Abstract
Cytochrome p450 enzymes (Cyps) are major phase-I xenobiotic-metabolizing enzymes. Cyps are regulated by many environmental chemicals and drugs. However, knowledge about regulation of Cyps by perfluorocarboxylic acids (PFCAs), which are persistent in the environment, is limited. Two days after a single i.p. administration (50 mg/kg) of perfluorooctanoic acid (PFOA) and perfluorodecanoic acid (PFDA) increased mRNA expression of Cyp2B10 (20-fold), 3A11 (two-fold), and 4A14 (32-fold), but not Cyp1A1/2 in mouse livers. PFDA and PFOA also markedly increased protein expression of Cyp2B (50-fold) and 4A (10-fold). PFDA increased Cyp4A14 mRNA expression at relatively low doses (0.5 mg/kg), but increased Cyp2B10 mRNA expression only at high doses (> 20 mg/kg). By using constitutive androstane receptor (CAR)-, pregnane-X receptor (PXR)-, peroxisome proliferator–activated receptor alpha (PPAR)-α-, and farnesoid X receptor-null mouse models, PPAR-α and CAR were shown to play central roles in the induction of Cyps by PFDA. Specifically, PFDA increased Cyp4A14 mRNA expression in wild-type (WT) mice, but much less in PPAR-α-null mice. PFDA increased Cyp2B10 mRNA expression in WT mice, but not in CAR-null mice. In addition, PFDA increased mRNA expression and nuclear translocation of the transcription factor CAR. Therefore, the current studies provide important insight into understanding the regulatory mechanisms initiated by PFCAs, and may help to better predict and understand the toxicokinetics and toxicodynamics of various PFCAs. In conclusion, PFCAs increased Cyp2B10 and 4A14 expression by activating PPAR-α and CAR nuclear receptors, respectively. PPAR-α is activated at much lower doses of PFDA than CAR.
Keywords: constitutive androstane receptor (CAR), cytochrome p450 enzymes (Cyps), perfluorocarboxylic acids (PFCAs), perfluorodecanoic acid (PFDA), perfluorooctanoic acid (PFOA), peroxisome proliferator–activated receptor alpha (PPAR-α)
Due to their surfactant properties as well as chemical and thermal stability, certain perfluorocarboxylic acids (PFCAs) have been used extensively in a number of commercial and customer products, such as Scotchgard products and in making Teflon brand products. These perfluorinated compounds have been detected in birds, fish, bears, and humans (Calafat et al., 2006; De Silva and Mabury, 2006; Falandysz et al., 2006; Sinclair et al., 2006; Smithwick et al., 2005; So et al., 2006). Perfluorochemicals have become a public health concern in recent years as studies have revealed their persistence in the environment, and as data have emerged about their toxicity (So et al., 2006). The liver is thought to be a prime organ of effect for perfluorocarboxylic acids, due to efficient hepatic uptake and persistence in the liver (Vanden Heuvel et al., 1991a, b; Van Rafelghem et al., 1987). PFCAs, such as perfluorooctanoic acid (PFOA, also known as C8) and perfluorodecanoic acid (PFDA, also known as C10), are peroxisome proliferator–activated receptor alpha (PPAR-α) activators and capable of triggering oxidative stress, and increasing proinflammatory cytokine tumor necrosis factor (TNF-α) secretion (Adinehzadeh and Reo, 1998; Langley, 1990; Takagi et al., 1991; Vanden Heuvel et al., 2006).
Cytochrome p450 enzymes (Cyps) are major phase-I xenobiotic-metabolizing enzymes. Cyps are induced by many environmental xenobiotics and drugs. For example, dioxin-related compounds activate the aryl hyrdrocarbon receptor (AhR), and thus induce Cyp1A1 expression (Denison and Deal, 1990; Whitlock, 1999). Polybrominated diphenyl ethers increase mouse Cyp3A11 expression, which is dependent on pregnane-X receptor (PXR) activation (Pacyniak et al., 2007). A recent paper showed that PFOA altered most genes in mouse liver through PPAR-α activation, and induced a similar subset of genes as those induced by phenobarbital and 1,4-bis-[2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP), two CAR activators (Rosen et al., 2008). However, the detailed regulatory mechanism was not further characterized in their studies. Thus, the purpose of this study was to determine whether the expression of mouse Cyp1A1/2, 2B10, 3A11, and 4A14 are altered by PFOA and PFDA, two widely used PFCAs, and to determine the underlying mechanisms accounting for the regulation of Cyps by PFCAs.
MATERIALS AND METHODS
Materials.
Sodium chloride, HEPES sodium salt, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) free acid, lithium lauryl sulfate, ethylenediaminetetraacetic acid, and D-(+)-glucose were purchased from Sigma-Aldrich (St Louis, MO). Micro-O-protect was purchased from Roche Diagnostics (Indianapolis, IN). Formaldehyde, 4-morpholinepropanesulfonic acid, sodium citrate, and NaHCO3 were purchased from Fischer Chemicals (Fairlawn, NJ). Chloroform, agarose, and ethidium bromide were purchased from AMRESCO, Inc. (Solon, OH). Perfluorooctanoic acid (96% purity) and perfluorodecanoic acid (98% purity) were both purchased from Sigma-Aldrich Co. (St. Louis, MO). All other chemicals, unless otherwise indicated, were purchased from Sigma-Aldrich Co.
Polyclonal antibodies of anti-rat Cyp2B1/2 (XenoTech, Inc., Lenexa, KS), anti-rat Cyp4A1/2/3 (Chemicon International, Inc., Temecula, CA), anti-mouse CAR1/2 (M-150) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), β-actin antibody (ab8227) (Abcam, Inc., Cambridge, MA), and goat anti-rabbit IgG horseradish peroxidase-linked secondary antibody (Sigma-Aldrich Co.) were purchased, individually. According to the manufacturer's instruction, anti-rat Cyp2B1/2 and anti-rat Cyp4A1/2/3 antibodies can recognize their mouse ortholog Cyp2B and Cyp4A, respectively.
