Abstract
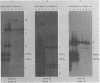
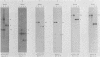
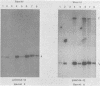
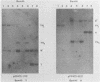
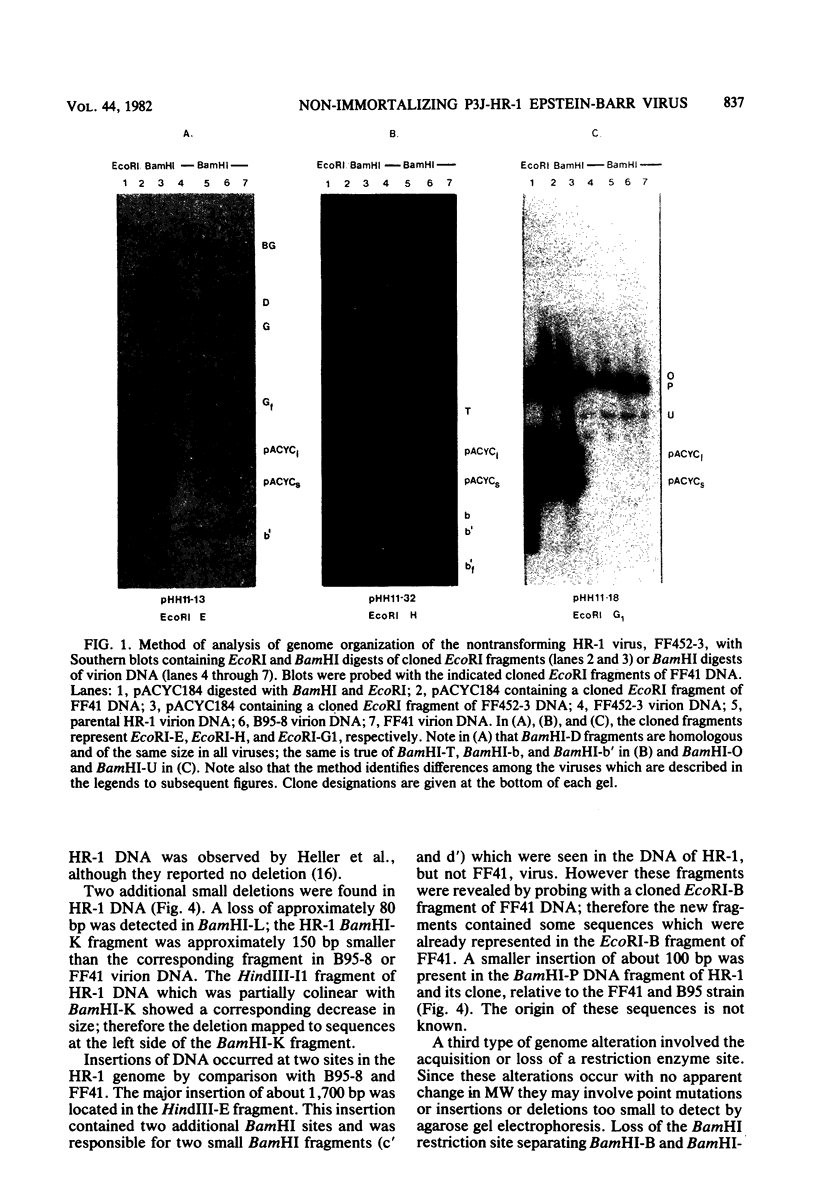
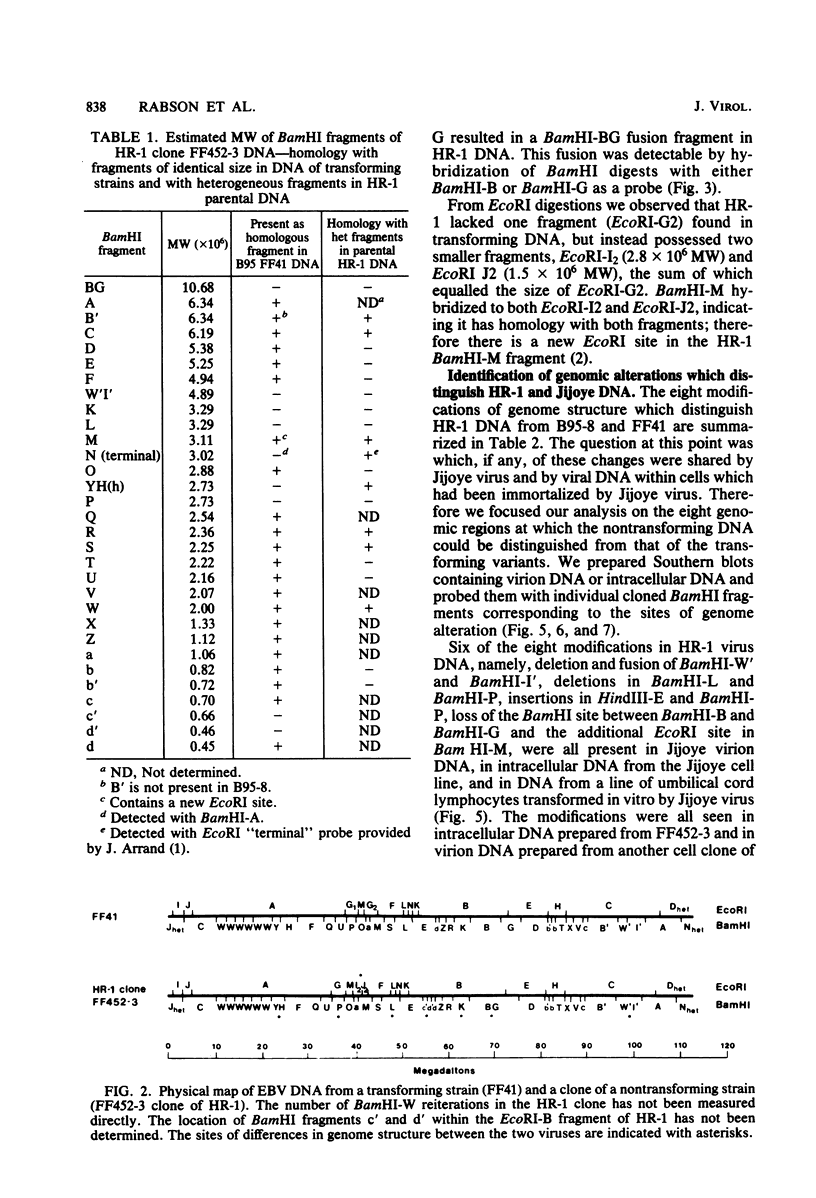
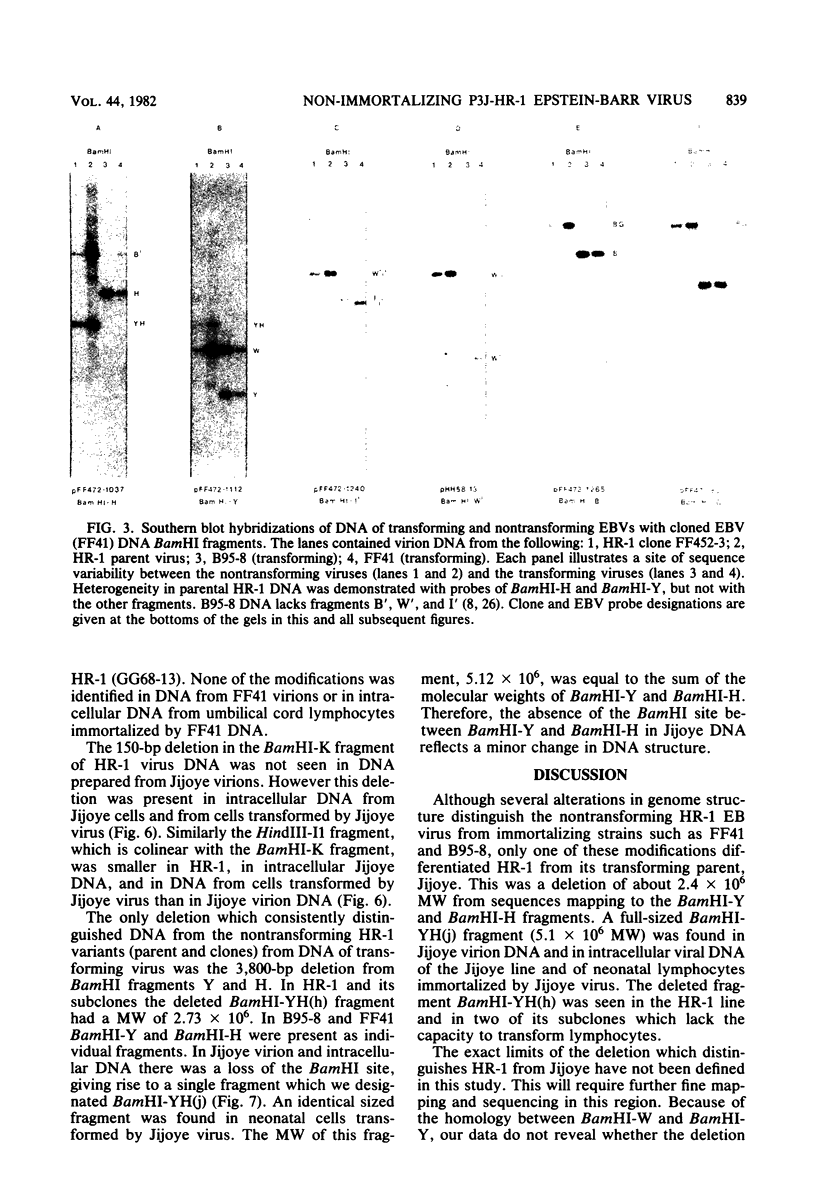
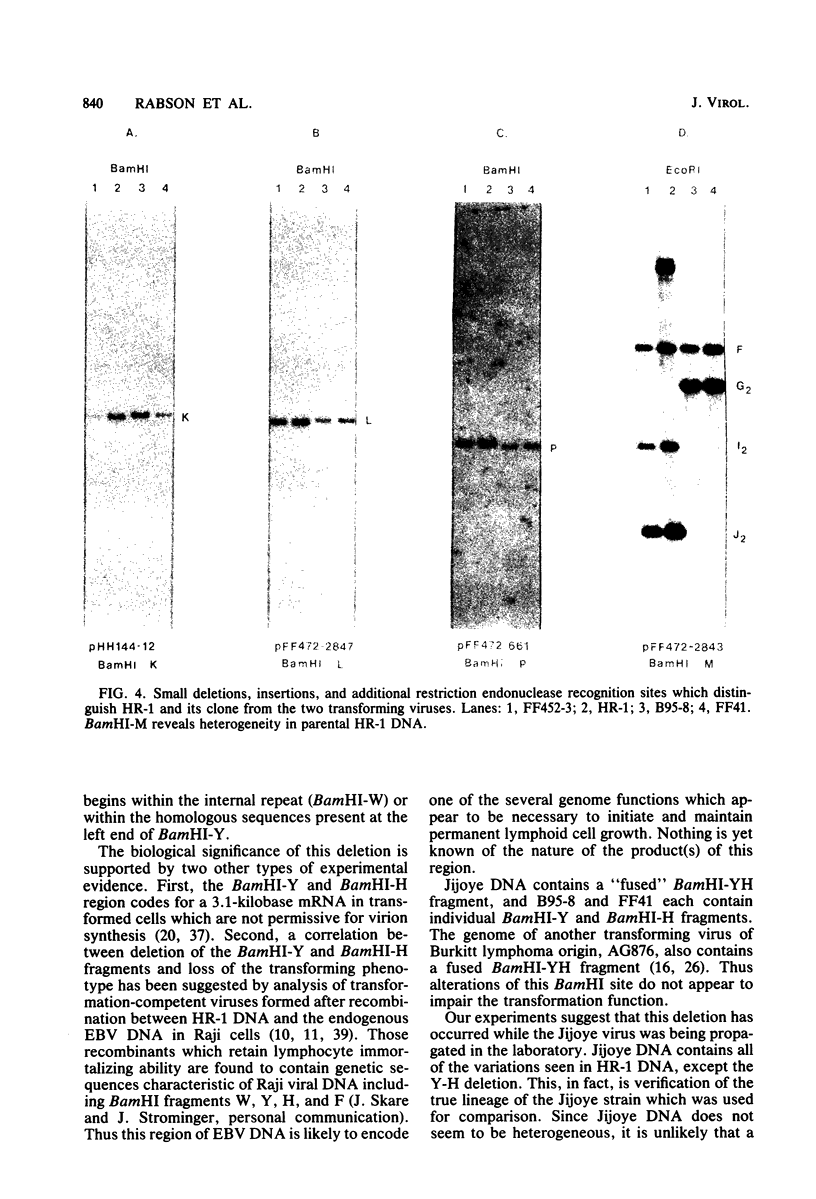
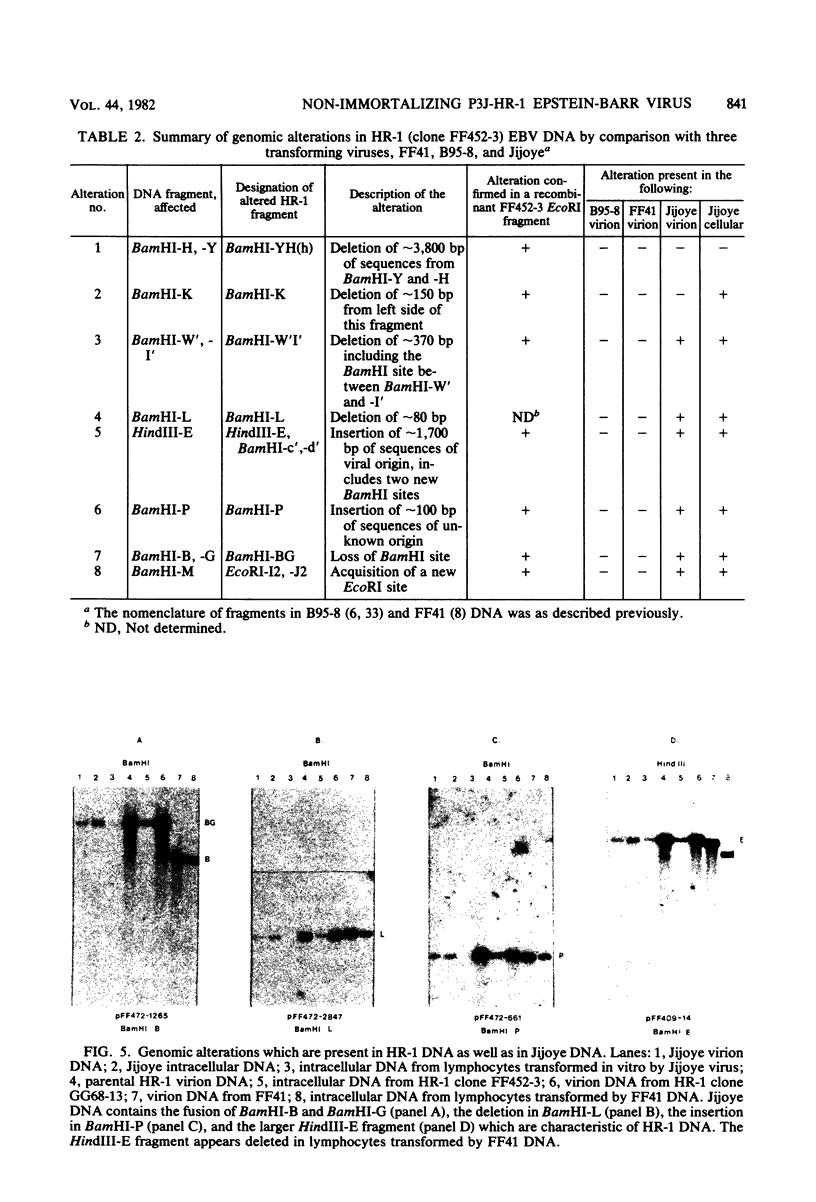
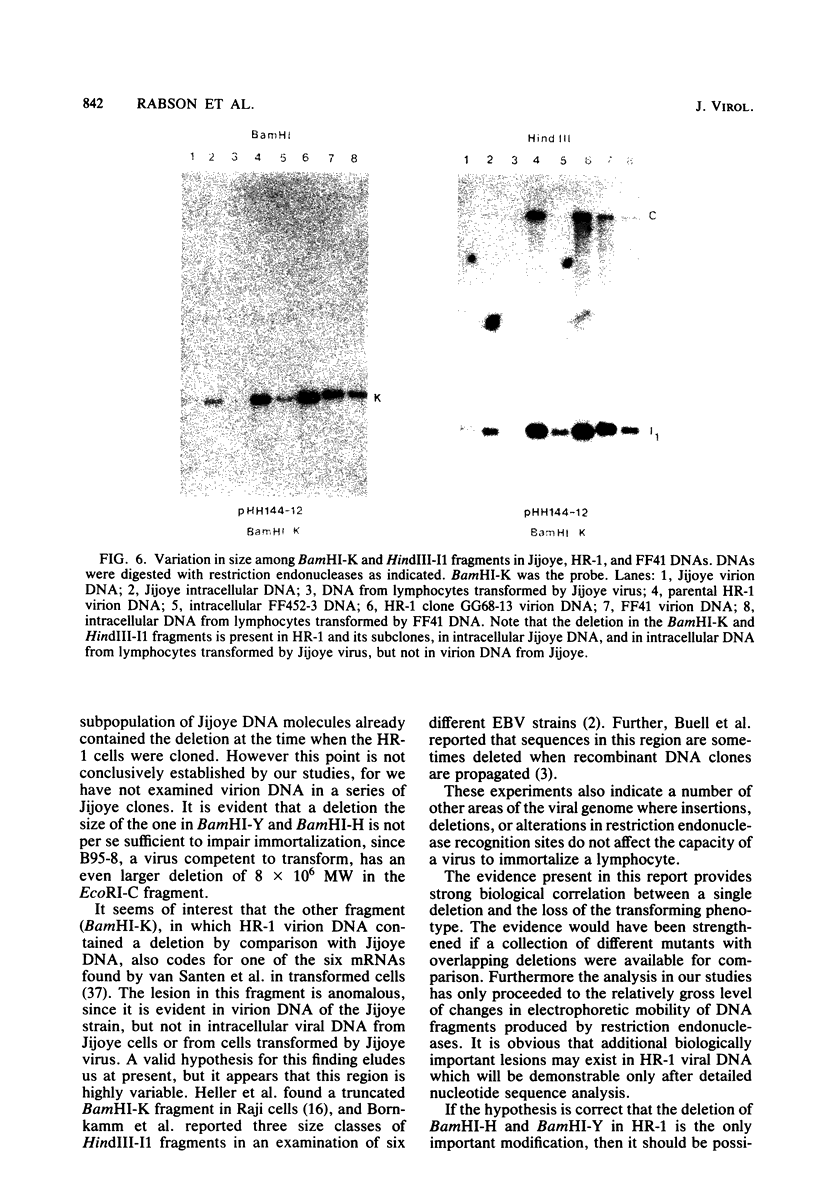
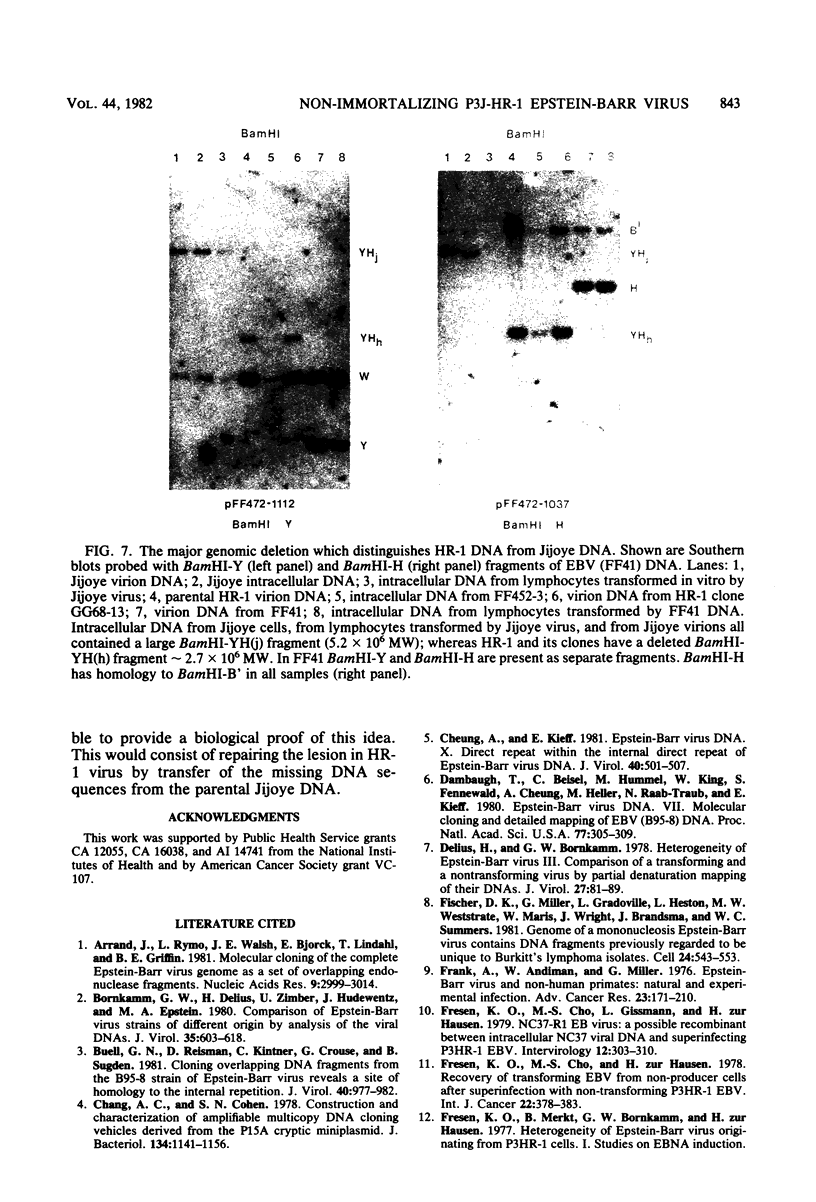
The P3J-HR-1 strain of Epstein-Barr virus (EBV) fails to immortalize human lymphocytes. We wished to understand the nature of the genomic alterations which correlated with the loss of this ability. As a first step, the heterogeneity of DNA molecules in the P3J-HR-1 line was eliminated by cell cloning. Then a physical map was prepared of virion DNA from one cell clone, designated FF452-3. By comparison with the genomes of two EBVs, B95-8 and FF41, which are competent to immortalize lymphocytes, we identified a total of eight modifications of BamHI and EcoRI restriction endonuclease fragments of EBV (FF452-3) DNA consisting of insertions, deletions, or loss of a restriction endonuclease recognition site. To