Abstract
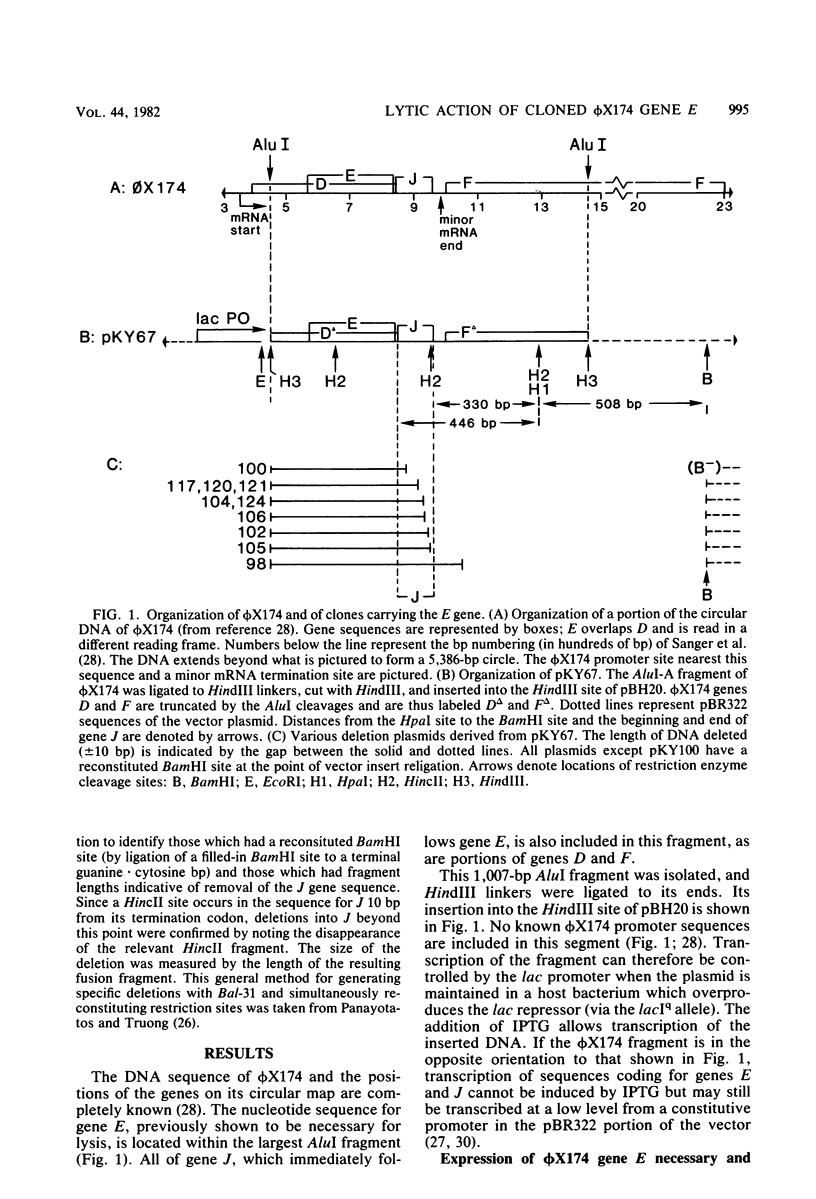
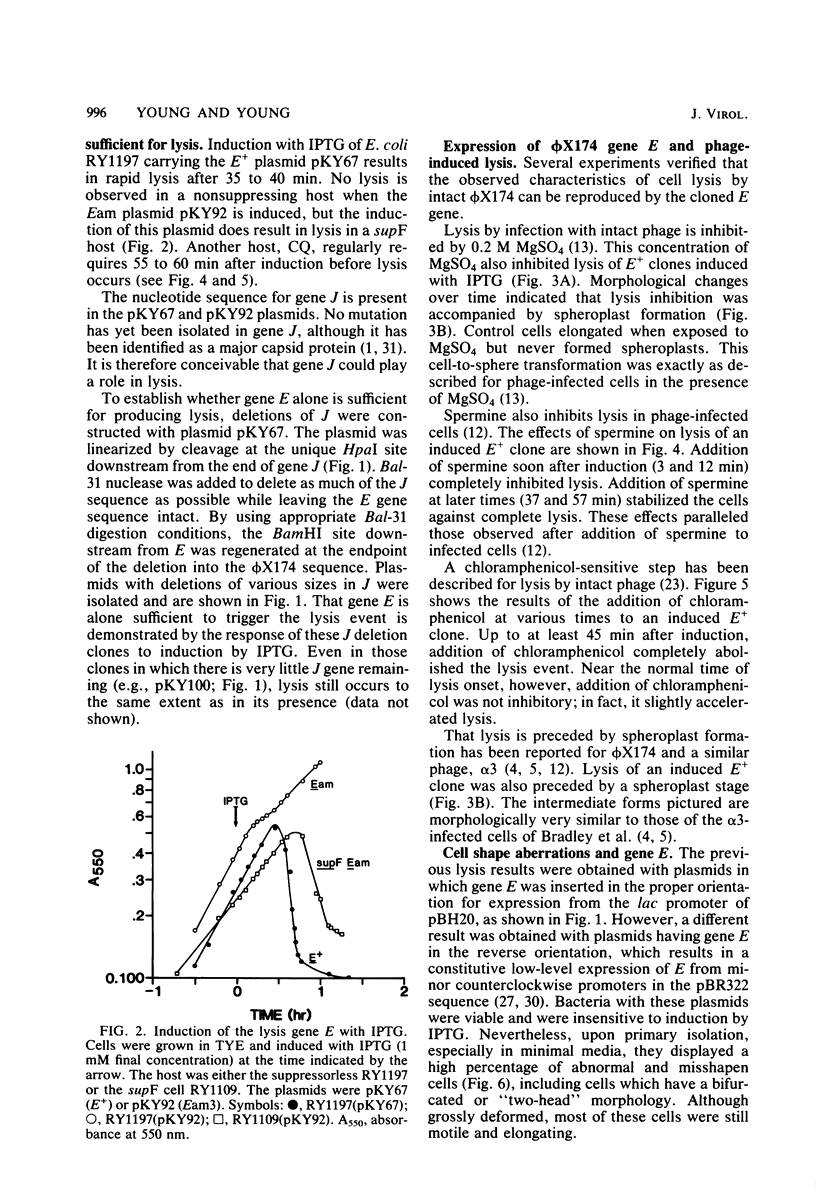
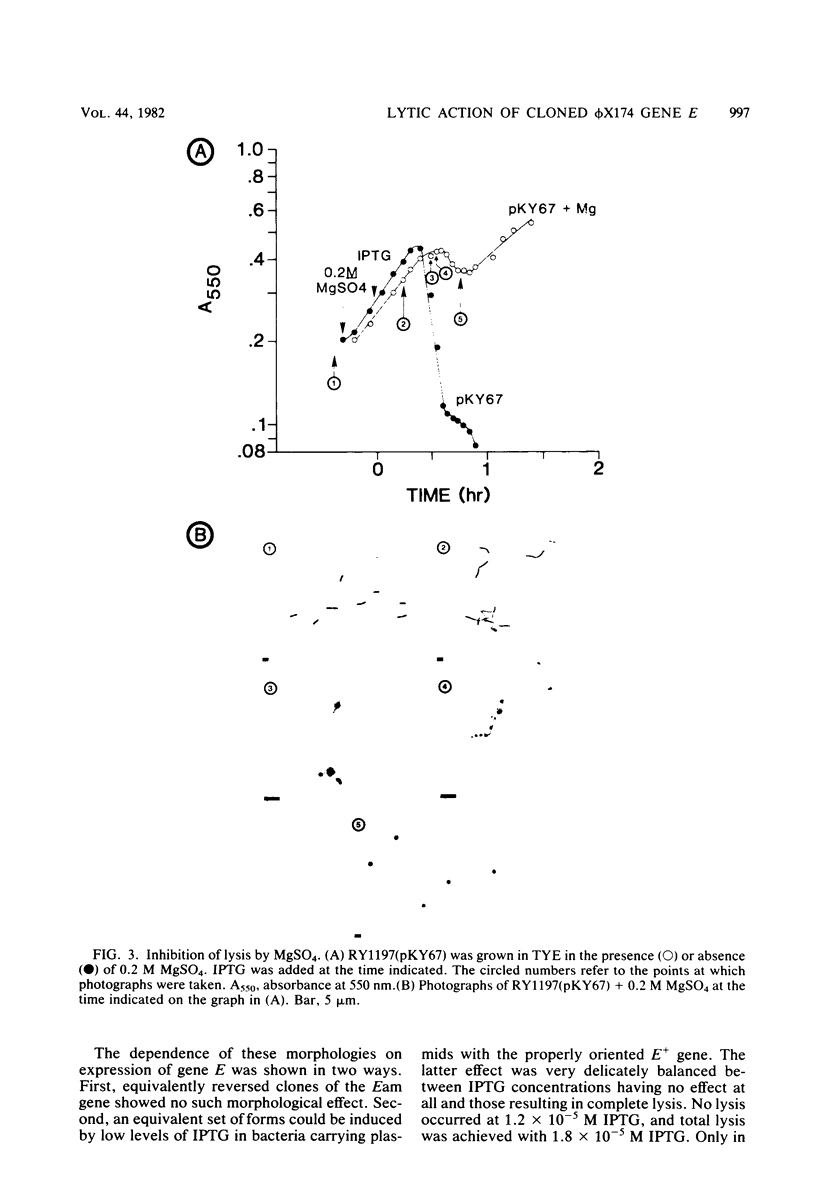
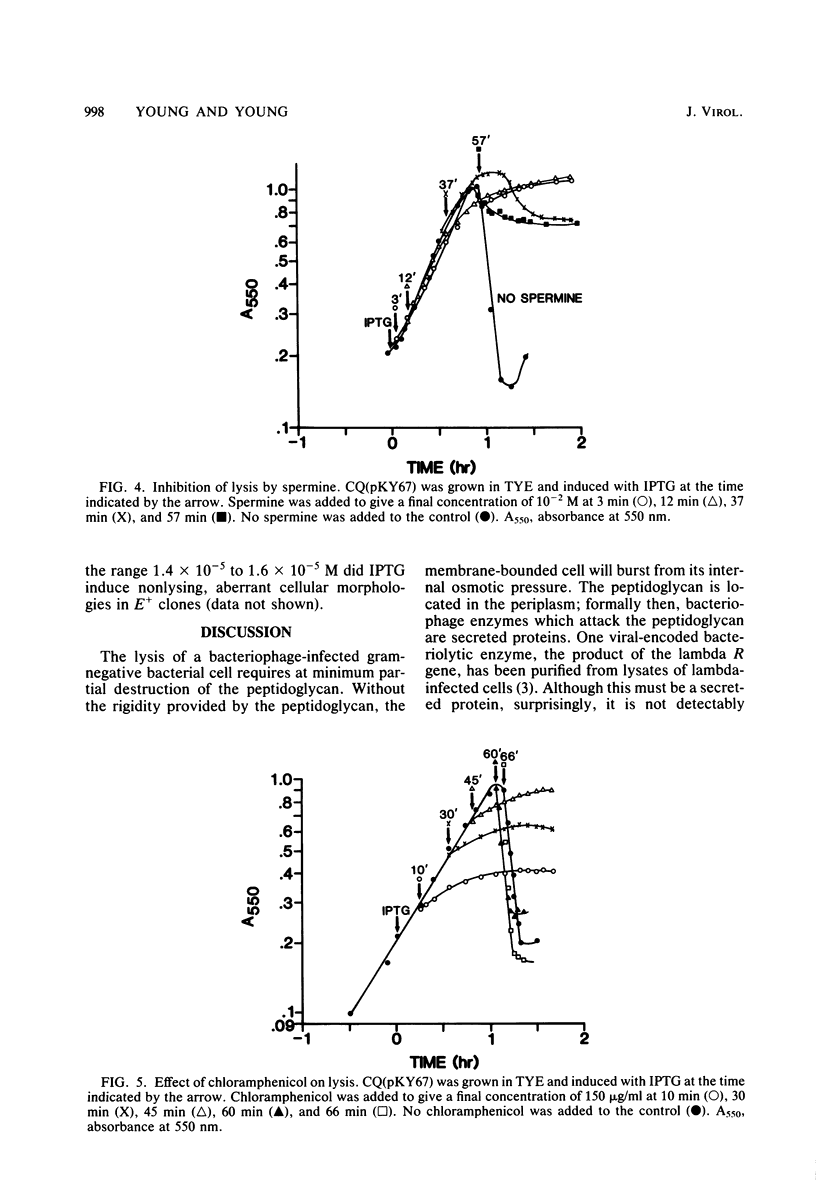
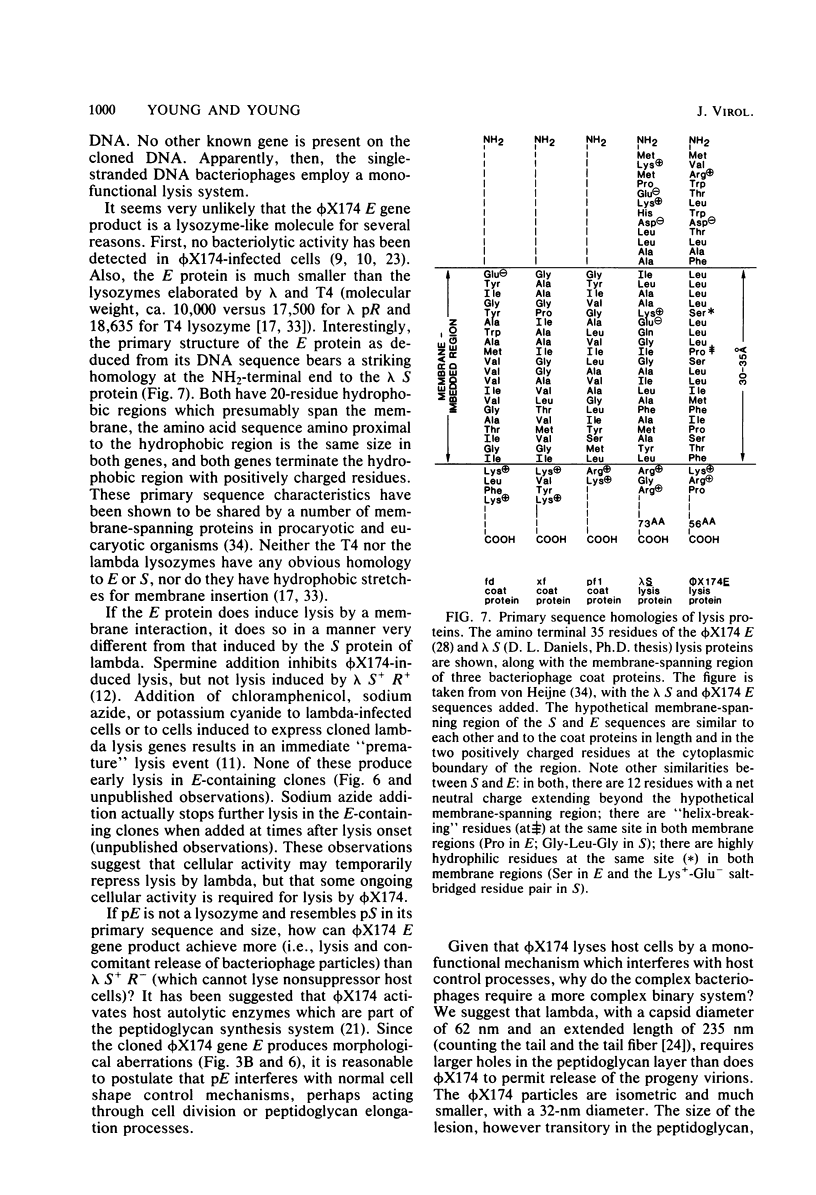
The phi X174 lysis gene E was placed under control of the lac promoter by cloning into the multicopy plasmid pBH20. Other phi X174 gene sequences were removed by nuclease digestion. Expression of gene E was shown to be necessary and sufficient to produce lysis phenomena exhibited by infection with intact phage. Lysis, its inhibition by MgSO4 and spermine, its progression through a spheroplasting stage, and its dependence on an early chloramphenicol-sensitive step were reproduced in clones induced for expression of the E gene product. Escherichia coli clones carrying the E gene not under lac control, and clones under lac control but only minimally induced for gene E expression, exhibited morphological aberrations consistent with the view that the mechanism by which gene E mediates cell lysis is related to host cell division