Abstract
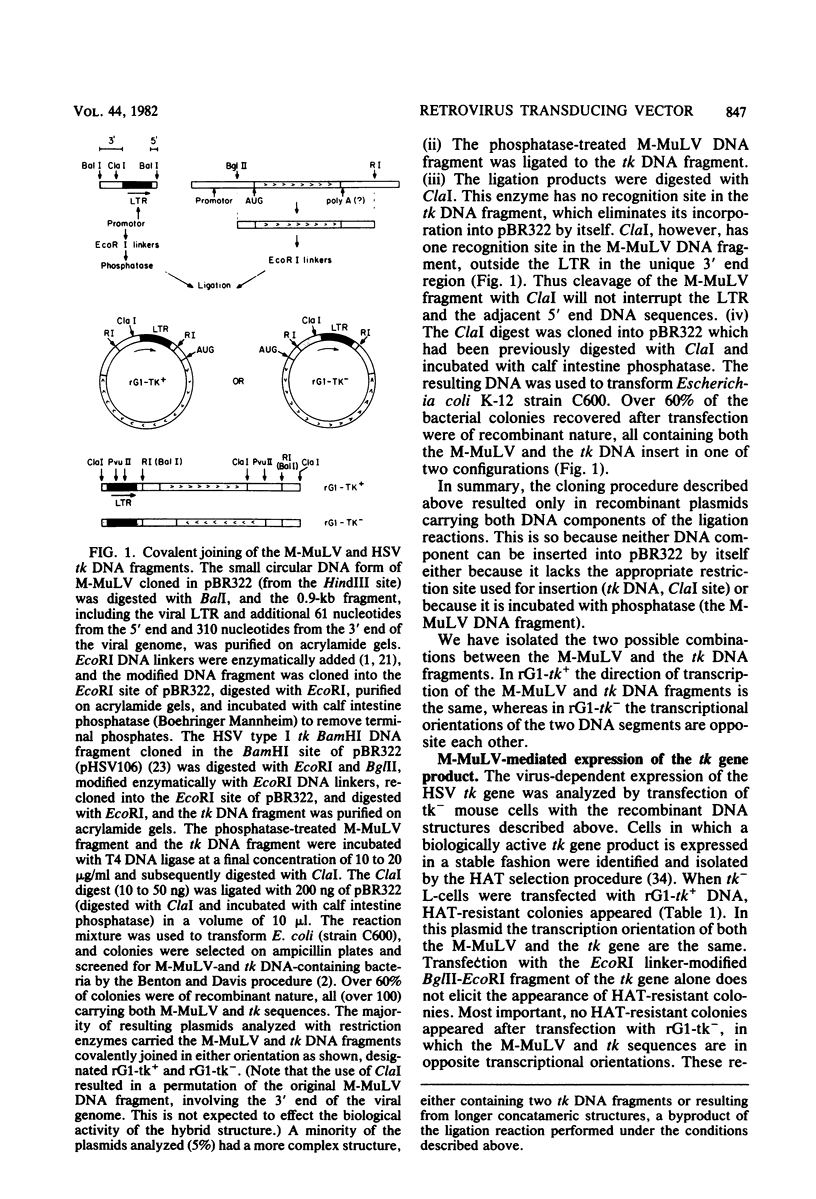
A 0.9-kilobase DNA fragment from the genome of Moloney murine leukemia virus, including the viral long terminal repeat, was covalently linked to the herpes simplex virus I thymidine kinase (tk) gene whose promoter was previously removed. The hybrid DNA structure was introduced into the chromosome of tk- mouse cells at single copy numbers, via transfection procedures. Cells expressing the newly introduced tk gene were identified by the HAT selection procedure and analyzed for tk- and moloney murine leukemia virus-specific DNA and RNA sequences by blot hybridization procedures. Expression of the tk gene is dependent on function(s) provided in cis by the viral DNA fragment. Vectors derived from this region are termed rGag (rG) vectors.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahl C. P., Marians K. J., Wu R. A general method for inserting specific DNA sequences into cloning vehicles. Gene. 1976;1(1):81–92. doi: 10.1016/0378-1119(76)90008-1. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Oskarsson M., Wood T. G., McClements W. L., Fischinger P. J., Vande Woude G. G. Activation of the transforming potential of a normal cell sequence: a molecular model for oncogenesis. Science. 1981 May 22;212(4497):941–943. doi: 10.1126/science.7233190. [DOI] [PubMed] [Google Scholar]
- Colbere-Garapin F., Chousterman S., Horodniceanu F., Kourilsky P., Garapin A. C. Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3755–3759. doi: 10.1073/pnas.76.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Generation of novel, biologically active Harvey sarcoma viruses via apparent illegitimate recombination. J Virol. 1981 Apr;38(1):136–150. doi: 10.1128/jvi.38.1.136-150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grosveld G. C., de Boer E., Shewmaker C. K., Flavell R. A. DNA sequences necessary for transcription of the rabbit beta-globin gene in vivo. Nature. 1982 Jan 14;295(5845):120–126. doi: 10.1038/295120a0. [DOI] [PubMed] [Google Scholar]
- Gruss P., Khoury G. Expression of simian virus 40-rat preproinsulin recombinants in monkey kidney cells: use of preproinsulin RNA processing signals. Proc Natl Acad Sci U S A. 1981 Jan;78(1):133–137. doi: 10.1073/pnas.78.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Expression of the chromosomal mouse Beta maj-globin gene cloned in SV40. Nature. 1979 Sep 6;281(5726):35–40. doi: 10.1038/281035a0. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. SV40 recombinants carrying a functional RNA splice junction and polyadenylation site from the chromosomal mouse beta maj globin gene. Cell. 1979 Jul;17(3):737–747. doi: 10.1016/0092-8674(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Hsiung N., Roginski R. S., Henthorn P., Smithies O., Kucherlapati R., Skoultchi A. I. Introduction and expression of a fetal human globin gene in mouse fibroblasts. Mol Cell Biol. 1982 Apr;2(4):401–411. doi: 10.1128/mcb.2.