determine which of these alterations might be responsible for the loss of transforming phenotype, we examined homologous DNA fragments of the Jijoye strain of EBV, the progenitor of the HR-1 strain which still retains the ability to immortalize lymphocytes. We also studied viral DNA in lymphocytes transformed in vitro by Jijoye virus. Six of the eight alterations were found both in Jijoye and in clonal HR-1 DNA and were presumably genomic traits characteristic of this lineage of EBV. A small deletion in the BamHI-K fragment of HR-1 DNA was not found in Jijoye virion DNA, but this deletion was present in intracellular Jijoye DNA. Thus only one major genomic lesion in HR-1 DNA, a deletion of at least 2.4 x 10(6) molecular weight of DNA from a fused BamHI-H-Y fragment, consistently distinguished Jijoye DNA from its non-immortalizing P3J-HR-1 derivative. This deletion is likely to affect EBV genes which are directly or indirectly involved in immortalizing lymphocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. R., Rymo L., Walsh J. E., Björck E., Lindahl T., Griffin B. E. Molecular cloning of the complete Epstein-Barr virus genome as a set of overlapping restriction endonuclease fragments. Nucleic Acids Res. 1981 Jul 10;9(13):2999–3014. doi: 10.1093/nar/9.13.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Zimber U., Hudewentz J., Epstein M. A. Comparison of Epstein-Barr virus strains of different origin by analysis of the viral DNAs. J Virol. 1980 Sep;35(3):603–618. doi: 10.1128/jvi.35.3.603-618.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G. N., Reisman D., Kintner C., Crouse G., Sugden B. Cloning overlapping DNA fragments from the B95-8 strain of Epstein-Barr virus reveals a site of homology to the internal repetition. J Virol. 1981 Dec;40(3):977–982. doi: 10.1128/jvi.40.3.977-982.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A., Kieff E. Epstein-Barr virus DNA. X. Direct repeat within the internal direct repeat of Epstein-Barr virus DNA. J Virol. 1981 Nov;40(2):501–507. doi: 10.1128/jvi.40.2.501-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Bornkamm G. W. Heterogeneity of Epstein-Barr virus. III. Comparison of a transforming and a nontransforming virus by partial denaturation mapping of their DNAs. J Virol. 1978 Jul;27(1):81–89. doi: 10.1128/jvi.27.1.81-89.