Abstract
Background
In a search for mutations of μ‐crystallin (CRYM), a taxion specific crystalline which is also known as an NADP regulated thyroid hormone binding protein, two mutations were found at the C‐terminus in patients with non‐syndromic deafness.
Objective
To investigate the mechanism of hearing loss caused by CRYM mutations
Methods
T3 binding activity of mutant μ‐crystallin was compared with that of wild‐type μ‐crystallin, because μ‐crystallin is known to be identical to T3 binding protein. To explore the sites within the cochlea where μ‐crystallin is functioning, its localisation in the mouse cochlea was investigated immunocytochemically using a specific antibody.
Results
One mutant was shown to have no binding capacity for T3, indicating that CRYM mutations cause auditory dysfunction through thyroid hormone binding properties. Immunocytochemical results indicated that μ‐crystallin was distributed within type II fibrocytes of the lateral wall, which are known to contain Na,K‐ATPase.
Conclusions
CRYM mutations may cause auditory dysfunction through thyroid hormone binding effects on the fibrocytes of the cochlea. μ‐Crystallin may be involved in the potassium ion recycling system together with Na,K‐ATPase. Future animal experiments will be necessary to confirm a causal relation between Na,K‐ATPase, T3, and deafness.
Keywords: μ‐crystallin; Na; K‐ATPase; 3,5,3′‐triiodo‐L‐thyronine (T3)‐binding protein (CTBP); spiral ligament; deafness
Recent advances in molecular genetics have revealed a number of genes responsible for hearing loss. So far, more than 100 genetic loci linked to non‐syndromic deafness have been described, and 37 genes whose mutations can cause deafness have been cloned (hereditary hearing loss homepage: http://www.uia.ac.be/dnalab/hhh/). Deafness is a highly heterogeneous disorder, and a large diversity of encoded molecules is expressed. Identification of genes responsible for deafness sheds light on the molecular basis of inner ear function and the mechanisms of sensorineural hearing loss. Recently, we have shown that mutations of CRYM, which is highly expressed in the cochlea and vestibule, cause hearing loss.1 To date, two different types of mutation of CRYM at the C‐terminus were found among non‐syndromic deafness patients.1 μ‐Crystallin was first identified as a major structural protein of the ocular lens.2 However, recently it was demonstrated that μ‐crystallin is identical to NADPH dependent cytosolic 3,5,3′‐triiodo‐L‐thyronine (T3) binding protein (p38CTBP).3 When T3 is incorporated into a target cell, it binds to cytosolic μ‐crystallin/p38CTBP in the presence of nicotinamide adenine dinucleotide phosphate (NADPH), and μ‐crystallin/p38CTBP retains the same amount of intracellular and intranuclear T3.4,5 Further, it promotes transcriptional activity of T3.6 The C‐terminal region of μ‐crystallin/p38CTBP functions to preserve the protein in cytoplasm.7
Although it has been shown that mutations of CRYM cause hearing loss, little is known about the precise role of μ‐crystallin in auditory function or its localisation in the inner ear. In this study, to further elucidate the mechanism of hearing loss caused by CRYM mutations, the effects of two mutations found in patients—X315Y and K314T—were evaluated by comparing their binding activity to T3 with that of wild type μ‐crystallin. In addition, the localisation of μ‐crystallin was immunocytochemically examined in the mouse cochlea. To date, Crym mRNA expression has been observed in the lateral fibrocytes of the spiral ligament and the spiral limbus fibrocytes in the mouse cochlea at postnatal day (Pn) P0.1 These cells maintain ionic gradient and regulate fluid homeostasis with Na,K‐ATPase, which is an integral plasma membrane bound enzyme. It is well known that Na,K‐ATPaseβ1 is regulated by T3 in various tissues,8,9 so whether a similar cellular/nuclear regulatory system operates within the inner ear is a question of interest. Therefore Na,K‐ATPaseβ1 was also double immunostained with μ‐crystallin in this study.