Animals and treatment.
Eight-week-old adult male C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME), and housed according to the American Animal Association Laboratory Animal Care guidance. For the dose-response study of PFDA, adult C57BL/6 male mice were administered a single i.p. dose (0.5, 1.0, 10, 20, 40, or 80 mg/kg of body weight). Control mice were treated i.p. with the vehicle, propylene glycol:water (1:1, vol/vol). Two days later, livers were removed, immediately frozen in liquid nitrogen, and stored at −80°C.
Breeding pairs of farnesoid X receptor (FXR)-null mice (Sinal et al., 2000) and PXR-null mice (Staudinger et al., 2001) in the C57BL/6 background were kindly provided by Dr Frank J. Gonzalez (National Institutes of Health/National Cancer Institute, Bethesda, MD). Breeding pairs of CAR-null mice in the C57BL/6 background were obtained from Dr Ivan Rusyn (University of North Carolina, Chapel Hill, NC), which were engineered by Tularik, Inc. (South San Francisco, CA), as described previously (Ueda et al., 2002). Each type of null and C57BL/6 mice (n = 5) were administered a single i.p. dose of PFOA (40 mg/kg), PFDA (80 mg/kg), or the vehicle propylene glycol:water (1:1, vol/vol). Two days later, livers were removed and snap-frozen in liquid nitrogen and stored at −80°C.
The PPAR-α-null mice originally engineered by the laboratory of Dr Frank J. Gonzalez (Lee et al., 1995), were back-crossed to a C57BL/6 background (Akiyama et al., 2001), and bred and housed at an the American Animal Association Laboratory Animal Care guidance-accredited facility at Pennsylvania State University (University Park, PA). The PPAR-α-null mice and their wild-type (WT) controls were treated in the laboratory of Dr Jeffrey M. Peters (Pennsylvania State University, University Park, PA); the dose of PFDA was decreased to 40 mg/kg due to increased lethality at the dose of 80 mg/kg. Two days later, livers were collected, frozen and shipped to the University of Kansas Medical Center (Kansas City, KS) for further studies.
Total RNA isolation.
Total RNA was isolated using RNA Bee reagent (Tel-Test, Inc., Friendswood, TX) per the manufacturer's protocol. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm.
Branched DNA signal amplification assay.
The mRNA of mouse genes were quantified using the branched DNA (bDNA) assay (Quantigene bDNA signal amplification kit; Panomics, Inc., Fremont, CA). The strategy of multiple oligonucleotide probe set design and the probe set of mouse Cyps have been described previously (Cheng et al., 2005; Hartley and Klaassen, 2000). The luminescence for each gene is reported as relative light units (RLUs) per 10 μg of total RNA. The data were normalized to glyceraldehyde-3-phosphate dehydrogenase.
Liver microsomes.
Microsomal samples were prepared from mouse livers as described previously (Chen et al., 2003). Briefly, 200–300 mg liver was minced in 10 ml of ice-cold homogenizing buffer (0.15M KCl, 50mM Tris-HCl [pH 7.4], containing 25 μg/ml leupeptin, 50 μg/ml aprotinin, 40 μg/ml phenylmethylsulphonyl fluoride, 0.5 μg/ml pepstatin, and 50 μg/ml antipain). The minced tissue was poured into a dounce homogenizer (Kontes, Vineland, NJ) and homogenized on ice for 10 strokes. Homogenates were filtered through one layer of gauze sponges (Tyco Healthcare Group LP, Mansfield, MA), and centrifuged at 10,000 × g for 20 min at 4°C. The supernatant was collected and centrifuged again at 100,000 × g for 1 h at 4°C. The resulting pellets were dissolved in resuspension buffer (0.25M sucrose, 10mM HEPES [pH 7.5], and 40 μg/ml phenylmethylsulphonyl fluoride) and stored at −80°C. Microsomal protein concentrations were determined using a Bradford protein assay kit from Sigma.
Nuclear protein extraction.
Nuclear proteins from mouse livers were extracted using NE-PER Nuclear and Cytoplasmic Extraction Reagent kit (Pierce Biotechnology, Inc., Rockford, IL), according to the manufacturer's instruction.
Western blots.
Microsomal protein samples mixed with sample loading buffer (75 μg protein/lane) were loaded after heating onto a 10% sodium dedecyl sulfate-polyacrylamide gel. Following electrophoresis, proteins in the gel were electrotransferred to a nitrocellulose membrane for 4.5 h at 34 V at 4°C. Membranes were blocked for 2 h at room temperature with 5% nonfat dry milk in Tris–buffered saline containing 0.1% Tween-20 (TBS-T). Blots were then incubated overnight with polyclonal antibody of each Cyp antibody at 4°C. Polyclonal antibodies of anti-rat Cyp2B1/2, anti-rat Cyp4A1/2/3, and anti-mouse CAR1/2 (M-150) were diluted (1:200) in 2.5% nonfat dry milk in TBS-T buffer. β-Actin protein was used as loading control using a β-actin antibody. After thorough washing (three 20-min washes with excess TBS-T), blots were incubated with goat anti-rabbit IgG horseradish peroxidase-linked secondary antibody (1:5000 dilution with 5% nonfat milk in TBS-T) for 1 h. Blots were washed again. Immunoreactive bands were detected with an enhanced chemical luminescence kit (Pierce Biotechnology, Inc., Rockford, IL). Protein bands of interest were visualized by exposure to Fuji Medical X-Ray film. Protein band intensities on the films were quantified with Quantity One 1-D Analysis Software (Bio-Rad Laboratories, Hercules, CA).
Statistical analysis.
Each data point represents the mean ± SEM. Data were analyzed by one-way ANOVA, followed by Duncan's post hoc test using Statistica software (StatSoft Inc., Tulsa, OK). Statistical significance was set at p < 0.05. When only the difference between control and PFDA treatment was determined, data were analyzed by Student's t-test, and statistical significance was considered at p < 0.05.
