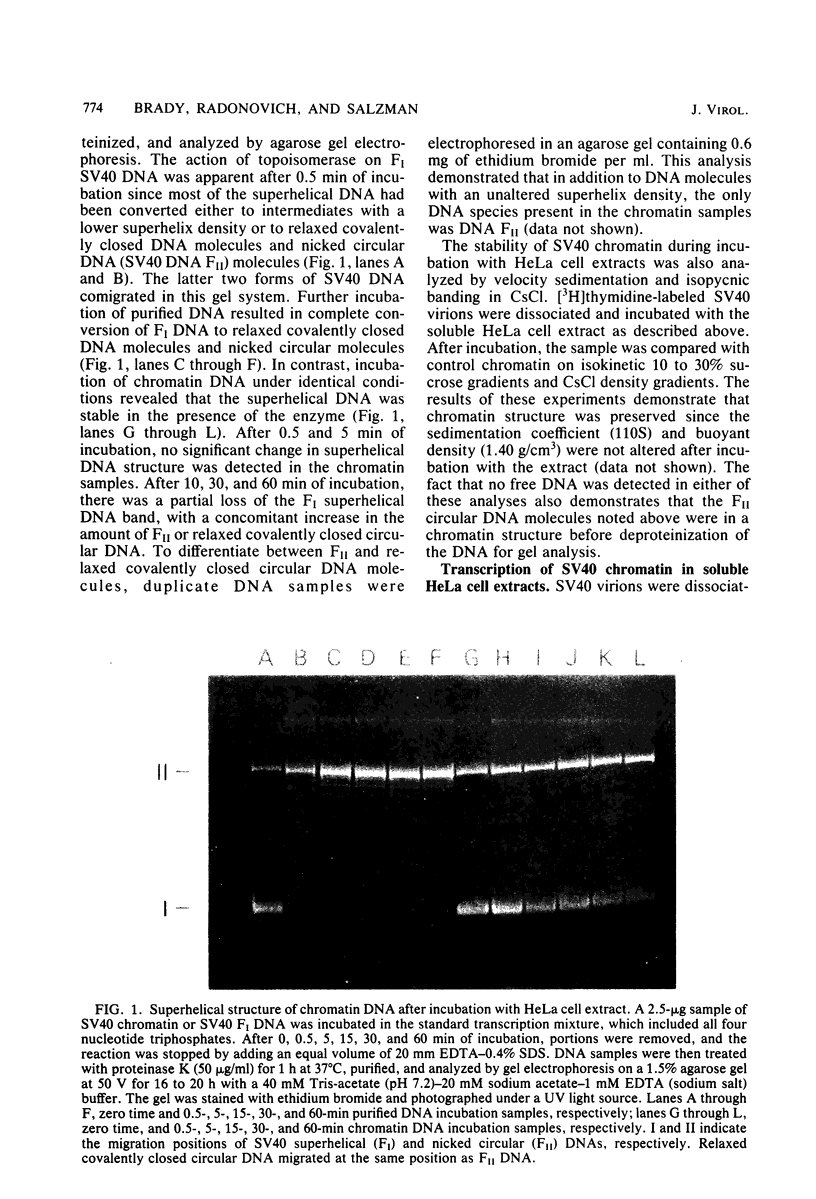
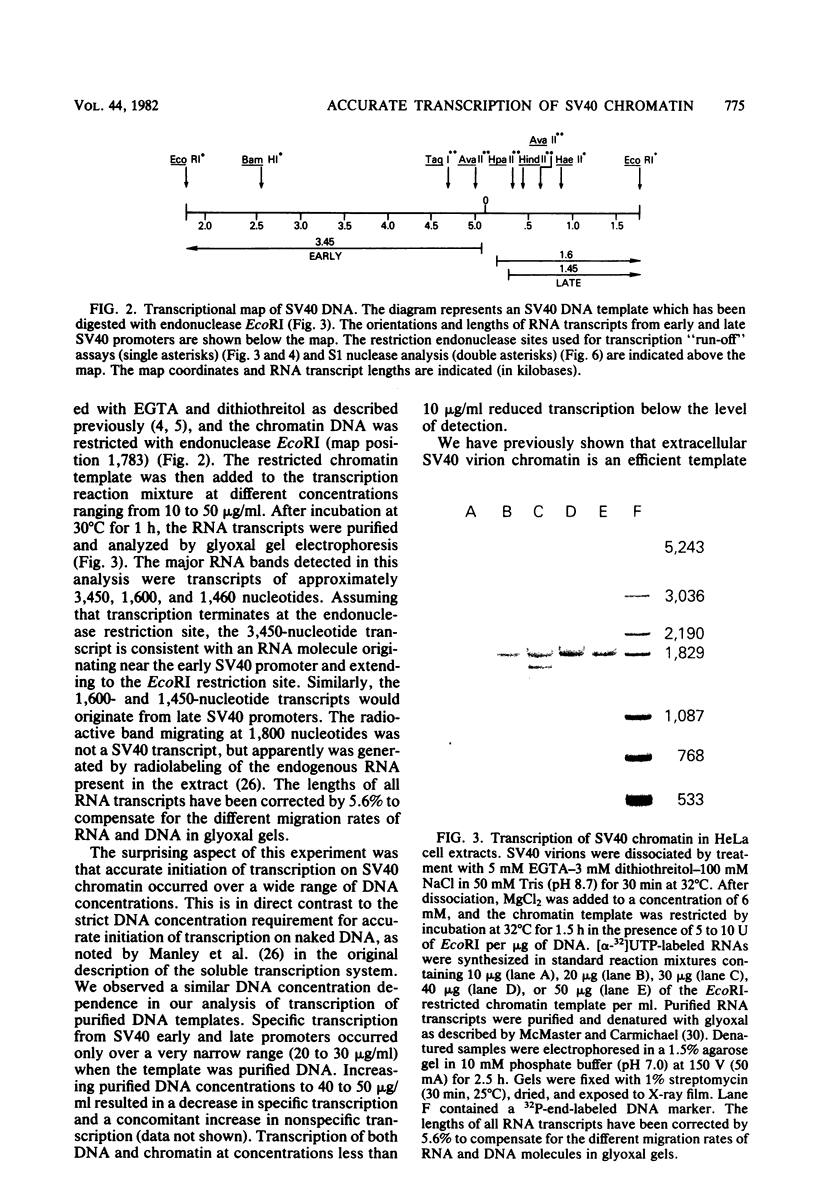
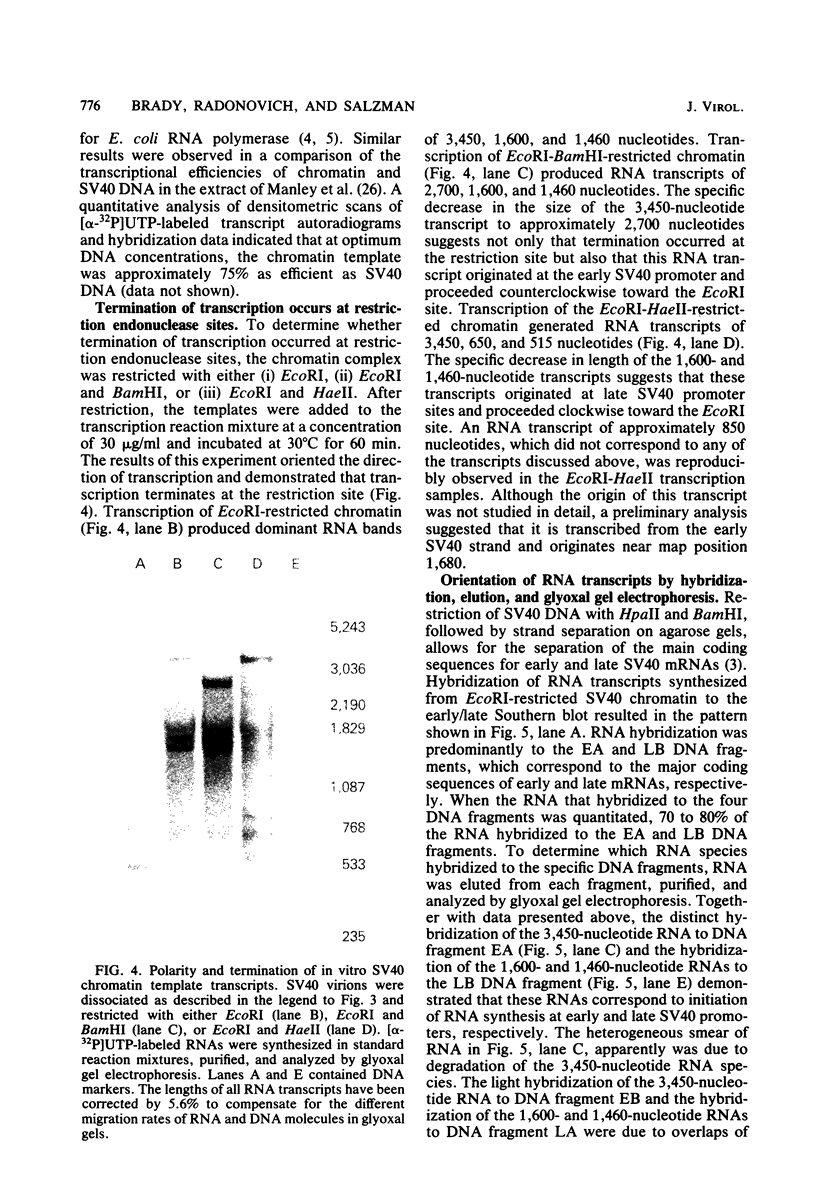
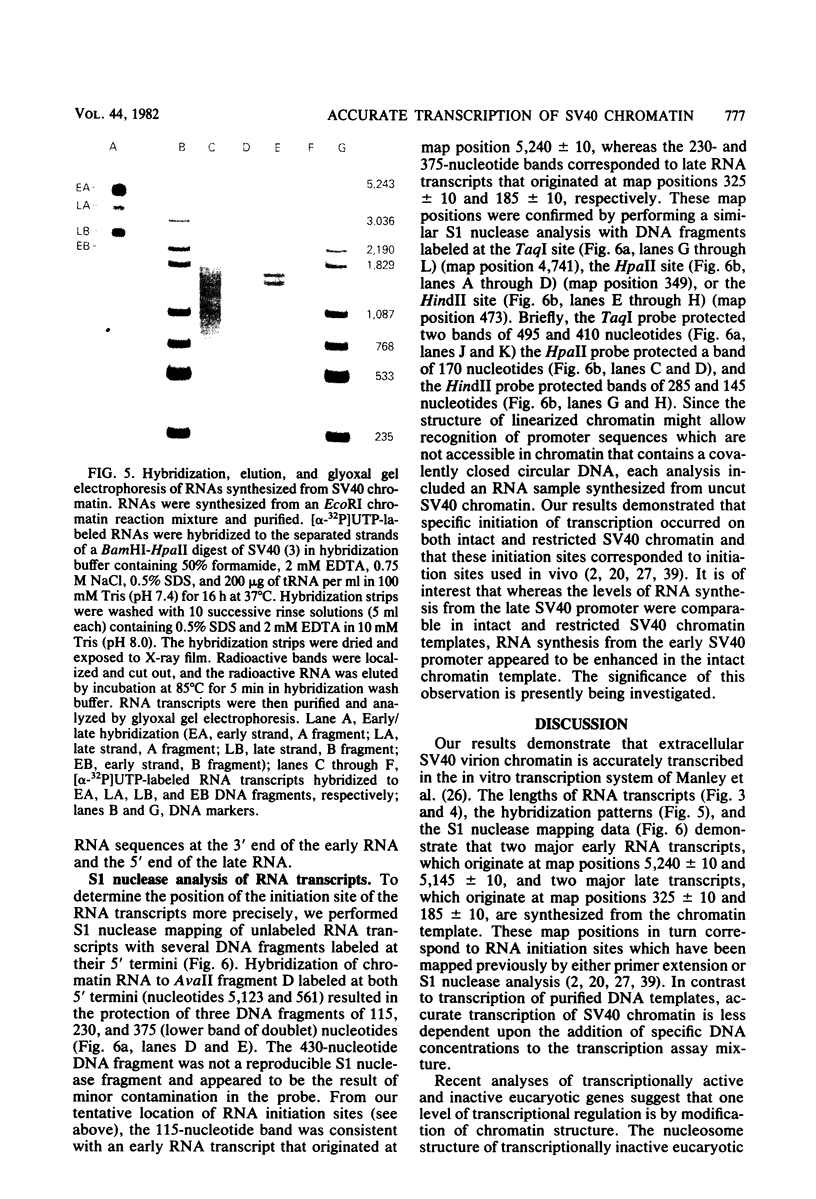
Abstract
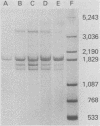
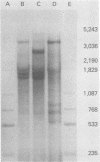
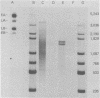
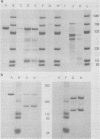
During simian virus 40 viral maturation, a series of modifications occur which alter the composition of viral nucleoprotein complexes. As a consequence, the chromatin that is extracted from extracellular simian virus 40 virions exhibits properties that are similar to those of transcriptionally active eucaryotic chromatin. The influence of this chromatin structure on specific RNA initiation by RNA polymerase II was examined by using the in vitro HeLa cell extract of Manley et al. (Proc. Natl. Acad. Sci. U.S.A. 77:3855-3859, 1980). The 5' ends of RNA transcripts were positioned by the "run-off" assay, in which transcripts extend from the initiation site to termination sites created by restriction cleavage and by S1 nuclease analysis, using DNA probes labeled at their 5' termini. Two major early RNA transcripts, which originated at map positions 5,240 +/- 10 and 5,145 +/- 10, and two major late RNA transcripts, which originated at map positions 325 +/- 10 and 185 +/- 10, were identified. Transcripts were initiated with comparable relative efficiencies at the same 5' site when either purified DNA or chromatin was used as the template. Our results suggest that extracellular simian virus 40 virion chromatin modifications do not regulate simian virus 40 promoter selection but function to increase the accessibility of RNA promoter sequences to RNA polymerase II and allow efficient elongation of the RNA chain after the initiation event.