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D. K., Miller G., Gradoville L., Heston L., Westrate M. W., Maris W., Wright J., Brandsma J., Summers W. C. Genome of a mononucleosis Epstein-Barr virus contains DNA fragments previously regarded to be unique to Burkitt's lymphoma isolates. Cell. 1981 May;24(2):543–553. doi: 10.1016/0092-8674(81)90345-7. [DOI] [PubMed] [Google Scholar]
- Frank A., Andiman W. A., Miller G. Epstein-Barr virus and nonhuman primates: natural and experimental infection. Adv Cancer Res. 1976;23:171–201. doi: 10.1016/s0065-230x(08)60546-1. [DOI] [PubMed] [Google Scholar]
- Fresen K. O., Cho M. S., Gissmann L., zur Hausen H. NC37-R1 Epstein-Barr virus (EBV): a possible recombinant between intracellular NC37 viral DNA and superinfecting P3HR-1 EBV. Intervirology. 1980;12(6):303–310. doi: 10.1159/000149089. [DOI] [PubMed] [Google Scholar]
- Fresen K. O., Cho M. S., zur Hausen H. Recovery of transforming EBV from non-producer cells after superinfection with non-transforming P3HR-1 EBV. Int J Cancer. 1978 Oct 15;22(4):378–383. doi: 10.1002/ijc.2910220403. [DOI] [PubMed] [Google Scholar]