RESULTS
Effects of PFOA and PFDA on Cyp1A1/2, 2B10, 3A11, and 4A14 mRNA Expression in Mouse Livers
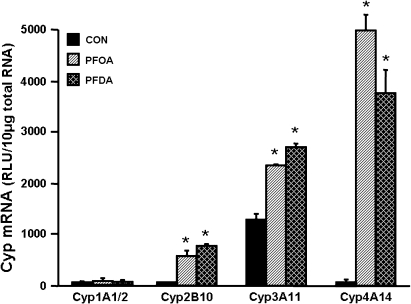
mRNA levels of cytochrome p450s after PFOA and PFDA administration were quantified (Fig. 1). PFOA increased mRNA expression of Cyp2B10 (18-fold), 3A11 (1.8-fold), and 4A14 (32.4-fold). PFDA increased mRNA levels of Cyp2B10, 3A11, and 4A14 approximately 24-, 2.1-, and 35-fold, respectively. However, neither PFOA nor PFDA altered mRNA expression of Cyp1A1/2. Therefore, at the transcriptional level, PFDA and PFOA markedly increased Cyp2B10 and 4A14 mRNA expression, increased Cyp3A11 somewhat, and did not increase Cyp1A1/2. Thus, regulation of Cyps by PFOA and PFDA was focused mainly on the regulation of Cyp2B10 and 4A14 in the following studies.
FIG. 1.
Effects of PFOA and PFDA on mRNA expression of Cyp1A1/2, 2B10, 3A11, and 4A14 in mouse livers. Adult male C57BL/6 mice (n = 5 per treatment) were administered a single i.p. dose of PFOA (40 mg/kg), PFDA (80 mg/kg), or the vehicle propylene glycol:water (1:1, vol/vol). After 4 days, mouse livers were collected. Total RNA from control or PFOA/PFDA-treated mice (n = 5 per treatment) was analyzed by the bDNA assay for the mRNA expression of each Cyp. The data are reported as RLU per 10 μg of total RNA, and are presented as mean ± SEM. Asterisks indicate statistically significant differences between control and PFOA- or PFDA-treated male mice (p < 0.05).
Effects of PFOA and PFDA on Cyp2B and 4A Protein Expression in Mouse Livers
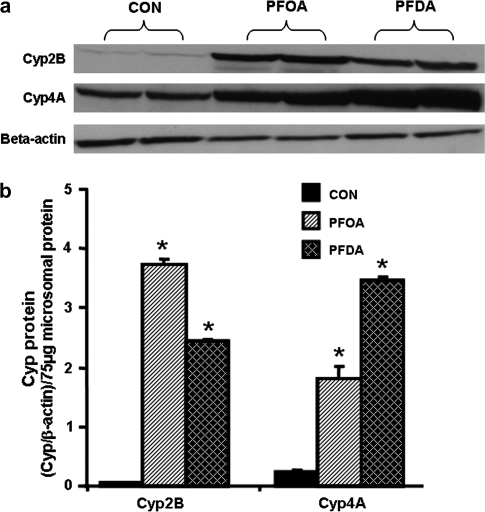
To determine whether the PFOA- and PFDA-induction in Cyp mRNA also results in increased protein, Cyp2B and 4A protein levels were evaluated after PFOA and PFDA administration (Fig. 2). Two days after administration, PFOA increased protein levels of Cyp2B and 4A about 68- and seven-fold, respectively. Two days after PFDA treatment, Cyp2B and 4A protein levels increased approximately 45- and 14-fold, respectively. Therefore, similar to regulation at the mRNA level, PFOA and PFDA markedly increase Cyp2B and 4A protein levels.
FIG. 2.
Effects of PFOA and PFDA on protein expression of Cyp2B and 4A in mouse livers. Adult male C57BL/6 mice (n = 5 per treatment) were administered a single i.p. dose of PFOA (40 mg/kg), PFDA (80 mg/kg), or the vehicle propylene glycol:water (1:1, vol/vol). After 4 days, mouse livers were collected. Microsomal protein from control or PFOA-/PFDA-treated mouse livers (n = 5 per treatment) was analyzed by western blots for the protein expression of each Cyp. (a) Protein levels of Cyp2B and 4A in mouse liver microsomes were analyzed by Western blotting. (b) Protein levels of Cyp2B and 4A in mouse liver microsomes are expressed as ratio of each Cyp to β-actin protein levels per 75 μg microsomal protein. Data are presented as mean ± SEM. Asterisks indicate statistically significant differences between control and PFOA- or PFDA-treated male mice (p < 0.05).
Due to the similarity in regulation of mouse Cyp2B10 and 4A14 by PFOA and PFDA, further experiments to determine the mechanism of induction of Cyps by PFOA and PFDA, focus on PFDA as the inducer.
Dose-Response of PFDA on Cyp2B10 and 4A14 mRNA Expression in Mouse Livers
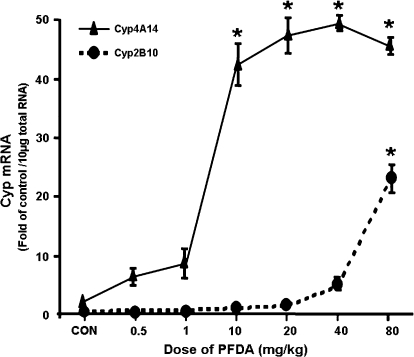
PFDA increased Cyp2B10 mRNA levels at the dose of 80 mg/kg, and increased Cyp4A14 mRNA levels at the dose of 10 mg/kg or higher (Fig. 3). Specifically, PFDA increased mRNA expression of Cyp2B10 (1.8-fold) and 4A14 (2.8-fold) at the dose of 10 and 0.25 mg/kg of body weight, respectively. As the dose of PFDA was increased, the mRNA expression of both Cyp2B10 and 4A14 increased. PFDA at 80 mg/kg increased the mRNA level of Cyp2B10 and 4A14 approximately 22- and 43-fold, respectively. The induction of mouse Cyp2B10 and 4A14 mRNA by PFDA have quite different dose-responses, with Cyp4A14 being induced at lower doses and Cyp2B10 at much higher doses.
