol.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito R, Bringas JR, Panner A, et al. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004;64:6858–6862. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
- 31.Saito R, Bringas JR, McKnight TR, et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64:2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- 32.Mamot C, Nguyen JB, Pourdehnad M, et al. Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J Neurooncol. 2004;68:1–9. doi: 10.1023/b:neon.0000024743.56415.4b. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham J, Oiwa Y, Nagy D, et al. Distribution of AAV-TK following intracranial convection-enhanced delivery into rats. Cell Transplant. 2000;9:585–594. doi: 10.1177/096368970000900504. [DOI] [PubMed] [Google Scholar]
- 34.Kawakami K, Kawakami M, Kioi M, Husain SR, Puri RK. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection- enhanced delivery in a murine model. J Neurosurg. 2004;101:1004– 1011. doi: 10.3171/jns.2004.101.6.1004. [DOI] [PubMed] [Google Scholar]
- 35.Degen JW, Walbridge S, Vortmeyer AO, Oldfield EH, Lonser RR. Safety and efficacy of convection-enhanced delivery of gemcitabine or carboplatin in a malignant glioma model in rats. J Neurosurg. 2003;99:893–898. doi: 10.3171/jns.2003.99.5.0893. [DOI] [PubMed] [Google Scholar]
- 36.Groothuis DR, Ward S, Itskovich AC, et al. Comparison of 14C-sucrose delivery to the brain by intravenous, intraventricular, and convection-enhanced intracerebral infusion. J Neurosurg. 1999;90:321–331. doi: 10.3171/jns.1999.90.2.0321. [DOI] [PubMed] [Google Scholar]
- 37.Puri RK, Hoon DS, Leland P, et al. Preclinical development of a recombinant toxin containing circularly permuted interleukin 4 and truncated Pseudomonas exotoxin for therapy of malignant astrocytoma. Cancer Res. 1996;56:5631–5637. [PubMed] [Google Scholar]
- 38.Patel SJ, Shapiro WR, Laske DW, et al. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: Initial experience in 51 patients. Neurosurgery. 2005;56:1243–1253. doi: 10.1227/01.neu.0000159649.71890.30. [DOI] [PubMed] [Google Scholar]
- 39.Rainov NG, Heidecke V. Long term survival in a patient with recurrent malignant glioma treated with intratumoral infusion of an IL4-targeted toxin (NBI-3001) J Neurooncol. 2004;66:197–201. doi: 10.1023/b:neon.0000013478.27604.01. [DOI] [PubMed] [Google Scholar]
- 40.Kunwar S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl. 2003;88:105–111. doi: 10.1007/978-3-7091-6090-9_16. [DOI] [PubMed] [Google Scholar]
- 41.Weber FW, Floeth F, Asher A, et al. Local convection enhanced delivery of IL4-Pseudomonas exotoxin (NBI-3001) for treatment of patients with recurrent malignant glioma. Acta Neurochir Suppl. 2003;88:93–103. doi: 10.1007/978-3-7091-6090-9_15. [DOI] [PubMed] [Google Scholar]
- 42.Weber F, Asher A, Bucholz R, et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol. 2003;64:125–137. doi: 10.1007/BF02700027. [DOI] [PubMed] [Google Scholar]
- 43.Rand RW, Kreitman RJ, Patronas N, et al. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res. 2000;6:2157–2165. [PubMed] [Google Scholar]
- 44.Oldfield EH, Youle RJ. Immunotoxins for brain tumor therapy. Curr Top Microbiol Immunol. 1998;234:97–114. doi: 10.1007/978-3-642-72153-3_7. [DOI] [PubMed] [Google Scholar]
- 45.Lidar Z, Mardor Y, Jonas T, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/ II clinical study. J Neurosurg. 2004;100:472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 46.Sampson JH, Reardon DA, Friedman AH, et al. Sustained radiographic and clinical response in patient with bifrontal recurrent glioblastoma multiforme with intracerebral infusion of the recombinant targeted toxin TP-38: case study. Neuro-Oncology. 2005;7:90–96. doi: 10.1215/S1152851703000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampson JH, Reardon DA, Akabani G, et al. A phase I study of intra-tumoral infusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin (TP-38) for the treatment of malignant brain tumors [abstract] Proc Am Assoc Cancer Res. 2002;43:746. [Google Scholar]
- 48.Torp SH, Helseth E, Ryan L, et al. Expression of the epidermal growth factor receptor gene in human brain metastases. APMIS. 1992;100:713–719. doi: 10.1111/j.1699-0463.1992.tb03989.x. [DOI] [PubMed] [Google Scholar]
- 49.Libermann TA, Razon N, Bartal AD, et al. Expression of epidermal growth factor receptors in human brain tumors. Cancer Res. 1984;44:753–760. [PubMed] [Google Scholar]
- 50.Torp SH, Helseth E, Dalen A, Unsgaard G. Epidermal growth factor receptor expression in human gliomas. Cancer Immunol Immunother. 1991;33:61–64. doi: 10.1007/BF01742530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 52.Murad G, Walbridge S, Morrison PF, et al. Real-time, image-guided, convection-enhanced delivery of interleukin 13 bound to pseudomonas exotoxin. Clin Cancer Res. 2006;12:3145–3151. doi: 10.1158/1078-0432.CCR-05-2583. [DOI] [PubMed] [Google Scholar]
- 53.Parney IF, Kunwar S, McDermott M, et al. Neuroradiographic changes following convection-enhanced delivery of the recombinant cytotoxin interleukin 13-PE38QQR for recurrent malignant glioma. J Neurosurg. 2005;102:267–275. doi: 10.3171/jns.2005.102.2.0267. [DOI] [PubMed] [Google Scholar]
- 54.Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 55.Love S, Plaha P, Patel NK, et al. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med. 2005;11:703–704. doi: 10.1038/nm0705-703. [DOI] [PubMed] [Google Scholar]
- 56.Patel NK, Bunnage M, Plaha P, et al. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol. 2005;57:298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- 57.Sampson JH, Akabani G, Archer GE, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65:27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 58.Sampson JH, Raghavan R, Brady ML, et al. Clinical utility of a patient-specific algorithm for simulating intracerebal drug infusions. Neuro-Oncology. 2007;9:343–353. doi: 10.1215/15228517-2007-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krauze MT, Saito R, Noble C, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg. 2005;103:923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]