Abstract
MicroRNAs (miRNAs) are postulated to be important regulators in cancers. Here, we report a genome-wide miRNA expression analysis in 52 acute myeloid leukemia (AML) samples with common translocations, including t(8;21)/AML1(RUNX1)-ETO(RUNX1T1), inv(16)/CBFB-MYH11, t(15;17)/PML-RARA, and MLL rearrangements. Distinct miRNA expression patterns were observed for t(15;17), MLL rearrangements, and core-binding factor (CBF) AMLs including both t(8;21) and inv(16) samples. Expression signatures of a minimum of two (i.e., miR-126/126*), three (i.e., miR-224, miR-368, and miR-382), and seven (miR-17–5p and miR-20a, plus the aforementioned five) miRNAs could accurately discriminate CBF, t(15;17), and MLL-rearrangement AMLs, respectively, from each other. We further showed that the elevated expression of miR-126/126* in CBF AMLs was associated with promoter demethylation but not with amplification or mutation of the genomic locus. Our gain- and loss-of-function experiments showed that miR-126/126* inhibited apoptosis and increased the viability of AML cells and enhanced the colony-forming ability of mouse normal bone marrow progenitor cells alone and particularly, in cooperation with AML1-ETO, likely through targeting Polo-like kinase 2 (PLK2), a tumor suppressor. Our results demonstrate that specific alterations in miRNA expression distinguish AMLs with common translocations and imply that the deregulation of specific miRNAs may play a role in the development of leukemia with these associated genetic rearrangements.
Keywords: apoptosis and cell viability and proliferation, core binding factor (CBF), microRNA expression profiling, miR-126, PLK2
Acute myeloid leukemia (AML) is a heterogeneous group of genetically diverse hematopoietic malignancies with variable response to treatment (1). Chromosome translocations are frequently observed in AML (2). Four major rearrangements in AML are the t(8;21), inv(16), t(15;17), and MLL/11q23 translocations, which account for ≈30% of all AML cases (3), and have been incorporated in the WHO classification as the criteria for subclassification of AML (4). The t(8;21), t(15;17), and inv(16) have been established as molecular indicators for favorable clinical outcome in AML, whereas MLL-rearrangement AML is classified as a disease of intermediate or poor prognosis (1, 5). Both the t(8;21) and inv(16) rearrangements result in the disruption of core-binding factor (CBF), a heterodimeric transcriptional regulator of normal hematopoiesis (6, 7) and, therefore, are collectively referred to as CBF AMLs, which represent 10–20% of primary AMLs (8, 9). Many expression profiling studies of protein-coding genes have been performed on AML by using DNA microarray analysis (e.g., refs. 10–13). However, the results of analyses of AML by different laboratories are not always consistent. For example, there is a relatively small overlap between genes relevant for prognosis reported by Bullinger et al. (11) and those reported by Valk et al. (12). Therefore, further validation of these observations in large cohorts and in independent studies is required before clinical application becomes feasible, and it seems unlikely that mRNA expression profiling alone can reveal the entire pathobiology of AML (5).
Recently, Lu et al. (14) described a new, bead-based flow-cytometric microRNA (miRNAs, miRs) expression profiling method that could successfully classify tumors and showed that identification of poorly differentiated tumors by using miRNA expression profiles was more accurate than by using mRNA expression profiles. This finding indicates that miRNA profiling could be superior to mRNA profiling in certain clinical circumstances and suggests that miRNA profiling is an important tool in molecular classification. miRNAs are endogenous ≈22-nt noncoding RNAs that can play important regulatory roles in development, cell proliferation, cell survival, and apoptosis (15). Moreover, evidence is emerging that miRNAs can function as oncogenes and tumor suppressor genes (16, 17).
To provide insights into the complex genetic alterations in leukemogenesis and to identify possible markers for the diagnosis and treatment of AML in the future, we performed a genome-wide bead-based miRNA expression analysis (14). The profiling data were analyzed with unsupervised and supervised clustering analyses, and a minimal set of miRNAs that can accurately predict the AML subtypes was identified. The expression pattern of the class-discriminator miRNAs was further validated by quantitative real-time PCR (qPCR). Finally, the mechanism of gene-expression regulation and function of miR-126, a class-discriminator miRNA for CBF AMLs, as well as its potential targets, was carefully studied.
Results
MiRNA Expression Profiling in AMLs.