FIG. 3.
Dose-response of PFDA on mouse Cyp2B10 and 4A14 mRNA expression. Adult C57BL/6 male mice were administered i.p. with PFDA at various doses (0.5, 1.0, 10, 20, 40, and 80 mg/kg of body weight) for two days, whereas control mice treated i.p. with the vehicle, propyleneglycerol:water (1:1, vol/vol). Individual RNA samples from treated male mouse livers (n = 4) were analyzed by the bDNA assay. Data are expressed as mean ± SEM of (fold of control) at each dose. Asterisks indicate statistically significant differences between control and PFDA-treated male mice (p < 0.05).
Effects of PFDA on Cyp2B10 and 4A14 mRNA Expression in WT and PPAR-α-Null Mice
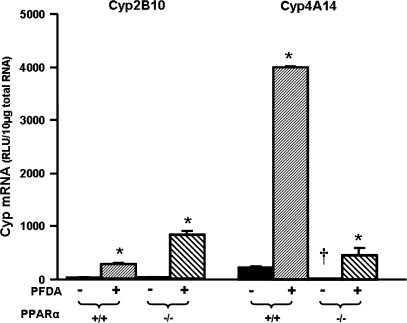
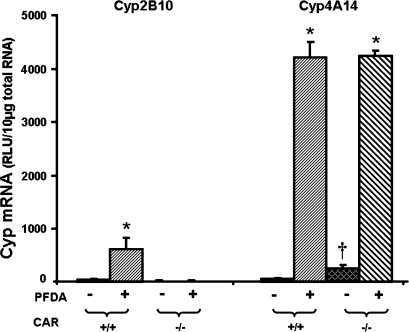
PFDA is a well-known peroxisome proliferator, and a PPAR-α agonist (Cai et al., 1995; Cimini et al., 2000; Johnson and Klaassen, 2002). Therefore, PPAR-α-null mice were used to determine the role of PPAR-α in PFDA's ability to induce Cyp2B10 and 4A14 by PFDA. As shown in Figure 4, disruption of PPAR-α did not alter constitutive Cyp2B10 mRNA abundance. However, PFDA increased Cyp2B10 mRNA 9.5-fold in WT mice, and more in PPAR-α-null mice (29-fold). Elimination of functional PPAR-α protein, as illustrated in the PPAR-α-null mice, decreased the constitutive mRNA of Cyp4A14. PFDA increased Cyp4A14 mRNA expression 17-fold in the WT mice. PFDA increased Cyp4A14 in the PPAR-α-null mice, but much less compared with that in the WT mice.
FIG. 4.
Effects of PFDA on Cyp2B10 and 4A14 mRNA expression in WT and PPAR-α-null mice. The mouse treatments were separated into 4 groups: WT + CON, WT mice treated with the vehicle, propyleneglycerol:water (1:1, vol/vol); WT + PFDA, WT mice treated with PFDA at dose 40 mg/kg of body weight; PPAR KO + CON, PPAR-α-null mice treated with the vehicle; PPAR KO + PFDA, PPAR-α-null mice treated with PFDA. The mice were treated for two days. Total RNA from treated mouse livers was analyzed by the bDNA assay. All data were expressed as mean ± SEM of four to five mice for each treatment. Asterisk indicates statistically significant difference between treated and control mice (p < 0.05). Single dagger (†) represents statistically significant differences (p < 0.05) between the vehicle-treated WT male mice and the vehicle-treated PPAR-α-null male mice.
Effects of PFDA on Cyp2B10 and 4A14 mRNA Expression in WT and CAR-Null Mice
Cyp2B10 is a known target gene of the CAR signaling pathway (Honkakoski et al., 1998). Therefore, CAR-null mice were used in the present study to determine the role of CAR in the ability of PFDA to induce Cyp2B10 and 4A14. As shown in Figure 5, PFDA treatment increased mRNA expression of Cyp2B10 and 4A14 in WT mice. However, in CAR-null mice, induction of Cyp2B10 by PFDA was completely abolished (Fig. 5). In contrast, Cyp4A14 upregulation by PFDA was maintained in CAR-null mice.
FIG. 5.
Effects of PFDA on Cyp2B10 and 4A14 mRNA expression in WT and CAR-null mice. The black and the dark-latticed bars represent regulation of each Cyp by vehicle treatment; the striated bars depict regulation of each Cyp by PFDA treatment. Adult C57BL/6 WT or CAR-null male mice were treated with the vehicle, propyleneglycerol:water (1:1, vol/vol) or PFDA at dose 50 mg/kg of body weight. The mice were treated for two days. Total RNA from untreated or treated mouse livers was analyzed by the bDNA assay. All data were expressed as mean ± SEM of five mice for each treatment. Asterisk indicates statistically significant difference between PFDA-treated and control mice (p < 0.05); Single dagger (†) represents statistically significant differences (p < 0.05) between the vehicle-treated WT male mice and the vehicle-treated CAR-null male mice.
Effects of PFDA on Cyp2B10, 3A11, and 4A14 mRNA Expression in WT and PXR-Null Mice
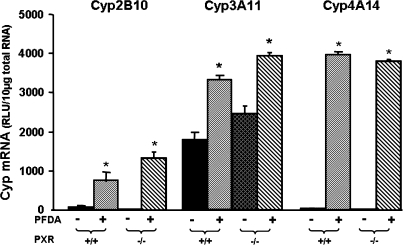
PXR is involved in the regulation of Cyps including the Cyp3A family and Cyp2B genes. Therefore, PXR-null mice were used to determine the role of PXR in the ability of PFDA to induce Cyp2B10, 3A11, and 4A14. As shown in Figure 6, PFDA treatment increased Cyp2B10, 3A11, and 4A14 mRNA in PXR-null mice, similar to the increase in WT mice.