Full text
PDF

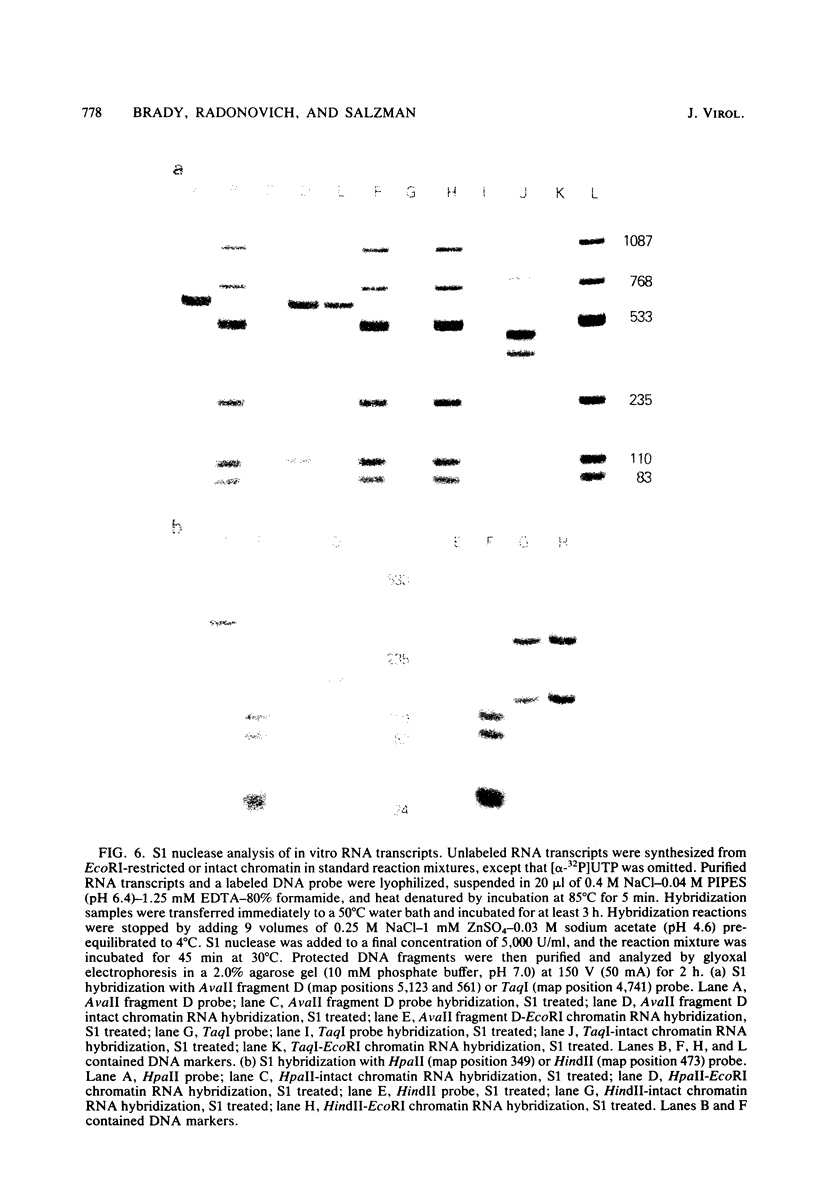



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. Deletions covering the putative promoter region of early mRNAs of simian virus 40 do not abolish T-antigen expression. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3865–3869. doi: 10.1073/pnas.77.7.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., May E., Salzman N. P. Characterization of simian virus 40 tsA58 transcriptional intermediates at restrictive temperatures: relationship between DNA replication and transcription. J Virol. 1977 Jun;22(3):702–710. doi: 10.1128/jvi.22.3.702-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Lavialle C., Salzman N. P. Efficient transcription of a compact nucleoprotein complex isolated from purified simian virus 40 virions. J Virol. 1980 Aug;35(2):371–381. doi: 10.1128/jvi.35.2.371-381.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J., Radonovich M., Lavialle C., Salzman N. P. Simian virus 40 maturation: chromatin modifications increase the accessibility of viral DNA to nuclease and RNA polymerase. J Virol. 1981 Aug;39(2):603–611. doi: 10.1128/jvi.39.2.603-611.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breindl M., Jaenisch R. Conformation of Moloney murine leukaemia proviral sequences in chromatin from leukaemic and nonleukaemic cells. Nature. 1979 Jan 25;277(5694):320–322. doi: 10.1038/277320a0. [DOI] [PubMed] [Google Scholar]