We performed a large-scale miRNA expression profiling analysis of 435 mammalian miRNAs on 57 samples, including 47 primary AML specimens, 7 AML cell lines, and 3 normal control samples (see Materials and Methods). The 47 primary AML specimens included 10 t(8;21)/AML1(RUNX1)-ETO(RUNX1T1), 7 inv(16)/CBFB-MYH11, 10 t(15;17)/PML-RARA, and 20 MLL-rearrangement AMLs [including one t(6;11)/MLL-AF6(MLLT4), 9 t(9;11)/MLL-AF9(MLLT3), 3 t(11;19)(q23;p13.1)/MLL-ELL, and 7 t(11;19)(q23;p13.3)/MLL-ENL(MLLT1)]. The seven AML cell lines included one t(8;21), one inv(16), and five MLL-rearrangement AMLs. Three normal bone marrow samples from healthy donors were used as normal controls, which included two mononuclear cell (MNC) samples and one purified CD15+ myeloid progenitor cell sample. Bead-based miRNA expression profiling detection was performed as described (14). Two samples with total fluorescence <15,000 were discarded as unsuccessful labeling/sample quality. After normalization and filtering [see supporting information (SI) Text], a total of 55 samples (including 52 AML and 3 normal controls) and 112 human miRNA genes with confidently detectable expression values were selected for further analyses.
We first performed an unsupervised, two-way (genes against samples), hierarchical cluster analysis (HCA). Remarkably, leukemia samples clearly grouped into three clusters: (i) t(8;21) and inv(16) samples grouped together as Cluster 1 with three exceptions; (ii) t(15;17) samples grouped together as Cluster 2 with two exceptions; and (iii) all of the MLL-rearrangement AML samples grouped together and formed Cluster 3. Normal control samples also grouped together and formed a subcluster within Cluster 3 (see Fig. S1A). The clustering was not associated with patient's age, gender, blast cell percentage, clinical state, or sample source (peripheral blood or bone marrow). Clustering of cases with MLL rearrangement did not correlate completely with either MLL partner genes or French–American–British (FAB) subtypes (Fig. S1a), which is consistent with observations from the mRNA-based expression assays (18, 19). In a three-dimensional view of a principal-component analysis (PCA), the three clusters are clearly separate (Fig. S1b).
MiRNAs Differentially Expressed Between the Subtypes of AMLs.
Gene expression data were further analyzed for significance by using ANOVA multiple-groups test and permutation tests. The ANOVA analysis identified 32 miRNAs that were significantly, differentially expressed between the subgroups (Fig. 1A). We also used significance analysis of microarray (SAM) (20) and permutation tests to identify differentially expressed genes with a “one-vs.-all of the others” approach. We identified 24, 19, 11, 2, 3, and 5 miRNAs differentially-expressed in MLL-rearrangement, t(15;17), t(8;21) plus inv(16), t(8;21), inv(16) AML samples, and normal controls, compared with all other samples, respectively. Each miRNA had at least a two-fold difference in expression between the two compared parts, along with a q value [a measure of the false discovery rate (FDR)] (21) <0.05. The overall FDR in each set of differentially expressed miRNAs is <5%. Together, there are a total of 41 unique differentially expressed miRNAs detected by ANOVA and/or SAM (Fig. 1A). The clustering pattern observed in Fig. 1A is similar to that in Fig. S1a. The 41 miRNAs presented a similar expression pattern between the AML cell lines and the relevant primary leukemia specimens, suggesting that the relevant critical regulatory pathways remain conserved in the cell lines despite numerous passages. As shown in Fig. 1A, miR-126/126* were specifically overexpressed in both t(8;21) and inv(16) samples, whereas miR-224, miR-368, and miR-382 were almost exclusively overexpressed in the t(15;17) samples. Among the miRNAs that were significantly overexpressed in MLL rearrangements, seven miRNAs including miR-17–5p, miR-17–3p, miR-18a, miR-19a, miR-20a, miR-19b, and miR-92 are from a unique polycistronic miRNA cluster, namely mir-17-92 (22, 23).
Fig. 1.
Expression profiling analysis and validation of miRNAs in AMLs with common translocations. (A) Expression profiling of the 41 differentially expressed miRNAs in 55 samples as detected by the bead-based miRNA expression assay. Unsupervised, average linkage hierarchical clustering was performed. Expression data were mean centered, and the relative value for each sample was represented by a color, with red representing a high expression and green representing a low expression (scale shown in the upper right). The three clusters of AMLs and the subcluster with normal controls were indicated by color bars across the top of the figure (four colors). (B) Expression profiling of the seven class-discriminatory miRNAs in 56 AML and 9 control samples as detected by TaqMan qPCR. Data are presented as ΔCT. “OLD” samples are those included in the bead-based miRNA expression assay, whereas “NEW” samples are independent samples that were not included in that assay. “cl_,” cell line; “N_,” normal control; “MNC_,” mononuclear cells; “CD34+,” CD34+ hematopoietic stem/progenitor cells; “CD15+,” CD15+ myeloid progenitor cells; “j-miR-2,” a miRNA identified by J.L., H.Z., and T.R.G. (unpublished data).
Class Prediction of Distinct AML Subtypes with a Minimal Number of 2∼7 miRNAs.