FIG. 6.
Effects of PFDA on Cyp2B10, 3A11, and 4A14 mRNA expression in WT and PXR-null mice. The black and the dark-latticed bars represent regulation of each Cyp by vehicle treatment; the striated bars depict regulation of each Cyp by PFDA treatment. Adult C57BL/6 WT or PXR-null male mice were treated with the vehicle, propyleneglycerol:water (1:1, vol/vol) or PFDA at dose 50 mg/kg of body weight. The mice were treated for two days. Total RNA from untreated or treated mouse livers was analyzed by the bDNA assay. All data were expressed as mean ± SEM of five mice for each treatment. Asterisk indicates statistically significant difference between PFDA-treated and control mice (p < 0.05). Single dagger (†) represents statistically significant differences (p < 0.05) between the vehicle-treated WT male mice and the vehicle-treated PXR-null male mice.
Effects of PFDA on Cyp2B10 and 4A14 mRNA Expression in WT and FXR-Null Mice
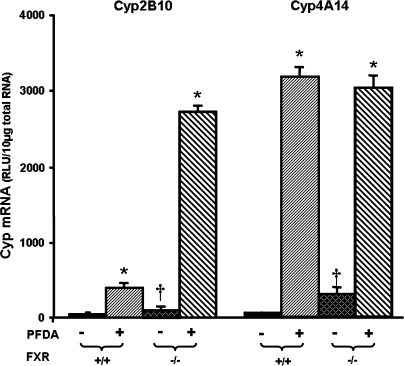
PFDA is a perfluorinated fatty acid. The main agonists for FXR are bile acids, however fatty acids are also ligands for FXR (Zhao et al., 2004). Therefore, it was determined whether upregulation of Cyps by PFDA is mediated through FXR activation. As shown in Figure 7, disruption of FXR increased constitutive Cyp2B10 mRNA 6.4-fold. PFDA increased Cyp2B10 mRNA levels 10.7-fold in WT mice, and even more in FXR-null mice (35-fold). Elimination of functional FXR protein also increased constitutive Cyp4A14 mRNA abundance about 7.6-fold. PFDA increased Cyp4A14 mRNA expression in both FXR-null and WT mice.
FIG. 7.
Effects of PFDA on Cyp2B10 and 4A14 mRNA expression in WT and FXR-null mice. The black and the dark-latticed bars represent regulation of each Cyp by vehicle treatment; the striated bars depict regulation of each Cyp by PFDA treatment. Adult C57BL/6 WT or FXR-null male mice were treated with the vehicle, propyleneglycerol:water (1:1, vol/vol) or PFDA at dose 50 mg/kg of body weight. The mice were treated for two days. Total RNA from untreated or treated mouse livers was analyzed by the bDNA assay. All data were expressed as mean ± SEM of five mice for each treatment. Asterisk indicates statistically significant difference between PFDA-treated and control mice (p < 0.05). Single dagger (†) represents statistically significant differences (p < 0.05) between the vehicle-treated WT male mice and the vehicle-treated FXR-null male mice.
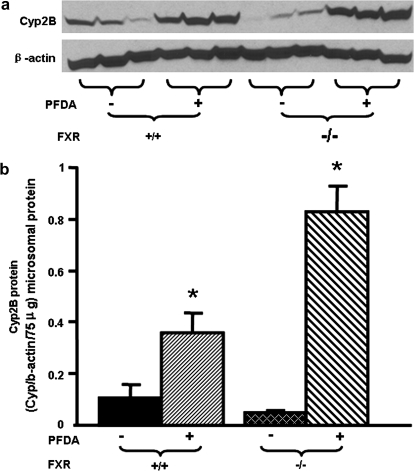
Regulation of Cyp2B by PFDA in FXR-null mouse liver was further characterized at the protein level. As shown in Figure 8, PFDA increased Cyp2B protein levels 3.4-fold in WT mice, and much more in FXR-null mice (17.6-fold).
FIG. 8.
Effects of PFDA on Cyp2B protein expression in WT and FXR-null mouse livers. The black and the dark-latticed bars represent regulation of Cyp2B protein by vehicle treatment; the striated bars depict regulation of Cyp2B protein by PFDA treatment. Adult C57BL/6 WT or FXR-null male mice were given a single i.p. administration with the vehicle, propyleneglycerol:water (1:1, vol/vol) or PFDA at dose 50 mg/kg of body weight. Mouse livers were collected after two days. Microsomal protein from control or PFDA-treated mouse livers (n = 3/treatment) was analyzed by Western blots for the protein expression of Cyp2B. (a) Protein levels of Cyp2B in mouse liver microsomes were analyzed by Western blotting. (b) Protein levels of Cyp2B in mouse liver microsomes are expressed as ratio of Cyp2B to β-actin protein levels per 75 μg microsomal protein. Data are presented as mean ± SEM. Asterisks indicate statistically significant differences between control and PFDA-treated male mice (p < 0.05).
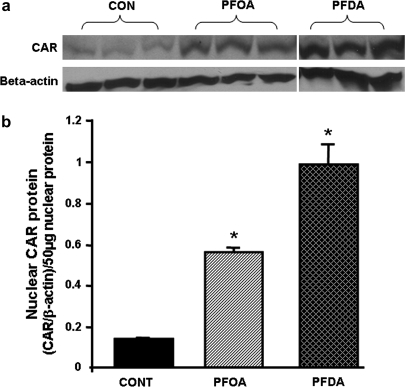
Effects of PFOA and PFDA on Nuclear Levels of CAR Protein
As shown in Figure 9, PFOA and PFDA increased nuclear CAR protein levels 4- and 7.3-fold, respectively.
