Abstract
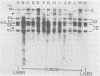
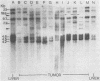
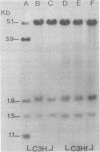
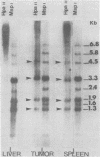
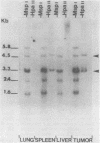
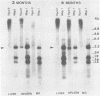
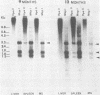
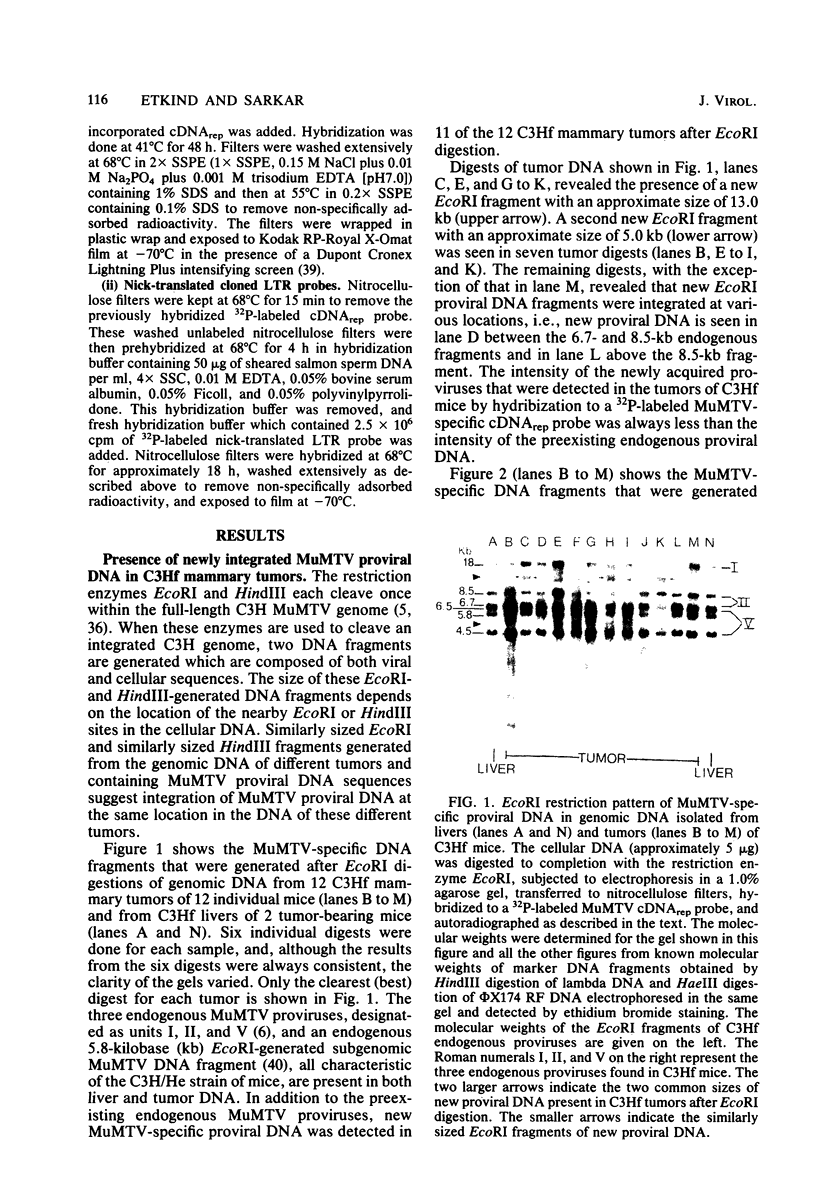
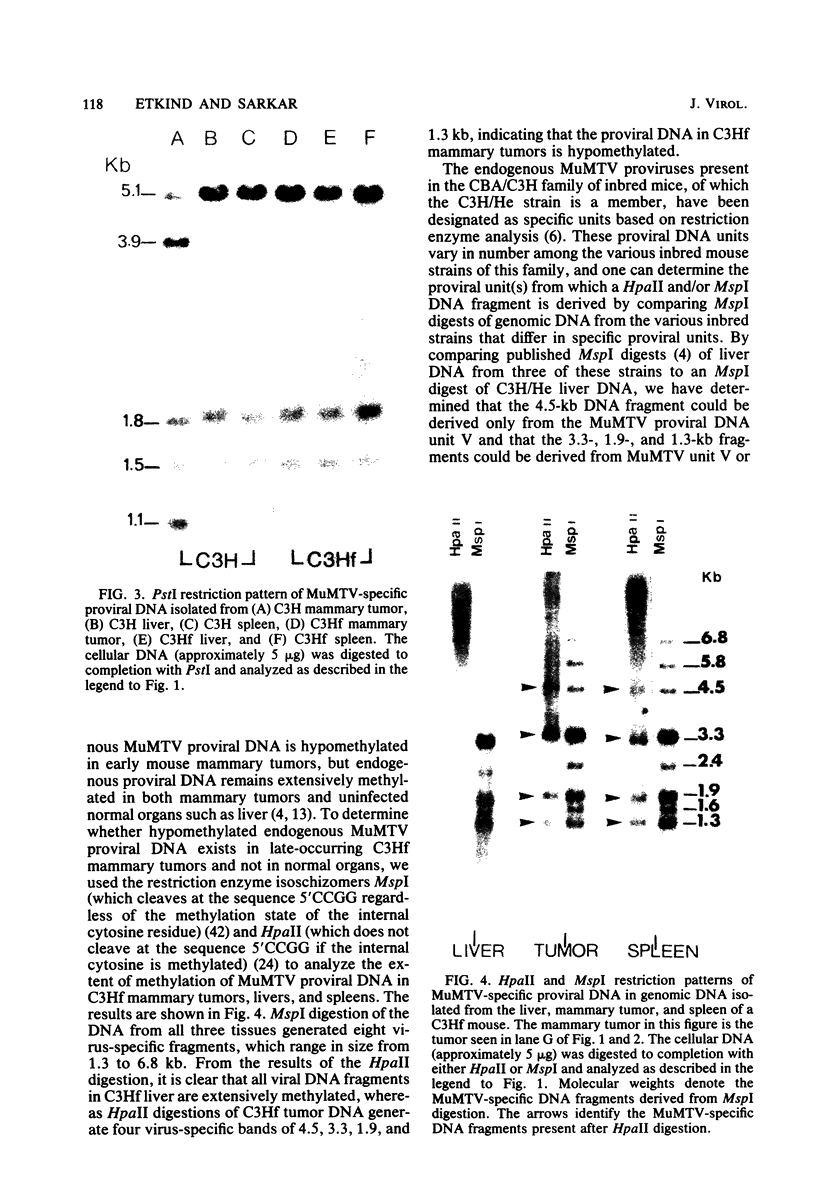
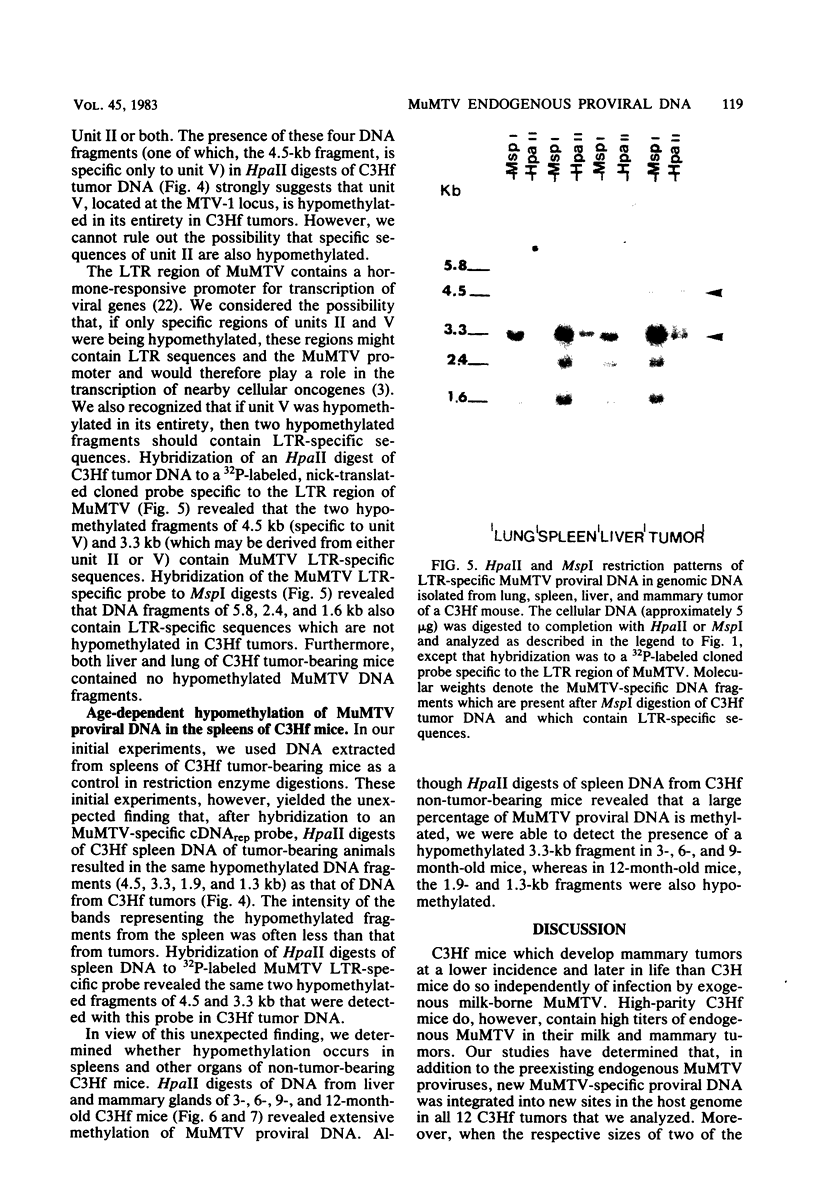
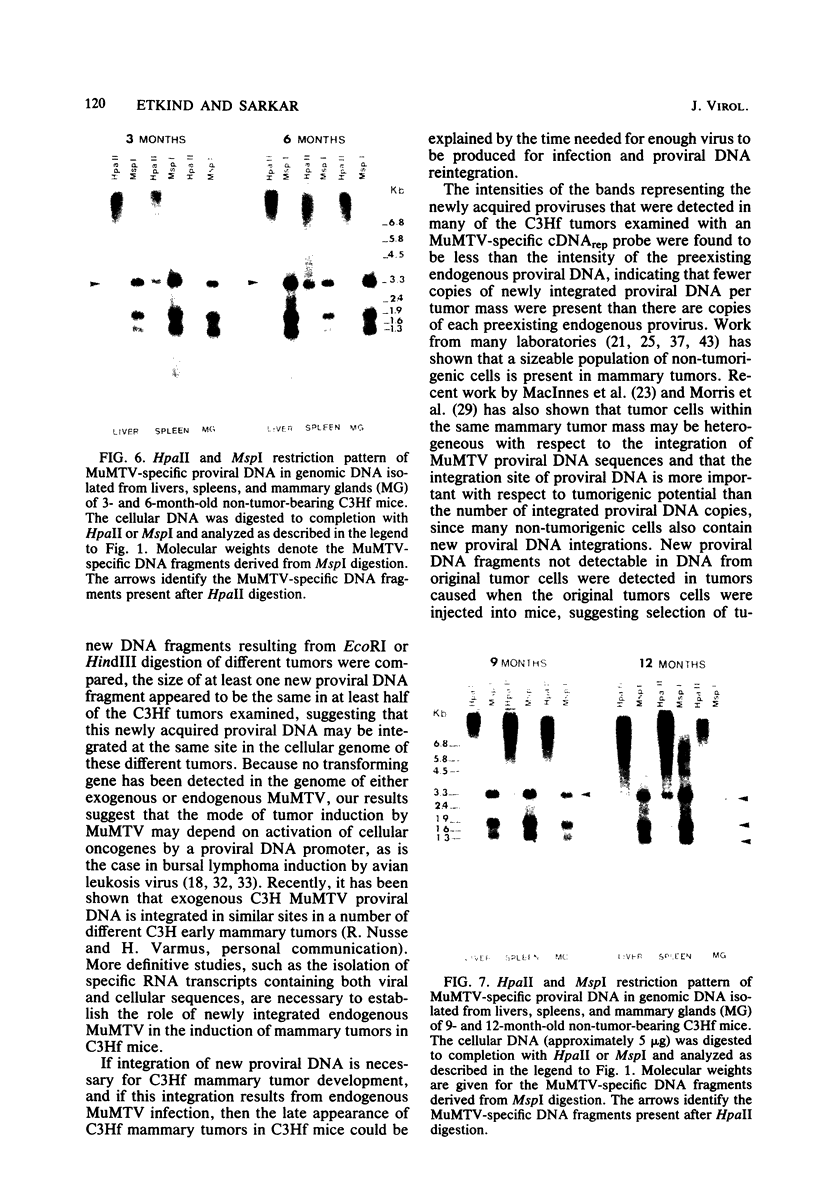
To understand the molecular mechanisms by which the endogenous murine mammary tumor virus (MuMTV) proviruses are expressed and produce late-occurring mammary tumors in C3Hf mice, we analyzed, by the use of restriction enzymes and the Southern transfer procedure, genomic DNA from normal organs of mammary tumor-bearing and tumor-free mice and from 12 late-occurring C3Hf mammary tumors. We found, by using the restriction enzymes EcoRI and HindIII, that in addition to the preexisting endogenous MuMTV proviruses, new MuMTV-specific proviral DNA was integrated into new sites in the host genome in all 12 of the tumors that we examined. PstI digests of C3Hf tumor DNA revealed that the new proviral DNA found in C3Hf tumors was of endogenous origin. Moreover, the respective sizes of at least one of the new DNA fragments generated by EcoRI or HindIII digestion were the same in at least 50% of the C3Hf tumors analyzed, suggesting that