- Grogan E., Miller G., Henle W., Rabson M., Shedd D., Niederman J. C. Expression of Epstein-Barr viral early antigen in monolayer tissue cultures after transfection with viral DNA and DNA fragments. J Virol. 1981 Dec;40(3):861–869. doi: 10.1128/jvi.40.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Kieff E. DNA of Epstein-Barr virus. II. Comparison of the molecular weights of restriction endonuclease fragments of the DNA of Epstein-Barr virus strains and identification of end fragments of the B95-8 strain. J Virol. 1977 Aug;23(2):421–429. doi: 10.1128/jvi.23.2.421-429.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Nogee L., Hayward G. S. Organization of repeated regions within the Epstein-Barr virus DNA molecule. J Virol. 1980 Jan;33(1):507–521. doi: 10.1128/jvi.33.1.507-521.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., Dambaugh T., Kieff E. Epstein-Barr virus DNA. IX. Variation among viral DNAs from producer and nonproducer infected cells. J Virol. 1981 May;38(2):632–648. doi: 10.1128/jvi.38.2.632-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Heston L., Rabson M., Brown N., Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982 Jan 14;295(5845):160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- King W., Thomas-Powell A. L., Raab-Traub N., Hawke M., Kieff E. Epstein-Barr virus RNA. V. Viral RNA in a restringently infected, growth-transformed cell line. J Virol. 1980 Nov;36(2):506–518. doi: 10.1128/jvi.36.2.506-518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Yajima Y., Nonoyama M. Mechanism of infection by Epstein-Barr virus. II. Comparison of viral DNA from HR-1 and superinfected Raji cells by restriction enzymes. Virology. 1977 Aug;81(1):17–24. doi: 10.1016/0042-6822(77)90054-x. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975 May;92(2):478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- Miller G., Grogan E., Heston L., Robinson J., Smith D. Epstein-Barr viral DNA: infectivity for human placental cells. Science. 1981 Apr 24;212(4493):452–455. doi: 10.1126/science.6259735. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Traub N., Dambaugh T., Kieff E. DNA of Epstein-Barr virus VIII: B95-8, the previous prototype, is an unusual deletion derivative. Cell. 1980 Nov;22(1 Pt 1):257–267. doi: 10.1016/0092-8674(80)90173-7. [DOI] [PubMed] [Google Scholar]