We further used the prediction analysis of microarrays (PAM) method (24) to determine the minimal number of miRNAs that can predict the AML subtypes accurately (see Materials and Methods). As shown in Table S1, the expression signature of a minimal number of two (i.e., miR-126, and miR-126*), three (i.e., miR-224, miR-368, and miR-382), and seven (i.e., miR-17–5p and miR-20a plus the aforementioned five) miRNAs can best characterize CBF, t(15;17), and MLL-rearrangement AMLs, respectively, resulting in a diagnostic accuracy >94%. The detailed cross-validated probabilities of the entire set of 52 AML samples is shown in Fig. S2; and see Table S2. A similar level of accuracy was achieved by using a different supervised learning algorithm, namely SVM (data not shown).
qPCR Confirmation of the Seven Class-Discriminatory miRNAs.
We then used a TaqMan qPCR (25) to validate the expression pattern of the above seven class-discriminatory miRNAs in a group of 56 AML and 9 normal control samples (see Materials and Methods). The 56 AML samples include 47 AML patient samples [10 t(8;21), 10 inv(16), 10 t(15;17), and 17 MLL-rearrangement AMLs] and 9 AML cell lines. The nine normal control samples include five CD34+ hematopoietic progenitor cells, one CD15+ myeloid progenitor, and three MNC. Forty-one (63%) of the 65 samples were not used in the bead-based expression assay. As shown in Fig. 1B, the differential expression pattern of the miRNAs detected by the bead-based expression assay (Fig. 1A) was confirmed by qPCR.
Overexpression of miR-126/126* in CBF AMLs Is Not a Consequence of DNA Amplification.
To reveal the mechanism underlying the overexpression of miR-126/126* in CBF AMLs, we first examined the DNA copy number of the miR-126/126* locus at 9q34.3 in 30 (12 CBF and 18 non-CBF) AML samples and two normal MNC controls using TaqMan qPCR (see Materials and Methods). As shown in Fig. S3, there was no amplification of genomic locus of miR-126/126* in CBF leukemia samples relative to either normal controls or non-CBF AMLs or to a deletion of this locus in non-CBF AMLs.
Differential Expression of miR-126/126* Might Be Associated with Epigenetic Regulation.
MiR-126/126* is located within intron 7 of the host gene, namely epidermal growth factor-like 7 (EGFL7) (Fig. S4a). Interestingly, the locus of the whole precursor of miR-126/126* is embedded in a 287-bp CpG island with 29 CpG dinucleotides, whereas a larger, 1201-bp CpG island with 97 CpG dinucleotides is located upstream (within the second intron of the host gene). We determined the DNA methylation status of both CpG island regions in 21 samples including 8 CBF AMLs, 10 non-CBF leukemia samples, and 3 normal MNC controls using bisulfite genomic sequencing methods (see Materials and Methods). The average methylation rate of the 287-bp CpG island in the CBF AMLs (70.8%) was significantly lower (t test; P < 0.005) than that (94.4%) in the non-CBF leukemias (Fig. S4b). In analysis of the 19 samples with both methylation (Fig. S4b) and expression (Fig. 1B) information available, we observed that the expression level of miR-126/126* was significantly negatively correlated (for both miRNAs, two-tailed P < 0.0005; rs = −0.75 for miR-126, rs = −0.83 for miR-126*; Spearman's rank correlation test) with the degree of methylation of the 287-bp CpG island (see Fig. S4c). In contrast, the average methylation rate of the 1,201-bp CpG island of the CBF AMLs (6.1%) was not significantly different (two-tailed P > 0.69; t test) from that (7.0%) of the non-CBF AMLs (see Fig. S4d). To confirm that the decreased expression of miR-126 is related to DNA methylation, we treated ME-1 and MV4-11 cell lines with decitabine (5-Aza-2′-deoxycytidine) and Trichostatin A (TSA). As shown in Fig. S4e, expression of miR-126 in the cell lines was increased significantly after the treatment. Therefore, the differential expression of miR-126/126* was, at least partly, associated with methylation regulation of the 287-bp CpG island.
Differential Expression of miR-126/126* Is Not Associated with Mutation.
Calin et al. (26) stated that mutations in miRNA sequences are common and may have functional importance. We therefore investigated whether differential expression of miR-126/126* is also associated with DNA sequence mutations. We sequenced the region of the 128-bp CpG island in the above 21 samples used for the methylation analysis. We did not identify any mutations in this region except for a known SNP at nucleotide 151 (A-to-G transition) of the CpG island fragment in one or both alleles of all of the samples. Although the biological function of this SNP needs to be clarified, it does not appear to be associated with differential expression of miR-126/126*, because this SNP existed in both CBF and non-CBF leukemia samples.
MiR-126 Inhibits Cell Apoptosis and Increases Cell Viability.