Methods
Assay of [125 I] T3 binding to µ‐crystallin
T3 binding activity of μ‐crystallin was measured as previously described.4 Briefly, fractions containing μ‐crystallin and its mutants were prepared by the TNT coupled reticulocyte lysate system (Promega, Madison, Wisconsin, USA). To these fractions was then added TED buffer (10 mM Tris‐HCl, pH 7.4, 0.5 mMEGTA, 0.5 mMDTT) up to a final volume of 170 μl, and the mixture was incubated at 4°C for 20 minutes, with or without 100 μM NADPH, and with [125I] labelled T3 (40 000 cpm added to each tube) and 0–10−6 M of unlabelled T3. The bound form of labelled T3 was separated by 30 μl dextran coated charcoal (DCC). The amount of μ‐crystallin‐bound T3 was determined by radioactivity. The non‐specific binding, which was determined by the radioactivity of 10−6 M unlabelled T3, was subtracted from each value. The dissociation constant and the maximum binding capacity were estimated by the method of Scatchard. Each value was calculated as the mean of triplicate.
Preparation of polyclonal antibodies to µ‐crystallin
Two sequences were chosen from mouse μ‐crystallin to synthesise peptides (AFLSAEEVQDHLRSC and EGHSNTAVPSHQASC). The peptides were coupled to keyhole limpet haemocyanin (KLH), using m‐maledo‐benzoyl‐N‐hydroxysuccinimide ester as a coupling agent. The peptide conjugates were mixed with complete adjuvant and injected into a rabbit. The antiserum had been affinity purified on a column carrying the peptide used for immunisation. Synthesised peptides (2 mg) were coupled to an activated thiol‐sepharose and applied to the peptide column. The serum from rabbits immunised with the peptides was passed through the column.
Immunocytochemistry
Localisation of μ‐crystallin in the mature mouse cochlea was examined. Mouse inner ears at four weeks were isolated and fixed by fresh 4% paraformaldehyde. The wax embedded frozen tissue sections (15 μm thick) were dewaxed with phosphate buffered saline (PBS) and stained with the anti‐μ‐crystallin antibodies. As a control experiment, the antiserum preabsorbed with the synthetic peptides was used. For double immunofluorescence immunostaining, the tissues were double stained with anti‐Na,K‐ATPaseβ1 antibodies and anti‐μ‐crystallin antibodies, and visualised with FITC conjugated anti‐goat antibodies (for Na,K‐ATPaseβ1) and TRITC conjugated anti‐rabbit antibodies (for μ‐crystallin).
Vector constructions and the transfection into COS1 cells
Amplified fragments of wild‐type, X315Y, and K314T of CRYM with HA tag were subcloned into pcDNA3.1(+) (Invitrogen, Carlsbad, California, USA) as described1 and verified by sequencing. These constructs and empty vector were transfected into COS1 cells with Lipofectamine 2000 (Invitrogen) using the manufacture's protocol.
Immunoblotting
CRYM wild type, X315Y, or K314T transfected COS1 cells and the mouse cochlear tissue were lysed in radioimmunoprecipitation assay buffer (PBS, 0.1% sodium dodecyl sulphate, 1% sodium deoxycholate, 1% TritonX‐100, pepstatin A 10 μg/ml). The lysates were incubated with Lammeri sample buffer containing dithiothreitol. Concentration of the protein was measured by Bradford's method. The same amount of protein (20 mg) was analysed by SDS gel electrophoresis followed by immunoblotting with anti‐μ‐crystallin antibodies or anti‐HA antibodies.
Results
Affinity constants to T3 in wild type, K314T, and X315Y expressed reticulocyte lysates
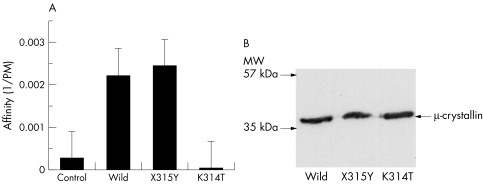
To assess the T3 binding activity of the mutant μ‐crystallin proteins, we carried out a Scatchard analysis with the lysates from the μ‐crystallin expressed reticulocytes. The mean value was obtained from three independent experiments. In the absence of NADPH, there was low binding activity to T3 in all the lysates we studied (data not shown). T3 binding activity increased in the lysates expressed with wild type and X315Y plasmids, but not in those with the pcDNA3.1 vector as a control or the K314T plasmids. The affinity constant of wild type CRYM to T3 was approximately 2/nM, which is similar to the constant previously described.4 There is no significant difference between wild type and X315Y. On the other hand, the calculated affinity constants of K314T mutant were lower than those of the wild type CRYM (p = 0.0008; table 1, fig 1A). The affinities of the K314Y mutant were not significantly altered when we added NADPH (data not shown).