processes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama A., Hamatake R. K., Hayashi M. Morphogenesis of phi X174: in vitro synthesis of infectious phage from purified viral components. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7285–7289. doi: 10.1073/pnas.78.12.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell B. G., Air G. M., Hutchison C. A., 3rd Overlapping genes in bacteriophage phiX174. Nature. 1976 Nov 4;264(5581):34–41. doi: 10.1038/264034a0. [DOI] [PubMed] [Google Scholar]
- Bieńkowska-Szewczyk K., Taylor A. Murein transglycosylase from phage lambda lysate. Purification and properties. Biochim Biophys Acta. 1980 Oct;615(2):489–496. doi: 10.1016/0005-2744(80)90515-x. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Dewar C. A., Robertson D. Structural changes in Escherichia coli infected with a phi X174 type bacteriophage. J Gen Virol. 1969 Jul;5(1):113–121. doi: 10.1099/0022-1317-5-1-113. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Spheroplast formation in cells of Escherichia coli infected with a phi-X 174 type bacteriophage. J Gen Virol. 1968 Jul;3(1):141–142. doi: 10.1099/0022-1317-3-1-141. [DOI] [PubMed] [Google Scholar]
- Breeden L., Yarus M., Cline S. A cloned suppressor tRNA gene relaxes stringent control. Mol Gen Genet. 1980;179(1):125–133. doi: 10.1007/BF00268454. [DOI] [PubMed] [Google Scholar]
- FUJIMURA R., KAESBERG P. The adsorption of bacteriophage phi-X174 to its host. Biophys J. 1962 Nov;2:433–449. doi: 10.1016/s0006-3495(62)86866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J., Fusselman R., Hise J., Chiou L., Smith-Grillo D., Schulz J., Young R. Cell lysis by induction of cloned lambda lysis genes. Mol Gen Genet. 1981;182(2):326–331. doi: 10.1007/BF00269678. [DOI] [PubMed] [Google Scholar]
- Groman N. B., Suzuki G. Effect of spermine on lysis and reproduction by bacteriophages phi-X174, lambda, and f2. J Bacteriol. 1966 Dec;92(6):1735–1740. doi: 10.1128/jb.92.6.1735-1740.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwender H. H., Hofschneider P. H. Lysis inhibition of phi-X174-, M12-, and Q-beta-infected Escherichia coli bacteria by magnesium ions. Biochim Biophys Acta. 1969 Oct 22;190(2):454–459. doi: 10.1016/0005-2787(69)90094-x. [DOI] [PubMed] [Google Scholar]