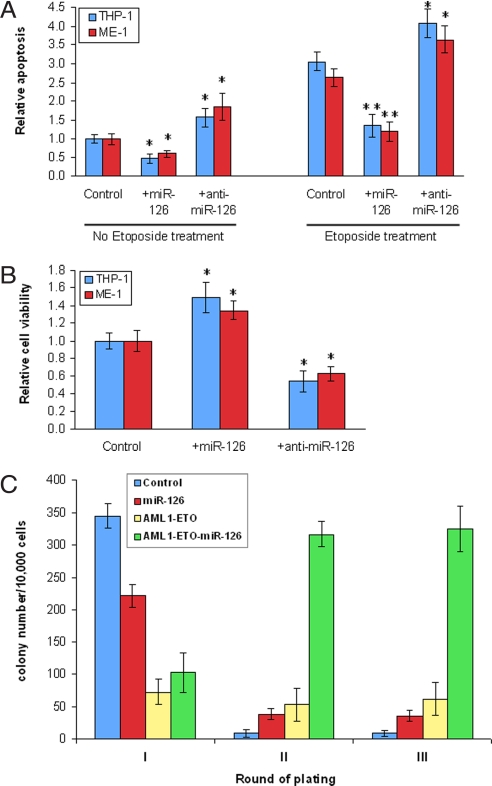
To examine the functional role of miR-126/126* in AML cells, we performed gain- and loss- of-function experiments. As shown in Fig. 2A, forced expression of miR-126 significantly inhibited apoptosis in THP-1/t(9;11) and ME-1/inv(16) cells with or without the treatment of Etoposide (VP16; a DNA topoisomerase II inhibitor); in contrast, knockdown of miR-126 by an anti-miR-126 inhibitor significantly increased apoptosis. As expected, we also observed that forced expression of miR-126 significantly increased cell viability in THP-1 and ME-1 cells, whereas the opposite effect was observed when miR-126 was down-regulated (Fig. 2B). The forced expression and knockdown of miR-126 were confirmed by qPCR, respectively (see Fig. S5a). Thus, miR-126 may function as an oncogene in leukemogenesis.
Fig. 2.
The functional role and target(s) of miR-126. (A) Forced expression of miR-126 by transduced MSCVpuro-miR-126 plasmid significantly inhibited, whereas down-regulation of miR-126 by anti-miR-126 inhibitor significantly increased apoptosis in THP-1 and ME-1 cells with or without Etoposide treatment. (B) Forced expression of miR-126 significantly increased, whereas down-regulation of miR-126 significantly decreased cell viability in THP-1 and ME-1 cells. Normalized mean values of three independent experiments and standard error (mean ± SE) are shown. *, P < 0.05; **, P < 0.001 (paired t test). (C) miR-126 enhanced colony forming ability of mouse normal bone marrow progenitor cells alone and particularly, in cooperation with AML1-ETO (AE). Only the colonies that each contained at least 50 cells were counted. Note, the colony numbers of the first round of plating largely reflected the transduction efficiency, which was related to the size of the plasmids, with a higher transduction efficiency for a smaller-sized plasmid. Thus, the low colony number of the control in the second and third rounds of plating is not due to a low efficiency of transduction.
MiR-126 Enhances Proliferation of Mouse Bone Marrow Progenitor Cells Alone and Particularly, in Cooperation with the t(8;21) Fusion Gene.
The AML1-ETO (AE) fusion gene resulting from the t(8;21) cannot cause leukemia alone in vivo (27). To investigate whether miR-126 has a synergistic action with AE in oncogenicity, we performed a colony-forming and replating assay. Mouse bone marrow progenitor cells transduced with MSCVpuro (empty vector; as a control), MSCVpuro-miR-126, MSCVpuro-AE, and MSCVpuro-AE-miR-126, respectively, were plated on methylcellulose medium (see SI Text for details). The forced expression of miR-126 and AML1-ETO were confirmed by qPCR (see Fig. S5b). The colonies were replated every 7 days under the same conditions. As shown in Fig. 2C, transduction of miR-126 or AE alone or both of them together caused significantly more colonies (4- to 36-fold; P < 0.001, t test) than transduction of MSCVpuro empty vector (i.e., control) after replating (i.e., in the second and third rounds of plating). Remarkably, after replating, the number of colonies of MSCVpuro-AE-miR-126 (>300) is significantly greater (>3-fold; P < 0.001, t test) than the sum of the colonies (<100) of MSCVpuro-AE and MSCVpuro-miR-126 (Fig. 2C), indicating that there is a very significantly synergistic effect between miR-126 and AE.
Identification of Potential Targets of miR-126 in AMLs.