FIG. 9.
Effects of PFOA and PFDA on nuclear levels of CAR protein in mouse livers. (a) Protein levels of CAR and beta-actin in mouse liver nuclei were analyzed by Western blotting. (b) Protein levels of CAR in mouse liver nuclei are expressed as ratio of CAR to β-actin protein levels per 50 μg nuclear protein. Data are presented as mean ± SEM. Asterisks indicate statistically significant differences between control and PFOA- or PFDA-treated male mice (p < 0.05).
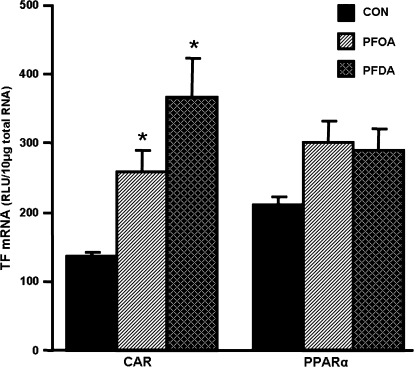
Effects of PFOA and PFDA on mRNA Expression of Transcription Factors CAR and PPAR-α
PFOA and PFDA increased CAR mRNA expression 1.9- and 2.7-fold, respectively (Fig. 10). PFOA and PFDA tended to increase PPAR-α mRNA, but it was less than 50%, and was not statistically significant.
FIG. 10.
Effects of PFOA and PFDA on CAR and PPAR-α mRNA expression in mouse livers. Total RNA from control or PFOA-/PFDA-treated male C57BL/6 mice (n = 5 per treatment) was analyzed by the bDNA assay for the mRNA expression of CAR and PPAR-α. The data are reported as RLU per 10 μg of total RNA, and are presented as mean ± SEM. Asterisks indicate statistically significant differences between control and PFOA- or PFDA-treated male mice (p < 0.05).
DISCUSSION
The present study demonstrates that two perfluorocarboxylic acids, PFOA and PFDA, markedly increase the expression of Cyp2B10 and 4A14 at both mRNA and protein levels. However, PFOA and PFDA only minimally increased Cyp3A11 mRNA, and did not alter Cyp1A1/2 mRNA expression. The dose-response for induction of Cyp2B10 and 4A14 mRNA by PFDA was different, with Cyp4A14 being induced by PFDA at doses as low as 0.25 mg/kg, whereas Cyp2B10 induction only occurred at high doses (> 10 mg/kg). Different potency for Cyp2B10 and 4A14 induction by PFDA suggests that different mechanisms are responsible for the induction. In addition, PFDA increased CAR mRNA level 2.7-fold (Fig. 10) and nuclear CAR protein 7.3-fold (Fig. 9), which is essential for Cyp2B10 induction.
Chemicals increase the expression of their target Cyps by distinctive signaling pathways: AhR ligands increase Cyp1A1; CAR activators increase Cyp2B10; PXR ligands increase Cyp2B10 and 3A11; and PPAR-α ligands increase Cyp4A14. Therefore, PFDA might increase Cyp2B10 and 4A14 expression via activation of CAR, PXR, and PPAR-α nuclear receptors. As demonstrated in the present study, induction of Cyp2B10 by PFDA is via activation of CAR, whereas upregulation of mouse Cyp4A14 by PFDA is mediated through activation of PPAR-α.
Induction of the Cyp1A family of proteins, in particular Cyp1A1, is a hallmark response of exposure to combustion products and other environmental contaminants that activate the AhR (Denison and Deal, 1990; Whitlock, 1999). However, the present study showed that PFDA and PFOA do not alter Cyp1A1 mRNA expression (Fig. 1). This is consistent with a previous report that showed that PFDA is not a AhR ligand (Brewster and Birnbaum, 1989).
Perfluorocarboxylic acids, including PFOA and PFDA, are well-known peroxisome proliferators and PPAR-α agonists. PPAR-α is activated by endogenous ligands, such as polyunsaturated fatty acids and by synthetic agonists, such as the fibrate class of drugs. PPAR-α is expressed mainly in liver, kidney, and skeletal muscle and it induces enzymes involved in fatty acid oxidation (Gouni-Berthold and Krone, 2005). In the present study, PFDA increased Cyp4A14 mRNA expression in mice, as has previously been shown in rats (Chinje et al., 1994; Johnson and Klaassen, 2002; Kudo et al., 2000). The data in this manuscript indicates that induction of Cyp4A by PFDA is mediated by activation of PPAR-α, as the induction of Cyp4A14 by PFDA is markedly attenuated in the PPAR-α-null mice (Fig. 4).
Cyp2B10 induction by PFDA is independent of PPAR-α activation (Fig. 4). However, induction of Cyp2B10 by PFDA requires CAR activation (Fig. 5). A recent manuscript showed that PFOA dose-dependently increased Cyp2B10 in both WT and PPAR-α-null mouse livers and that there is a high correlation in gene regulation by PFOA, TCPOBOP, and phenobarbital (Rosen et al., 2008). However, they did not clarify whether induction of Cyp2B10 by PFOA is through CAR activation. In the present study, by using CAR-null mice, as depicted in Figure 5, Cyp2B10 induction by PFDA did not occur in the CAR-null mice. In addition, PFDA treatment increased mRNA expression and nuclear translocation of CAR (Figs. 9 and 10). Therefore, in addition to activating PPAR-α, PFDA can induce some Cyps by activating the nuclear receptor CAR. A recent paper showed that PPAR-α agonists Wy-14,643, ciprofibrate, and clofibrate function as CAR antagonists by interrupting coactivator recruitment to the CAR ligand binding domain, reduce the transactivation of CAR, but do not alter Cyp2b10 expression in mouse liver (Guo et al., 2007). However in the present study, PFOA and PFDA, two other PPAR-α agonists, promote nuclear translocation of CAR and its activation, as well as induce Cyp2b10 expression in mouse liver (Figs. 1, 2, 5, 9), suggesting that PFOA and PFDA work as agonists of CAR. Therefore, individually, PPAR-α ligands can work as either agonists or antagonists of CAR.