the integration site of this new proviral DNA could be at the same location in the host genome of these tumors. Our results may imply that mammary tumorigenesis in C3Hf mice results from activation of cellular oncogenes by an MuMTV proviral DNA promoter. Specific hypomethylation of MuMTV proviral DNA was detected in the mammary tumors and spleens of C3Hf tumor-bearing mice. Our results indicated that most, if not all, of the hypomethylated MuMTV proviral DNA sequences were derived from the endogenous MuMTV provirus located at the MTV-1 locus, a locus responsible for the production of MuMTV antigens and increased incidence of mammary carcinoma in C3Hf mice. In spleens of non-tumor-bearing mice of ages 3, 6, 9, and 12 months, there was progressive hypomethylation of proviral DNA with increasing age, suggesting a possible correlation between demethylation of MuMTV proviral DNA in the spleens of C3Hf mice and the expression of endogenous MuMTV.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentvelzen P. Host-virus interactions in murine mammary carcinogenesis. Biochim Biophys Acta. 1974 Dec 31;355(3-4):236–259. doi: 10.1016/0304-419x(74)90012-2. [DOI] [PubMed] [Google Scholar]
- Breznik T., Cohen J. C. Altered methylation of endogenous viral promoter sequences during mammary carcinogenesis. Nature. 1982 Jan 21;295(5846):255–257. doi: 10.1038/295255a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Proviruses of mouse mammary tumor virus in normal and neoplastic tissues from GR and C3Hf mouse strains. J Virol. 1980 Aug;35(2):298–305. doi: 10.1128/jvi.35.2.298-305.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUX A., MUEHLBOCK O. THE MAMMARY TUMOR AGENT IN COMPLETELY MAMMECTOMIZED MICE. Proc Soc Exp Biol Med. 1964 Feb;115:433–435. doi: 10.3181/00379727-115-28933. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dux A., Mühlbock O. Propagation of the mammary tumor agent (Bittner virus) in the absence of mammary glands in mice. J Natl Cancer Inst. 1968 Jun;40(6):1309–1312. [PubMed] [Google Scholar]
- Etkind P. R., Szabo P., Sarkar N. H. Restriction endonuclease mapping of the proviral DNA of the exogenous RIII murine mammary tumor virus. J Virol. 1982 Mar;41(3):855–867. doi: 10.1128/jvi.41.3.855-867.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning T. G., Puma J. P., Cardiff R. D. Selective amplification of mouse mammary tumor virus in mammary tumors of GR mice. J Virol. 1980 Oct;36(1):109–114. doi: 10.1128/jvi.36.1.109-114.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning T. G., Vassos A. B., Cardiff R. D. Methylation and amplification of mouse mammary tumor virus DNA in normal, premalignant, and malignant cells of GR/A mice. J Virol. 1982 Mar;41(3):1007–1013. doi: 10.1128/jvi.41.3.1007-1013.