Recent findings indicate that animal miRNAs may not only repress protein synthesis but also induce mRNA degradation of a large portion of targets (28, 29). Thus, we used qPCR to determine whether a candidate target exhibits a significant inverse correlation of expression at RNA level with miR-126. From 674 predicted targets of miR-126 by at least one of four currently available major prediction programs, including TargetScanS (30), Miranda (31), PicTar (32), and MAMI (http://mami.med.harvard.edu/), we selected all of the 12 genes (i.e., CRK, FBXO33, IRS1, DIP2C, GOLPH3, PHF15, PLK2, PTPN9, RGS3, SLC7A5, SPRED1, and TOM1) that were predicted by at least three different programs for qPCR (Table S3). We found that only PLK2 and SPRED1 exhibited a significant inverse correlation (for PLK2: rs = −0.42, P < 0.01; for SPRED1: rs = −0.66, P < 0.0001; Spearman's rank correlation test) with the expression of miR-126 in the whole set of 41 samples tested (see Fig. S6 a–c). We then performed a luciferase reporter assay to validate the potential regulatory relationship. A significantly negative effect (P < 0.01; paired t test) on luciferase activity was observed in the presence of miR-126 on the 3′ UTR of PLK2 but not on that of SPRED1; such repression disappeared when the predicted target site in the 3′ UTR of PLK2 was mutated (Fig. S7 a and b). Moreover, the down- and up-regulation of PLK2 was associated with overexpression and down-regulation of miR-126, respectively (Fig. S7c). These results indicate that PLK2, but not SPRED1, is a bona fide target of miR-126.
Discussion
Here, we demonstrate that the expression signatures of as few as seven miRNAs accurately distinguish subtypes of AMLs with common translocations (Table S1). Interestingly, our findings that t(8;21) and inv(16) samples grouped together in both unsupervised and supervised analyses (see Fig. S1a and Fig. 1A) and that two miRNAs (i.e., miR-126 and miR-126*) could be used as predictors to discriminate CBF AMLs from non-CBF AMLs (Table S1 and Fig. S2), provide compelling evidence to support the notion that there is a common leukemogenic pathway existing in CBF AMLs (9, 33, 34). In fact, clinical studies have usually stratified t(8;21) and inv(16) patients together into one favorable-risk prognosis category, and treated them similarly (35, 36).
Several differentially expressed miRNAs identified here, including miR-10a, miR-10b, miR-17, miR-20, miR-29a, miR-29c, miR-126, miR-191, miR-181, and let-7d (see Fig. 1A) were also reported previously in the studies of hematopoietic cells (37–39), leukemia cell lines (39, 40), acute promyelocytic leukemia (41), or morphological subgroups of AMLs (42). During the preparation of this article, we became aware that several other articles regarding miRNAs in AML were published. Fazi et al. (43) observed a down-regulation of miR-223 in t(8;21) AML samples and showed that there was an epigenetic silencing of the myelopoiesis regulator miR-223 by the AML1-ETO oncoprotein. We also found that miR-223 was expressed at a lower level in the majority of t(8;21) samples than in other AML-M2 and AML-M4 samples, but it was significantly differentially expressed between AML and ALL (44), not between the subtypes of AML we studied. Fontana et al. (45) reported that miRNAs 17–5p-20a-106a control monocytopoiesis through suppressing AML1 protein expression, leading to M-CSF receptor down-regulation and thereby enhancing blast proliferation and inhibition of monocytic differentiation and maturation. Therefore, their study together with our finding (see Fig. 1) that the mir-17-92 cluster is particularly overexpressed in MLL-rearrangement AMLs (M4/M5, with the majority of leukemic cells being monoblasts) suggest that mir-17-92 may play an important role in the development of such AML. Using a qPCR method, Isken et al. (46) identified that miR-23b was down-regulated, whereas miR-221/222 and miR-34a were overexpressed in AMLs compared with normal controls. In a large-scale miRNA microarray study, Garzon et al. (47) found that miRNA expression in AML is closely associated with cytogenetics such as t(11q23) and FLT3-ITD mutations. Jongen-Lavrencic et al. (48) recently analyzed 215 AML cases including those bearing the common translocations using qPCR for 260 miRNAs, and notably, consistent with our findings, they also identified miR-126 and miR-382 as a class predictor for CBF and t(15;17) leukemia, respectively.
A current challenge is to clarify the mechanism(s) underlying regulation of miRNA expression (17). Here, we found that the overexpression of miR-126/126* in CBF AML is not a consequence of amplification or mutation of the genomic locus but is associated with partial de-methylation of the CpG island in which miR-126/126* is embedded. In addition, as shown in Fig. S5b, forced expression of AML1-ETO can significantly up-regulate expression of endogenous miR-126 (>2-fold), suggesting that, besides being related to DNA demethylation, overexpression of miR-126 in CBF leukemia might be also attributed to a direct up-regulation of AML1-ETO or CBFB-MYH11.
It is believed that the deregulation of miRNAs contributes to tumorigenesis by negatively regulating expression of their targets (15, 17). We identified PLK2 as a valid target of miR-126. PLK2 is one of the Polo-like kinase (PLK) family members that function in regulation of the cell cycle and DNA damage-induced checkpoints in mammals (49, 50). PLK2 expression is up-regulated directly by wild-type p53 after DNA damage and activates a G2 checkpoint in these circumstances (50, 51). Down-regulation of PLK2 was frequently observed in B-cell malignancies, and ectopic expression of PLK2 in Burkitt lymphoma cells resulted in apoptosis (49), suggesting that PLK2 may function as a tumor suppressor gene in hematologic malignancies (49, 50). Thus, miR-126 may play a role in leukemogenesis through negatively regulating PLK2. Our finding that miR-126 may function as a oncogene is supported further by an observation that miR-126 is significantly up-regulated in acute megakaryoblastic leukemia cell lines compared with in vitro differentiated megakaryocytes and CD34+ hematopoietic progenitors (39).