PXR, a xenobiotic sensor, has been shown to play roles in regulating detoxification enzymes (Cheng and Klaassen, 2006), is a major transcription factor for Cyp3A gene regulation, and can also regulate Cyp2B gene expression (Xie et al., 2000). However, as shown in the present study, PXR is not required for induction of Cyp2B10 and 4A14 by PFDA (Fig. 6).
PFDA is a perfluorinated fatty acid. Fatty acids can activate FXR (Zhao et al., 2004). In the present study, FXR was shown not to be essential for PFDA to induce Cyps. However, disruption of FXR increased constitutive mRNA expression of Cyp2B10 and 4A14, consistent with previous reports (Guo et al., 2003; Marschall et al., 2006; Schuetz et al., 2001). In addition, disruption of FXR enhanced the induction of Cyp2B by PFDA in mouse livers at both the mRNA and protein levels (Figs. 7 and 8). It has been previously shown that FXR-null mice have increased CAR mRNA (Guo et al., 2003). Therefore, both PFDA administration and loss of FXR function (as demonstrated in FXR-null mice) induces CAR expression. The marked induction of Cyp2B mRNA and protein by PFDA in FXR-null mice might be due to the increased CAR levels.
Taken together, PFDA and PFOA increase the expression of Cyp2B10 and 4A14 in mouse liver. At relatively low doses (0.25 mg/kg), PFDA activates PPAR-α, and thus increases Cyp4A14 expression. In contrast, at high doses (> 10 mg/kg), PFDA activates both CAR and PPAR-α nuclear receptors, and increases the expression of Cyp2B10 and 4A14, respectively. Therefore, PPAR-α and CAR play central roles in regulating mouse Cyps by PFDA. Considering the previous industrial use and the public health concern of perfluorochemicals, the current studies provide important insight into the mechanisms by which perfluorocarboxylic acids alter the physiology of animals.
FUNDING
National Institutes of Health grants (ES013714, ES09649, ES09716, and RR021940).
Acknowledgments
We thank Dr Jonathan M. Maher and Dr Lauren M. Aleksunes, and Xiaohong (Lucy) Lei for their help with animal care and sample processing.
References
- Adinehzadeh M, Reo NV. Effects of peroxisome proliferators on rat liver phospholipids: Sphingomyelin degradation may be involved in hepatotoxic mechanism of perfluorodecanoic acid. Chem. Res. Toxicol. 1998;11:428–440. doi: 10.1021/tx970155t. [DOI] [PubMed] [Google Scholar]
- Akiyama TE, Nicol CJ, Fievet C, Staels B, Ward JM, Auwerx J, Lee SS, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-alpha regulates lipid homeostasis, but is not associated with obesity: Studies with congenic mouse lines. J. Biol. Chem. 2001;276:39088–39093. doi: 10.1074/jbc.M107073200. [DOI] [PubMed] [Google Scholar]
- Brewster DW, Birnbaum LS. The biochemical toxicity of perfluorodecanoic acid in the mouse is different from that of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 1989;99:544–554. doi: 10.1016/0041-008x(89)90161-0. [DOI] [PubMed] [Google Scholar]
- Cai Y, Appelkvist EL, DePierre JW. Hepatic oxidative stress and related defenses during treatment of mice with acetylsalicylic acid and other peroxisome proliferators. J. Biochem. Toxicol. 1995;10:87–94. doi: 10.1002/jbt.2570100205. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Caudill SP, Reidy JA, Needham LL. Perfluorochemicals in pooled serum samples from United States residents in 2001 and 2002. Environ. Sci. Technol. 2006;40:2128–2134. doi: 10.1021/es0517973. [DOI] [PubMed] [Google Scholar]
- Chen C, Staudinger JL, Klaassen CD. Nuclear receptor, pregnane X receptor, is required for induction of UDP-glucuronosyl tranferases in mouse liver by pregnenolone-16 alpha-carbonitrile. Drug Metab. Dispos. 2003;31:908–915. doi: 10.1124/dmd.31.7.908. [DOI] [PubMed] [Google Scholar]
- Cheng X, Klaassen CD. Regulation of mRNA expression of xenobiotic transporters by the pregnane-X receptor (PXR) in mouse liver, kidney, and intestine. Drug Metab. Dispos. 2006;34:1863–1867. doi: 10.1124/dmd.106.010520. [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Chen C, Klaassen CD. Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps) Drug Metab. Dispos. 2005;33:1062–1073. doi: 10.1124/dmd.105.003640. [DOI] [PubMed] [Google Scholar]
- Chinje E, Kentish P, Jarnot B, George M, Gibson G. Induction of the CYP4A subfamily by perfluorodecanoic acid: The rat and the guinea pig as susceptible and non-susceptible species. Toxicol. Lett. 1994;71:69–75. doi: 10.1016/0378-4274(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Cimini A, Cristiano L, Bernardo A, Farioli-Vecchioli S, Stefanini S, Ceru MP. Presence and inducibility of peroxisomes in a human glioblastoma cell line. Biochim. Biophys. Acta. 2000;1474:397–409. doi: 10.1016/s0304-4165(00)00036-2. [DOI] [PubMed] [Google Scholar]
- De Silva AO, Mabury SA. Isomer distribution of perfluorocarboxylates in human blood: Potential correlation to source. Environ. Sci. Technol. 2006;40:2903–2909. doi: 10.1021/es0600330. [DOI] [PubMed] [Google Scholar]
- Denison MS, Deal RM. The binding of transformed aromatic hydrocarbon (Ah) receptor to its DNA recognition site is not affected by metal depletion. Mol. Cell. Endocrinol. 1990;69:51–57. doi: 10.1016/0303-7207(90)90088-p. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Taniyasu S, Gulkowska A, Yamashita N, Schulte-Oehlmann U. Is fish a major source of fluorinated surfactants and repellents in humans living on the Baltic Coast? Environ. Sci. Technol. 2006;40:748–751. doi: 10.1021/es051799n. [DOI] [PubMed] [Google Scholar]