- Bustin M. Binding of E. coli RNA polymerase to chromatin subunits. Nucleic Acids Res. 1978 Mar;5(3):925–932. doi: 10.1093/nar/5.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae C. B., Wong T. K., Gadski R. A. Transcription, processing and structure of chromatin in SV40-transformed cell. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1518–1524. doi: 10.1016/0006-291x(78)91393-1. [DOI] [PubMed] [Google Scholar]
- Crémisi C., Chestier A., Dauguet C., Yaniv M. Transcription of SV 40 nucleoprotein complexes in vitro. Biochem Biophys Res Commun. 1977 Sep 9;78(1):74–82. doi: 10.1016/0006-291x(77)91223-2. [DOI] [PubMed] [Google Scholar]
- Defer N., Crepin M., Terrioux C., Kruh J., Gros F. Comparison of non histone proteins selectively associated with nucleosomes with proteins released during limited DNase digestions. Nucleic Acids Res. 1979 Mar;6(3):953–966. doi: 10.1093/nar/6.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Coca-Prados M., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. I. Methods of isolation and characterization in CV-1 cells. J Virol. 1979 Feb;29(2):612–623. doi: 10.1128/jvi.29.2.612-623.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Weintraub H. M. An altered subunit configuration associated with the actively transcribed DNA of integrated adenovirus genes. Cell. 1977 Nov;12(3):783–794. doi: 10.1016/0092-8674(77)90277-x. [DOI] [PubMed] [Google Scholar]
- Frolova E. I., Zalmanzon E. S., Lukanidin E. M., Georgiev G. P. Studies of the transcription of viral genome in adenovirus 5 transformed cells. Nucleic Acids Res. 1978 Jan;5(1):1–11. doi: 10.1093/nar/5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova E. I., Zalmanzon E. S. Transcription of viral sequences in cells transformed by adenovirus Type 5. Virology. 1978 Sep;89(2):347–359. doi: 10.1016/0042-6822(78)90177-0. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Seidman M. M., Levine A. J. The detection and characterization of multiple forms of SV40 nucleoprotein complexes. Virology. 1978 Oct 15;90(2):305–316. doi: 10.1016/0042-6822(78)90315-x. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Lebowitz P., Frisque R. J., Gluzman Y. Identification of a promoter component involved in positioning the 5' termini of simian virus 40 early mRNAs. Proc Natl Acad Sci U S A. 1981 Jan;78(1):100–104. doi: 10.1073/pnas.78.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Kaufman R. J., Manley J., Gefter M., Sharp P. A. Transcription of Simian virus 40 DNA in a HeLa whole cell extract. J Biol Chem. 1981 Jan 10;256(1):478–482. [PubMed] [Google Scholar]
- Hu S. L., Manley J. L. DNA sequence required for initiation of transcription in vitro from the major late promoter of adenovirus 2. Proc Natl Acad Sci U S A. 1981 Feb;78(2):820–824. doi: 10.1073/pnas.78.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits E. B., Saragosti S., Yaniv M., Aloni Y. Escherichia coli RNA polymerase in vitro mimics simian virus 40 in vivo transcription when the template is viral nucleoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6556–6560. doi: 10.1073/pnas.77.11.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Bella F., Vesco C. Late modifications of simian virus 40 chromatin during the lytic cycle occur in an immature form of virion. J Virol. 1980 Mar;33(3):1138–1150. doi: 10.1128/jvi.33.3.1138-1150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavialle C., Reuveni Y., Thoren M., Salzman N. P. Molecular interaction between simian virus 40 DNA and Escherichia coli RNA polymerase. Mapping of the initiation sites on supercoiled and linear DNA. J Biol Chem. 1982 Feb 10;257(3):1549–1557. [PubMed] [Google Scholar]