Acute leukemias, like other human cancers, occur as the consequence of more than one mutation. Primary oncogenic events, such as those triggered by chromosomal rearrangements, are generally insufficient by themselves to cause leukemia and require secondary cooperating mutations to generate a fully transformed cell (52). For example, conditional expression of knockin CBFβ-MYH11 or AML1-ETO fusion gene, or expression of AML1-ETO after retroviral transduction/transplantation does not result in leukemia without a “second hit,” such as those induced by N-ethyl-N-nitrosourea (ENU) (27, 53). Because miR-126/126* were almost uniquely overexpressed in CBF AMLs, we hypothesize that their overexpression may contribute to the development of CBF AML as a second hit by cooperating with the primary oncogenic events (i.e., CBFβ-MYH11 or AML1-ETO), which is supported by our finding that miR-126 inhibits apoptosis and increases cell viability in AML cells and enhances proliferation of mouse normal bone marrow progenitor cells alone and particularly, in cooperation with the t(8;21) fusion genes. Clearly, further in vitro and in vivo assays are necessary to validate such a hypothesis. Similarly, miR-224, miR-368, and miR-382 may contribute to the development of APL in cooperation with the t(15;17), whereas the mir-17-92 cluster may contribute to MLL-rearrangement AML through cooperating with the MLL rearrangements.
Materials and Methods
See SI Text for more details on materials and methods used.
Bead-Based miRNA Expression Profiling Assay and Data Analysis.
Bead-based miRNA expression profiling assay was performed as described (14) with some modifications (44). The TIGR Mutiple Array Viewer software package (54) was used to perform data analysis and to visualize the results. The class prediction analysis was performed by using PAM software (24).
qPCR Assays of miRNAs, mRNAs, and DNA Locus Copy Number.
The TaqMan qPCR method (25) was used to validate the differential expression patterns of miRNAs by using kits from Applied Biosystems. qPCR with SYBR green dye (Qiagen) was used to determine expression of mRNA genes. U6 RNA and PGK1 (or GAPDH) were used as endogenous controls for qPCR of miRNA and mRNA, respectively. PCRs were performed in an Applied Biosystems 7900HT system according to the relevant manufacturer's recommendation, and each sample was detected in triplicate. We followed a method described by He et al. (23) to analyze DNA copy number of the miR-126/126* locus with modification as described (44).
Bisulfite Genomic Sequencing and Mutation Screening.
Genomic DNA (1 μg) from each of sample was treated with sodium bisulfite (55). Thereafter, the relevant genomic DNA regions were PCR amplified. After PCR amplification, DNA methylation levels were analyzed by bisulfite genomic sequencing with M13 primer as described (56). For mutation screening, the genomic regions were PCR amplified from genomic DNA samples with no sodium bisulfite treatment, and PCR products were then purified and sequenced directly.
Apoptosis Assay.
THP-1 or ME-1 cells were plated at a concentration of 10,000 cells per well in triplicate for each condition in a 96-well plate 24 h after transfection with MSCVpuro-miR-126, the control plasmid MSCVpuro, anti-miR126 inhibitor (Dharmacon), or inhibitor control (i.e., scrambled oligonucleotides; Dharmaconcomx). Etoposide (5 μM; Sigma) or the same volume of DMSO (mock treatment) was added to each well, and caspase-3 and caspase-7 activation was detected by using ApoONE Homogenous Caspase 3/7 Assay (Promega) 24 h later, by following the manufacturer's manual.
Cell Viability Assay.
THP-1 or ME-1 cells were plated at a concentration of 10,000 cells per well in triplicate for each condition in a 96-well plate 24 h after transfection with MSCVpuro-miR-126, MSCVpuro, anti-miR126 inhibitor, or inhibitor control. Metabolic activity of the cells was determined by using CellTiter-blue Reagent (Promega) 24 h later by following the manufacturer's manual.
Colony-Forming and Replating Assay.
In vitro colony-forming (i.e., immortalization) assays were performed as described (57) with some modifications.
Luciferase Reporter and Mutagenesis Assays.
MiR-126 expression plasmid (i.e., MSCVpuro-miR-126) or its control plasmid (i.e., MSCVpuro-PIG) (23) was cotransfected into HEK293T cells with a single report plasmid (pMIR-Report plasmid; Ambion) containing either the wild-type or mutated 3′ UTR of an individual predicted target gene. Luciferase was measured 42 h after transfection. The firefly luciferase activity was then normalized to β-galactosidase activity. Experiments were repeated three times independently.