- HUTCHISON C. A., SINSHEIMER R. L. KINETICS OF BACTERIOPHAGE RELEASE BY SINGLE CELLS OF PHI X174-INFECTED E. COLI. J Mol Biol. 1963 Aug;7:206–208. doi: 10.1016/s0022-2836(63)80046-7. [DOI] [PubMed] [Google Scholar]
- Henrich B., Lubitz W., Plapp R. Lysis of Escherichia coli by induction of cloned phi X174 genes. Mol Gen Genet. 1982;185(3):493–497. doi: 10.1007/BF00334146. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. X. Mutations in a phi-X Lysis gene. J Mol Biol. 1966 Jul;18(3):429–447. doi: 10.1016/s0022-2836(66)80035-9. [DOI] [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Josslin R. The lysis mechanism of phage T4: mutants affecting lysis. Virology. 1970 Mar;40(3):719–726. doi: 10.1016/0042-6822(70)90216-3. [DOI] [PubMed] [Google Scholar]
- MARKERT A., ZILLIG W. STUDIES ON THE LYSIS OF ESCHERICHIA COLI C BY BACTERIOPHAGE PHI-X174. Virology. 1965 Jan;25:88–97. doi: 10.1016/0042-6822(65)90256-4. [DOI] [PubMed] [Google Scholar]
- Mazza A., Felluga B. Electron microscope observations on lambda bacteriophage ultrastructure. J Ultrastruct Res. 1973 Nov;45(3):259–278. doi: 10.1016/s0022-5320(73)80052-8. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Truong K. Specific deletion of DNA sequences between preselected bases. Nucleic Acids Res. 1981 Nov 11;9(21):5679–5688. doi: 10.1093/nar/9.21.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Rosenberg M. A promoter of pBR322 activated by cAMP receptor protein. Nucleic Acids Res. 1981 Jul 24;9(14):3365–3377. doi: 10.1093/nar/9.14.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Tsugita A., Inouye M. Complete primary structure of phage lysozyme from Escherichia coli T4. J Mol Biol. 1968 Oct 14;37(1):201–212. doi: 10.1016/0022-2836(68)90083-1. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Membrane proteins: the amino acid composition of membrane-penetrating segments. Eur J Biochem. 1981 Nov;120(2):275–278. doi: 10.1111/j.1432-1033.1981.tb05700.x. [DOI] [PubMed] [Google Scholar]