Table 1 Affinity constant (Ka, ×109 M−1) obtained by estimation with Scatchard analysis. There are significant differences between K314T and the others.
| Ka (×109 M−1) | |
|---|---|
| Empty vector | 0.25 (0.12) |
| Wild | 2.21 (1.1) |
| X315Y | 2.44 (1.2) |
| K314T | 0.015 (0.1)* |
Ka value indicates mean (SD) of three separate determinations.
*p = 0.0008, determined by t test.
Figure 1 (A) Affinity of CRYM and its mutants to T3. The X315Y mutant showed normal binding activity. On the other hand the K314T mutant did not show any binding activity that was similar to the empty vector (control). (B) Immunoblotting with anti‐HA antibodies shows that all constructs were expressed at the expected size.
In order to confirm expression of wild type CRYM, X315Y, and K314T constructs in cells, each construct was transfected into COS1 cells and 20 μg of protein lysates were prepared and immunoblotted with anti‐HA antibodies. All constructs were expressed at the expected size (fig 1B).
μ‐Crystallin localisation in the cochlea
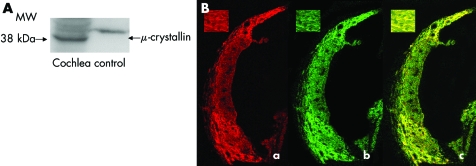
In order to clarify the μ‐crystallin protein localisation in the cochlea, we used immunocytochemical methods. For this purpose, we first made anti‐μ‐crystallin antiserum. Immunoblotting with this showed a strong and single band at the appropriate molecular weight, which means that the antiserum we prepared exclusively recognised μ‐crystallin (fig 2A). With this antiserum, we next undertook immunocytochemistry, and showed that mouse μ‐crystallin was specifically localised in the type II fibrocytes of the spiral ligament (fig 2B, a). Other portions of the cochlea—for example, the spiral limbus, the stria vascularis, and spiral prominent—were negative for immunostaining. As a control study, primary antiserum was preabsorbed with the synthetic peptides used for immunisation, and no immunoreactivity was detected (data not shown).
Figure 2 (A) Immunoblotting with anti‐μ‐crystallin antibodies showed strong and specific staining of a band at appropriate molecular weight. The control lane is the immunoreactivity of the protein from CRYM transfected COS1 cells. As the protein from CRYM transfected COS1 cells is co‐expressed with HA‐tag, the control lane showed staining of a band at heavier molecular weight. (B) Panel (a): Immunoreactivity of μ‐crystallin in the mature mouse cochlea visualised with TRITC conjugated anti‐rabbit antibodies distributed in spiral ligament particularly in type II fibrocytes. μ‐Crystallin had no immunoreactivity to other fibrocytes of spiral ligament . Inset: higher magnification view of type II fibrocytes. Panel (b): Immunoreactivity of Na,K‐ATPaseβ1 visualised with FITC conjugated anti‐goat antibodies distributed in spiral ligament particularly in type II fibrocytes. Panel (c): TRITC labelled μ‐crystallin was merged in FITC labelled Na,K‐ATPaseβ1. They co‐localised in type II fibrocytes in spiral ligament.
μ‐Crystallin and Na,K‐ATPaseβ1 co‐localisation in the cochlea
Type II fibrocytes of the spiral ligament, in which we found μ‐crystallin expression, are said to be important for K+ recycling. Because the expression of Na,K‐ATPaseβ1, which is a key enzyme of K+ recycling, is regulated by T3, and CRYM enhances transcription activity of T3, we compared the localisation of Na,K‐ATPaseβ1 with that of μ‐crystallin in the mouse cochlea. Confocal laserscan microscopy revealed co‐localisation of TRITC labelled μ‐crystallin (red) and FITC labelled Na,K‐ATPaseβ1 (green) in type II fibrocytes in the spiral ligament (fig 2B, panels (b) and (c)).
Discussion
In this study, to explore the mechanism of deafness caused by CRYM mutations, the functional role of μ‐crystallin was evaluated from the following viewpoints: first, the differences in T3 binding activity between wild type μ‐crystallin and its mutants found in the deaf patients, because of a plausible functional significance of μ‐crystallin as a T3 carrier protein; and second, immunocytochemical analysis to identify the possible sites in the cochlea where μ‐crystallin functions. The T3 binding assay showed that the K315Y mutation did not affect the NADPH dependent T3 binding. In contrast, addition of NADPH did not increase the T3 binding activity in K314T lysates, indicating that the K314T mutation impaired the NADPH dependent T3 binding.