Supplementary Material
Acknowledgments.
We thank Drs. G. J. Hannon, S. M. Hammond, and L. He at Cold Spring Harbor Laboratory for providing MSCVpuro-PIG vector, Dr. P. P. Liu at National Human Genome Research Institute for providing the ME-1 cell line, and Drs. Y. Sato and K. Sugita at Yamanashi University for providing the KOCL-48 cell line. We also thank Dr. L. Pelloso at the University of Chicago for helpful discussion and Drs. J. Xu and Y. Chen at the University of Chicago for technical help with the methylation analysis. This work was supported in part by National Institutes of Health (NIH) Grant CA127277 (to J.C.), the G. Harold and Leila Y. Mathers Charitable Foundation (J.C.), a Cancer Research Foundation Young Investigator Award (to J.C.), a Leukemia and Lymphoma Society Translational Research Grant (to J.D.R.), the Spastic Paralysis Foundation of the Illinois, the Eastern Iowa Branch of Kiwanis International (J.D.R.), NIH Grants CA40046 (to M.M.L.B. and R.A.L) and CA104509 (to D.-E.Z.). T.R.G. is an Investigator of the Howard Hughes Medical Institute. The sequencing was done by the Cancer Research Center DNA Sequencing Facility with the support of NIH Cancer Center Support Grant CA014599.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808266105/DCSupplemental.
References
- 1.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Rowley JD. Chromosome translocations: Dangerous liaisons revisited. Nat Rev Cancer. 2001;1:245–250. doi: 10.1038/35106108. [DOI] [PubMed] [Google Scholar]
- 3.Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer. 2007;7:233–245. doi: 10.1038/nrc2091. [DOI] [PubMed] [Google Scholar]
- 4.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 5.Bullinger L, Valk PJ. Gene expression profiling in acute myeloid leukemia. J Clin Oncol. 2005;23:6296–6305. doi: 10.1200/JCO.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Liu P, et al. Fusion between transcription factor CBF beta/PEBP2 beta and a myosin heavy chain in acute myeloid leukemia. Science. 1993;261:1041–1044. doi: 10.1126/science.8351518. [DOI] [PubMed] [Google Scholar]
- 7.de Bruijn MF, Speck NA. Core-binding factors in hematopoiesis and immune function. Oncogene. 2004;23:4238–4248. doi: 10.1038/sj.onc.1207763. [DOI] [PubMed] [Google Scholar]
- 8.Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 9.Strout MP, Marcucci G, Caligiuri MA, Bloomfield CD. Core-binding factor (CBF) and MLL-associated primary acute myeloid leukemia: biology and clinical implications. Ann Hematol. 1999;78:251–264. doi: 10.1007/s002770050511. [DOI] [PubMed] [Google Scholar]
- 10.Ross ME, et al. Gene expression profiling of pediatric acute myelogenous leukemia. Blood. 2004;104:3679–3687. doi: 10.1182/blood-2004-03-1154. [DOI] [PubMed] [Google Scholar]
- 11.Bullinger L, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004;350:1605–1616. doi: 10.1056/NEJMoa031046. [DOI] [PubMed] [Google Scholar]
- 12.Valk PJ, et al. Prognostically useful gene-expression profiles in acute myeloid leukemia. N Engl J Med. 2004;350:1617–1628. doi: 10.1056/NEJMoa040465. [DOI] [PubMed] [Google Scholar]
- 13.Haferlach T, et al. Global approach to the diagnosis of leukemia using gene expression profiling. Blood. 2005;106:1189–1198. doi: 10.1182/blood-2004-12-4938. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 15.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 16.Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 17.Wu W, Sun M, Zou GM, Chen J. MicroRNA and cancer: Current status and prospective. Int J Cancer. 2007;120:953–960. doi: 10.1002/ijc.22454. [DOI] [PubMed] [Google Scholar]
- 18.Kohlmann A, et al. New insights into MLL gene rearranged acute leukemias using gene expression profiling: Shared pathways, lineage commitment, and partner genes. Leukemia. 2005;19:953–964. doi: 10.1038/sj.leu.2403746. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong SA, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 20.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ota A, et al. Identification and characterization of a novel gene, C13orf25, as a target for 13q31–q32 amplification in malignant lymphoma. Cancer Res. 2004;64:3087–3095. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- 23.He L, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci USA. 2002;99:6567–6572. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calin GA, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 27.Yuan Y, et al. AML1-ETO expression is directly involved in the development of acute myeloid leukemia in the presence of additional mutations. Proc Natl Acad Sci USA. 2001;98:10398–10403. doi: 10.1073/pnas.171321298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagga S, et al. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Nilsen TW. Mechanisms of microRNA-mediated gene regulation in animal cells. Trends Genet. 2007;23:243–249. doi: 10.1016/j.tig.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 31.Krek A, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 32.John B, et al. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloomfield CD, et al. Core binding factor acute myeloid leukemia. Cancer and Leukemia Group B (CALGB) Study 8461. Ann Hematol. 2004;83(Suppl 1):S84–S85. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 34.Ichikawa H, et al. Common gene expression signatures in t(8;21)- and inv(16)-acute myeloid leukaemia. Br J Haematol. 2006;135:336–347. doi: 10.1111/j.1365-2141.2006.06310.x. [DOI] [PubMed] [Google Scholar]