- Gouni-Berthold I, Krone W. Peroxisome proliferator-activated receptor alpha (PPARalpha) and athero-sclerosis. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005;5:513–523. doi: 10.2174/156800605774962022. [DOI] [PubMed] [Google Scholar]
- Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Jr, Kliewer SA, Gonzalez FJ, Sinal CJ. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J. Biol. Chem. 2003;278:45062–45071. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- Guo D, Sarkar J, Suino-Powell K, Xu Y, Matsumoto K, Jia Y, Yu S, Khare S, Haldar K, Rao MS, et al. Induction of nuclear translocation of constitutive androstane receptor by peroxisome proliferator-activated receptor alpha synthetic ligands in mouse liver. J. Biol. Chem. 2007;282:36766–36776. doi: 10.1074/jbc.M707183200. [DOI] [PubMed] [Google Scholar]
- Hartley DP, Klaassen CD. Detection of chemical-induced differential expression of rat hepatic cytochrome P450 mRNA transcripts using branched DNA signal amplification technology. Drug Metab. Dispos. 2000;28:608–616. [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol. Cell. Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DR, Klaassen CD. Regulation of rat multidrug resistance protein 2 by classes of prototypical microsomal enzyme inducers that activate distinct transcription pathways. Toxicol. Sci. 2002;67:182–189. doi: 10.1093/toxsci/67.2.182. [DOI] [PubMed] [Google Scholar]
- Kudo N, Bandai N, Suzuki E, Katakura M, Kawashima Y. Induction by perfluorinated fatty acids with different carbon chain length of peroxisomal beta-oxidation in the liver of rats. Chem. Biol. Interact. 2000;124:119–132. doi: 10.1016/s0009-2797(99)00150-7. [DOI] [PubMed] [Google Scholar]
- Langley AE. Effects of perfluoro-n-decanoic acid on the respiratory activity of isolated rat liver mitochondria. J. Toxicol. Environ. Health. 1990;29:329–336. doi: 10.1080/15287399009531395. [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall HU, Wagner M, Bodin K, Zollner G, Fickert P, Gumhold J, Silbert D, Fuchsbichler A, Sjovall J, Trauner M. Fxr(-/-) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J. Lipid Res. 2006;47:582–592. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- Pacyniak EK, Cheng X, Cunningham ML, Crofton K, Klaassen CD, Guo GL. The flame retardants, polybrominated diphenyl ethers, are pregnane X receptor activators. Toxicol. Sci. 2007;97:94–102. doi: 10.1093/toxsci/kfm025. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, Abbott BD, Lau C, Corton JC. Toxicogenomic Dissection of the perfluorooctanoic acid transcript profile in mouse liver: Evidence for the involvement of nuclear receptors PPAR{alpha} and CAR. Toxicol. Sci. 2008;103:46–56. doi: 10.1093/toxsci/kfn025. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Strom S, Yasuda K, Lecureur V, Assem M, Brimer C, Lamba J, Kim RB, Ramachandran V, Komoroski BJ, et al. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J. Biol. Chem. 2001;276:39411–39418. doi: 10.1074/jbc.M106340200. [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Sinclair E, Mayack DT, Roblee K, Yamashita N, Kannan K. Occurrence of perfluoroalkyl surfactants in water, fish, and birds from New York State. Arch. Environ. Contam. Toxicol. 2006;50:398–410. doi: 10.1007/s00244-005-1188-z. [DOI] [PubMed] [Google Scholar]
- Smithwick M, Muir DC, Mabury SA, Solomon KR, Martin JW, Sonne C, Born EW, Letcher RJ, Dietz R. Perfluoroalkyl contaminants in liver tissue from East Greenland polar bears (Ursus maritimus) Environ. Toxicol. Chem. 2005;24:981–986. doi: 10.1897/04-258r.1. [DOI] [PubMed] [Google Scholar]
- So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, Lam PK. Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ. Sci. Technol. 2006;40:2924–2929. doi: 10.1021/es060031f. [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A, Sai K, Umemura T, Hasegawa R, Kurokawa Y. Short-term exposure to the peroxisome proliferators, perfluorooctanoic acid and perfluorodecanoic acid, causes significant increase of 8-hydroxydeoxyguanosine in liver DNA of rats. Cancer Lett. 1991;57:55–60. doi: 10.1016/0304-3835(91)90063-n. [DOI] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol. Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, Peterson RE. Disposition of perfluorodecanoic acid in male and female rats. Toxicol. Appl. Pharmacol. 1991a;107:450–459. doi: 10.1016/0041-008x(91)90308-2. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, Peterson RE. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J. Biochem. Toxicol. 1991b;6:83–92. doi: 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: A comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol. Sci. 2006;92:476–489. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- Van Rafelghem MJ, Mattie DR, Bruner RH, Andersen ME. Pathological and hepatic ultrastructural effects of a single dose of perfluoro-n-decanoic acid in the rat, hamster, mouse, and guinea pig. Fundam. Appl. Toxicol. 1987;9:522–540. [PubMed] [Google Scholar]
- Whitlock JP., Jr Induction of cytochrome P4501A1. Annu. Rev. Pharmacol. Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Simon CM, Pierce AM, Safe S, Blumberg B, Guzelian PS, Evans RM. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev. 2000;14:3014–3023. doi: 10.1101/gad.846800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A, Yu J, Lew JL, Huang L, Wright SD, Cui J. Polyunsaturated fatty acids are FXR ligands and differentially regulate expression of FXR targets. DNA Cell. Biol. 2004;23:519–526. doi: 10.1089/1044549041562267. [DOI] [PubMed] [Google Scholar]