- Levy B. W., Connor W., Dixon G. H. A subset of trout testis nucleosomes enriched in transcribed DNA sequences contains high mobility group proteins as major structural components. J Biol Chem. 1979 Feb 10;254(3):609–620. [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Chambon P. The SV40 early region TATA box is required for accurate in vitro initiation of transcription. Nature. 1981 Mar 26;290(5804):310–315. doi: 10.1038/290310a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Rio D. C., Robbins A. K., Tjian R. SV40 gene expression is modulated by the cooperative binding of T antigen to DNA. Cell. 1981 Aug;25(2):373–384. doi: 10.1016/0092-8674(81)90056-8. [DOI] [PubMed] [Google Scholar]
- Nelson D., Perry M. E., Chalkley R. A correlation between nucleosome spacer region susceptibility to DNase I and histone acetylation. Nucleic Acids Res. 1979 Feb;6(2):561–574. doi: 10.1093/nar/6.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Cedar H. Selective degradation of integrated murine leukemia proviral DNA by deoxyribonucleases. Cell. 1977 Aug;11(4):933–940. doi: 10.1016/0092-8674(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D., Robbins A., Myers R., Tjian R. Regulation of simian virus 40 early transcription in vitro by a purified tumor antigen. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5706–5710. doi: 10.1073/pnas.77.10.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stalder J., Seebeck T., Braun R. Degradation of the ribosomal genes by DNAse I in Physarum polycephalum. Eur J Biochem. 1978 Oct;90(2):391–395. doi: 10.1111/j.1432-1033.1978.tb12616.x. [DOI] [PubMed] [Google Scholar]