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M. Incorporation of oligodeoxynucleotides into DNA. Proc Natl Acad Sci U S A. 1968 Sep;61(1):284–291. doi: 10.1073/pnas.61.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers J., Nowinski R. C., Geering G., Hardy W. Detection of avian and mammalian oncogenic RNA viruses (oncornaviruses) by immunofluorescence. Cancer Res. 1972 Jan;32(1):98–106. [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Macinnes J. I., Chan E. C., Percy D. H., Morris V. L. Mammary tumors from GR mice contain more than one population of mouse mammary tumor virus-infected cells. Virology. 1981 Aug;113(1):119–129. doi: 10.1016/0042-6822(81)90141-0. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Smith H. O. Specificity of Hpa II and Hae III DNA methylases. Nucleic Acids Res. 1977 Dec;4(12):4211–4221. doi: 10.1093/nar/4.12.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., van Nie R., Nusse R., Hynes N. E., Groner B. Mammary tumor induction loci in GR and DBAf mice contain one provirus of the mouse mammary tumor virus. Cell. 1981 Jan;23(1):165–173. doi: 10.1016/0092-8674(81)90281-6. [DOI] [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Moore D. H., Sarkar N. H., Charney J. Bioactivity and virions in the blood of mice with mammary tumor virus. J Natl Cancer Inst. 1970 Apr;44(4):965–973. [PubMed] [Google Scholar]