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung N., Warrick H., deRiel J. K., Tuan D., Forget B. G., Skoultchi A., Kucherlapati R. Cotransfer of circular and linear prokaryotic and eukaryotic DNA sequences into mouse cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4852–4856. doi: 10.1073/pnas.77.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Joyner A., Yamamoto Y., Bernstein A. Retrovirus long terminal repeats activate expression of coding sequences for the herpes simplex virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1573–1577. doi: 10.1073/pnas.79.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Maitland N. J., McDougall J. K. Biochemical transformation of mouse cells by fragments of herpes simplex virus DNA. Cell. 1977 May;11(1):233–241. doi: 10.1016/0092-8674(77)90334-8. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Howard B. H., Berg P. Synthesis of rabbit beta-globin in cultured monkey kidney cells following infection with a SV40 beta-globin recombinant genome. Nature. 1979 Jan 11;277(5692):108–114. doi: 10.1038/277108a0. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Benoist C., Breathnach R. Transformation of mouse fibroblasts to methotrexate resistance by a recombinant plasmid expressing a prokaryotic dihydrofolate reductase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1527–1531. doi: 10.1073/pnas.78.3.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Henderson L. E., Stephenson J. R., Copeland T. D., Long C. W., Ihle J. N., Gilden R. V. Amino- and carboxyl-terminal amino acid sequences of proteins coded by gag gene of murine leukemia virus. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1404–1408. doi: 10.1073/pnas.75.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SZYBALSKA E. H., SZYBALSKI W. Genetics of human cess line. IV. DNA-mediated heritable transformation of a biochemical trait. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2026–2034. doi: 10.1073/pnas.48.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Temin H. M. Formation of infectious progeny virus after insertion of herpes simplex thymidine kinase gene into DNA of an avian retrovirus. Cell. 1981 Oct;26(1 Pt 1):67–77. doi: 10.1016/0092-8674(81)90034-9. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Shoemaker C., Goff S., Gilboa E., Paskind M., Mitra S. W., Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. Competition hybridization by "pre-saturation" of HeLa cell DNA. J Mol Biol. 1969 Sep 28;44(3):551–562. doi: 10.1016/0022-2836(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Hoffmann J. W., Goff S. P., Weinberg R. A. Adaptation of a retrovirus as a eucaryotic vector transmitting the herpes simplex virus thymidine kinase gene. Mol Cell Biol. 1982 Apr;2(4):426–436. doi: 10.1128/mcb.2.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Gibson M., Spear P. G., Scolnick E. M. Construction and isolation of a transmissible retrovirus containing the src gene of Harvey murine sarcoma virus and the thymidine kinase gene of herpes simplex virus type 1. J Virol. 1981 Sep;39(3):935–944. doi: 10.1128/jvi.39.3.935-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]