- Talkington C. A., Nishioka Y., Leder P. In vitro transcription of normal, mutant, and truncated mouse alpha-globin genes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7132–7136. doi: 10.1073/pnas.77.12.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. A., Radonovich M. F., Salzman N. P. Characterization of the 5'-terminal structure of simian virus 40 early mRNA's. J Virol. 1979 Aug;31(2):437–446. doi: 10.1128/jvi.31.2.437-446.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Derbyshire R., Guy A., Molko D., Roget A., Téoule R., Chambon P. Specific in vitro transcription of conalbumin gene is drastically decreased by single-point mutation in T-A-T-A box homology sequence. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7024–7028. doi: 10.1073/pnas.77.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Kédinger C., Corden J., Brison O., Chambon P. Specific in vitro initiation of transcription on conalbumin and ovalbumin genes and comparison with adenovirus-2 early and late genes. Nature. 1980 Jun 5;285(5764):367–373. doi: 10.1038/285367a0. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of actively transcribed nucleosomes using immobilized HMG 14 and 17 and an analysis of alpha-globin chromatin. Cell. 1981 Feb;23(2):391–400. doi: 10.1016/0092-8674(81)90134-3. [DOI] [PubMed] [Google Scholar]
- Williamson P., Felsenfeld G. Transcription of histone-covered T7 DNA by Escherichia coli RNA polymerase. Biochemistry. 1978 Dec 26;17(26):5695–5705. doi: 10.1021/bi00619a015. [DOI] [PubMed] [Google Scholar]
- Wu C., Bingham P. M., Livak K. J., Holmgren R., Elgin S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell. 1979 Apr;16(4):797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]