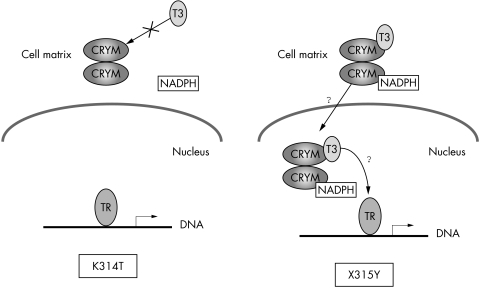
μ‐Crystallin was immunocytochemically distributed in the type II fibrocytes of the spiral ligament. These results suggested that it may carry T3 into the nucleus of the type II fibrocytes of the spiral ligament and that the K314T mutant of CRYM causes the dysfunction the transfer of T3 from cytoplasm to nucleus (fig 3). It is well known that the action of thyroid hormone is initiated through the activation of gene expression by binding to its nuclear receptor.10 Therefore, we speculate that the K314T mutant abrogates the affinity to T3, and the translocation of T3 from cytosol to nucleus is interfered with. However, the X315Y mutant may cause different effects on the T3 transportation process—for example, the releasing property or the entering process into the nucleus, or both (fig 3). Although any precise abnormal behaviour of the X315Y mutant is unknown, the subcellular expression patterns of the two mutant proteins have been shown to differ—that is, when transfecting into COS7 cells, the X315Y mutant showed a vacuolation pattern in the cytoplasm, and cells expressing the K314T mutant showed strong staining predominantly in perinuclear regions.1 Therefore, it may be possible to hypothesise that the two mutants cause hearing loss by different cellular mechanisms.
Figure 3 Schema of the hypothesis of CRYM mutants dysfunction. CRYM binds T3 in the cytoplasm and brings it to the nucleus. Thyroid hormone action is mediated by nuclear thyroid hormone receptors. The binding experiments suggested that the two mutations cause hearing loss by different mechanisms, as in this picture. The K314T mutant cannot bind to T3 and therefore cannot bring T3 into the nucleus where thyroid hormone receptor exists. The X315Y mutant may be able to bind T3, but may be in trouble when entering the nucleus or releasing on thyroid hormone receptor.
Immunocytochemical results clearly showed that μ‐crystallin is distributed within type II fibrocytes of the lateral wall in the cochlea, supporting the localisation of Crym mRNA in the previous in situ hybridisation findings.1 Type II fibrocytes are known to contain Na,K‐ATPaseβ1 subunits and play an important role in transporting and maintaining high K+ ion concentration in the endolymph.11 It is well established that a functional T3 response element was identified in the promoter region of the Na,K‐ATPaseβ1 gene,9 and Na,K‐ATPase activity was stimulated by T3 in various tissues. As the two molecules are likely to be co‐localised in type II fibrocytes, μ‐crystallin might be involved in the potassium ion recycling system through Na,K‐ATPase. This may indicate that dysfunction of μ‐crystallin interferes with potassium ion recycling, thus disturbing the maintenance of K+‐rich endolymph and a positive electrical potential.12,13
The hypothesis mentioned above is also supported by the previous reports describing the relation between T3 and Na,K‐ATPase. In cultured rat mesangial cells, Na,K‐ATPase mRNA expression was increased by T3 exposure.8 According to this study, after exposure of mesangial cells to T3, α1 mRNA was soon increased threefold and β1 mRNA was increased more gradually. The data suggest that T3 stimulates Na,K‐ATPase gene expression, protein production, and enzymatic activity in the corresponding cells. In contrast, Na,K‐ATPaseα1 and β1 immunoreactivity was reported to be reduced in the inner ear of hypothyroid animals, indicating that a decreased level of T3 may affect hearing by reducing Na,K‐ATPase level, maintaining ion and fluid transport.14
In conclusion, our results suggest that mutations of CRYM may affect binding properties with thyroid hormone (T3), that the possible site in the cochlea where μ‐crystallin functions is the type II fibrocyte of the spiral ligament, and that μ‐crystallin may interfere with K+ ionic disequilibrium through Na,K‐ATPaseβ1 in the type II fibrocytes.