- Morris V. L., Gray D. A., Jones R. F., Chan E. C., McGrath C. M. Mouse mammary tumor virus DNA sequences in tumorigenic and nontumorigenic cells from a mammary adenocarcinoma. Virology. 1982 Apr 15;118(1):117–127. doi: 10.1016/0042-6822(82)90325-7. [DOI] [PubMed] [Google Scholar]
- Nandi S., Helmich C., Haslam S. Hemic cell-associated mammary tumor virus activity in BALB-cfC3H mice. J Natl Cancer Inst. 1974 Apr;52(4):1277–1283. doi: 10.1093/jnci/52.4.1277. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. D., Maloney T., McGrath C. M. Phenotypic variance among cells isolated from spontaneous mouse mammary tumors in primary suspension culture. Cancer Res. 1981 Mar;41(3):1154–1164. [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Traina V. L., Taylor B. A., Cohen J. C. Genetic mapping of endogenous mouse mammary tumor viruses: locus characterization, segregation, and chromosomal distribution. J Virol. 1981 Dec;40(3):735–744. doi: 10.1128/jvi.40.3.735-744.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. MspI, an isoschizomer of hpaII which cleaves both unmethylated and methylated hpaII sites. Nucleic Acids Res. 1978 Sep;5(9):3231–3236. doi: 10.1093/nar/5.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nie R., Verstraeten A. A. Studies of genetic transmission of mammary tumour virus by C3Hf mice. Int J Cancer. 1975 Dec 15;16(6):922–931. doi: 10.1002/ijc.2910160606. [DOI] [PubMed] [Google Scholar]