- 35.Slovak ML, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 36.Byrd JC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 37.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 38.Ramkissoon SH, et al. Hematopoietic-specific microRNA expression in human cells. Leuk Res. 2006;30:643–647. doi: 10.1016/j.leukres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Garzon R, et al. MicroRNA fingerprints during human megakaryocytopoiesis. Proc Natl Acad Sci USA. 2006;103:5078–5083. doi: 10.1073/pnas.0600587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J, et al. Human microRNA clusters: Genomic organization and expression profile in leukemia cell lines. Biochem Biophys Res Commun. 2006;349:59–68. doi: 10.1016/j.bbrc.2006.07.207. [DOI] [PubMed] [Google Scholar]
- 41.Garzon R, et al. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148–4157. doi: 10.1038/sj.onc.1210186. [DOI] [PubMed] [Google Scholar]
- 42.Debernardi S, et al. MicroRNA miR-181a correlates with morphological sub-class of acute myeloid leukaemia and the expression of its target genes in global genome-wide analysis. Leukemia. 2007;21:912–916. doi: 10.1038/sj.leu.2404605. [DOI] [PubMed] [Google Scholar]
- 43.Fazi F, et al. Epigenetic silencing of the myelopoiesis regulator microRNA-223 by the AML1/ETO oncoprotein. Cancer Cell. 2007;12:457–466. doi: 10.1016/j.ccr.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Mi S, et al. MicroRNA expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc Natl Acad Sci USA. 2007;104:19971–19976. doi: 10.1073/pnas.0709313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fontana L, et al. MicroRNAs 17–5p-20a–106a control monocytopoiesis through AML1 targeting and M-CSF receptor upregulation. Nat Cell Biol. 2007;9:775–787. doi: 10.1038/ncb1613. [DOI] [PubMed] [Google Scholar]
- 46.Isken F, et al. Identification of acute myeloid leukaemia associated microRNA expression patterns. Br J Haematol. 2008;140:153–161. doi: 10.1111/j.1365-2141.2007.06915.x. [DOI] [PubMed] [Google Scholar]
- 47.Garzon R, et al. MicroRNA signatures associated with cytogenetics and prognosis in acute myeloid leukemia. Blood. 2008;111:3138–3189. doi: 10.1182/blood-2007-07-098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jongen-Lavrencic M, Sun SM, Dijkstra MK, Valk PJ, Lowenberg B. MicroRNA expression profiling in relation to the genetic heterogeneity of acute myeloid leukemia. Blood. 2008;111:5078–5085. doi: 10.1182/blood-2008-01-133355. [DOI] [PubMed] [Google Scholar]
- 49.Syed N, et al. Transcriptional silencing of Polo-like kinase 2 (SNK/PLK2) is a frequent event in B-cell malignancies. Blood. 2006;107:250–256. doi: 10.1182/blood-2005-03-1194. [DOI] [PubMed] [Google Scholar]
- 50.Smith P, Syed N, Crook T. Epigenetic inactivation implies a tumor suppressor function in hematologic malignancies for Polo-like kinase 2 but not Polo-like kinase 3. Cell Cycle. 2006;5:1262–1264. doi: 10.4161/cc.5.12.2813. [DOI] [PubMed] [Google Scholar]
- 51.Burns TF, Fei P, Scata KA, Dicker DT, El-Deiry WS. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol Cell Biol. 2003;23:5556–5571. doi: 10.1128/MCB.23.16.5556-5571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pui CH, Jeha S. New therapeutic strategies for the treatment of acute lymphoblastic leukaemia. Nat Rev Drug Discov. 2007;6:149–165. doi: 10.1038/nrd2240. [DOI] [PubMed] [Google Scholar]
- 53.Castilla LH, et al. The fusion gene Cbfb-MYH11 blocks myeloid differentiation and predisposes mice to acute myelomonocytic leukaemia. Nat Genet. 1999;23:144–146. doi: 10.1038/13776. [DOI] [PubMed] [Google Scholar]
- 54.Saeed AI, et al. TM4 microarray software suite. Methods Enzymol. 2006;411:134–193. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- 55.Frommer M, et al. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci USA. 1992;89:1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saito Y, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9:435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 57.Lavau C, Luo RT, Du C, Thirman MJ. Retrovirus-mediated gene transfer of MLL-ELL transforms primary myeloid progenitors and causes acute myeloid leukemias in mice. Proc Natl Acad Sci USA. 2000;97:10984–10989. doi: 10.1073/pnas.190167297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.