Acknowledgements
We thank Ms A C Apple‐Mathews for help in preparing the manuscript, and Dr J Mitsushita for helpful comments and continuous encouragement. This study was supported by a Health Sciences Research Grant (Research on Eye and Ear Science, Immunology, Allergy and Organ Transplantation) from the Ministry of Health and Welfare of Japan and by the Acute Profound Deafness Research Committee of the Ministry of Health and Welfare of Japan.
Footnotes
Conflicts of interest: none declared
References
- 1.Abe S, Katagiri T, Saito‐Hisaminato A, Usami S, Inoue Y, Tsunoda T, Nakamura Y. Identification of CRYM as a candidate responsible for nonsyndromic deafness, through cDNA microarray analysis of human cochlear and vestibular tissues. Am J Hum Genet 20037273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim R Y, Gasser R, Wistow G J. Mu‐crystallin is a mammalian homologue of Agrobacterium ornithine cyclodeaminase and is expressed in human retina. Proc Natl Acad Sci USA 1992899292–9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vie M P, Evrard C, Osty J, Breton‐Gilet A, Blanchet P, Pomerance M, Rouget P, Francon J, Blondeau J P. Purification, molecular cloning, and functional expression of the human nicodinamide‐adenine dinucleotide phosphate‐regulated thyroid hormone‐binding protein. Mol Endocrinol 1997111728–1736. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi M, Hashizume K, Suzuki S, Ichikawa K, Takeda T. A novel NADPH‐ dependent cytosolic 3,5,3′‐triiodo‐L‐thyronine (T3)‐binding protein (CTBP; 5.1S) in rat liver. A comparison with 4.7SNADPH‐ dependent CTBP. Endocrinology 19911291701–1708. [DOI] [PubMed] [Google Scholar]
- 5.Hashizume K, Suzuki S, Ichikawa K, Takeda T. Purification of cytosolic 3,5,3′‐ triiodo‐L‐thyronine(T3)‐binding protein(CTBP), which regulates nuclear T3 translocation. Biochem Biophys Res Commun 19911741084–1089. [DOI] [PubMed] [Google Scholar]
- 6.Mori J, Suzuki S, Kobayashi M, Inagaki T, Komatsu A, Takeda T, Miyamoto T, Ichikawa K, Hashizume K. Nicotinamide adenine dinucleotide phosphate‐dependent cytosolic T3 binding protein as a regulator for T3‐mediated transactivation. Endocrinology 20021431538–1544. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki S, Mori J, Kobayashi M, Inagaki T, Komatsu A, Yamashita K, Takeda T, Miyamoto T, Ichikawa K, Hashizume K. Presence of functional domains in NADPH‐dependent cytosolic 3,5,3'‐triiodo‐L‐thyronine‐binding protein (p38CTBP) molecule: analysis with deletion mutants. Horm Metab Res 200335577–582. [DOI] [PubMed] [Google Scholar]
- 8.Ohara T, Ikeda U, Muto S, Oguchi A, Tsuruya Y, Yamamoto K, Kawakami K, Shimada K, Asano Y. Thyroid hormone stimulates Na(+)‐K(+)‐ATPase gene expression in cultured rat mesangial cells. Am J Physiol 1993265F370–F376. [DOI] [PubMed] [Google Scholar]
- 9.Feng J, Orlowski J, Lingrel J B. Identification of a functional thyroid hormone response element in the upstream flanking region of the human Na,K‐ATPase beta 1 gene. Nucleic Acids Res 1993212619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazar M A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 199314184–193. [DOI] [PubMed] [Google Scholar]
- 11.ten Cate W J, Curtis L M, Rarey K E. Effects of low‐sodium, high‐potassium dietary intake on cochlear lateral wall Na+,K(+)‐ATPase. Eur Arch Otorhinolaryngol 19942516–11. [DOI] [PubMed] [Google Scholar]
- 12.Spicer S S, Schulte B A. The fine structure of spiral ligament cells relates to ion return to the stria and varies with place‐frequency. Hear Res 199610080–100. [DOI] [PubMed] [Google Scholar]
- 13.Steel K P, Kros C J. A genetic approach to understanding auditory function. Nat Genet 200127143–149. [DOI] [PubMed] [Google Scholar]
- 14.Zuo J, Rarey K E. Responsiveness of alpha 1 and beta 1 cochlear Na, K‐ATPase isoforms to thyroid hormone. Acta Otolaryngol 1996116422–428. [DOI] [PubMed] [Google Scholar]