- Ragona G., Ernberg I., Klein G. Induction and biological characterization of the Epstein-Barr virus (EBV) carried by the Jijoye lymphoma line. Virology. 1980 Mar;101(2):553–557. doi: 10.1016/0042-6822(80)90473-0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rymo L., Forsblom S. Cleavage of Epstein-Barr virus DNA by restriction endonucleases EcoRI, HindIII and BamI. Nucleic Acids Res. 1978 Apr;5(4):1387–1402. doi: 10.1093/nar/5.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymo L., Lindahl T., Adams A. Sites of sequence variability in Epstein-Barr virus DNA from different sources. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2794–2798. doi: 10.1073/pnas.76.6.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairenji T., Hinuma Y. Re-evaluation of a transforming strain of Epstein-Barr virus from the Burkitt lymphoma cell line, Jijoye. Int J Cancer. 1980 Sep 15;26(3):337–342. doi: 10.1002/ijc.2910260313. [DOI] [PubMed] [Google Scholar]
- Skare J., Strominger J. L. Cloning and mapping of BamHi endonuclease fragments of DNA from the transforming B95-8 strain of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3860–3864. doi: 10.1073/pnas.77.7.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinitz M., Bakács T., Klein G. Interaction of the B95-8 and P3HR-1 substrains of Epstein-Barr virus (EBV) with peripheral human lymphocytes. Int J Cancer. 1978 Sep 15;22(3):251–257. doi: 10.1002/ijc.2910220306. [DOI] [PubMed] [Google Scholar]
- Sugden B., Summers W. C., Klein G. Nucleic acid renaturation and restriction endonuclease cleavage analyses show that the DNAs of a transforming and a nontransforming strain of Epstein-Barr virus share approximately 90% of their nucleotide sequences. J Virol. 1976 May;18(2):765–775. doi: 10.1128/jvi.18.2.765-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Yajima Y., Marczynska B., Nonoyama M. Transforming activity of Epstein-Barr virus obtained by superinfection of Raji cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2008–2010. doi: 10.1073/pnas.75.4.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen V., Cheung A., Kieff E. Epstein-Barr virus RNA VII: size and direction of transcription of virus-specified cytoplasmic RNAs in a transformed cell line. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1930–1934. doi: 10.1073/pnas.78.3.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]