Abstract
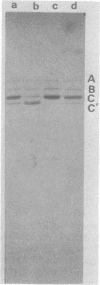
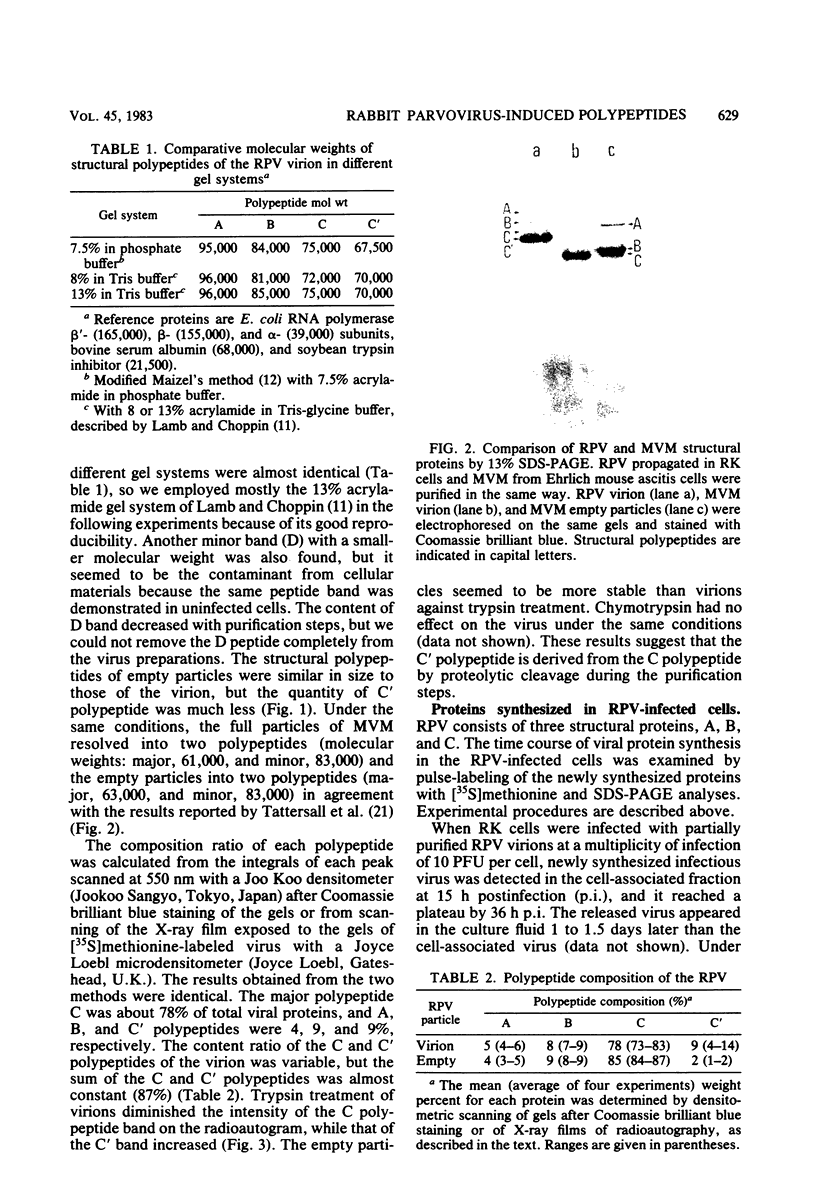
The structural and nonstructural polypeptides of a rabbit parvovirus (RPV) (F-7-9 strain) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The virion contained three polypeptide components, A (molecular weight, 96,000), B (85,000), and C (75,000). A part of the polypeptide C was cleaved into the smaller-molecular-weight polypeptide C' by proteolysis during purification steps. The major polypeptide C together with C' constituted about 87% of the total viral proteins, and the minor polypeptides, A and B, constituted 4 and 9%, respectively. The structural polypeptides of empty particles were similar in size and composition to those of the virion, but the content of the C' polypeptide was very low. When rabbit kidney cell cultures were infected with RPV, the C polypeptide was detected as early as 15 h postinfection, whereas A and B were first demonstrated at 18 h. The C' polypeptide was not detected for 44 h. In addition to the three structural polypeptides, at least three nonstructural polypeptides, E, F, and G, were demonstrated in the RPV-infected cells. Polypeptide E (molecular weight, 49,000), detected mostly in cytoplasm, seemed to be a cellular protein. The F (25,000) and G (22,000) polypeptides seemed to be virus-coded proteins since they were precipitated with the anti-RPV rabbit immunoglobulin. According to partial proteolysis and peptide mapping, the F and G polypeptides shared the same peptide components.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C., Crettol-Järvinen A. Peptide mapping of heterogeneous protein samples. J Biol Chem. 1979 Apr 25;254(8):2565–2567. [PubMed] [Google Scholar]
- Buller R. M., Rose J. A. Characterization of adenovirus-associated virus-induced polypeptides in KB cells. J Virol. 1978 Jan;25(1):331–338. doi: 10.1128/jvi.25.1.331-338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Hayashi M. The parovivirus MVM: particles with altered structural proteins. Virology. 1975 Jul;66(1):261–261. doi: 10.1016/0042-6822(75)90196-8. [DOI] [PubMed] [Google Scholar]
- Gautschi M., Siegl G. Structural proteins of parvovirus Lu 3. Evidence for only two protein components within infectious virions. Arch Gesamte Virusforsch. 1973;43(4):326–333. [PubMed] [Google Scholar]
- Hampton E. G. H-1 virus growth in synchronized rat embryo cells. Can J Microbiol. 1970 Apr;16(4):266–268. doi: 10.1139/m70-049. [DOI] [PubMed] [Google Scholar]
- Handa H., Carter B. J. Adeno-associated virus DNA replication complexes in herpes simplex virus or adenovirus-infected cells. J Biol Chem. 1979 Jul 25;254(14):6603–6610. [PubMed] [Google Scholar]
- Johnson F. B., Hoggan M. D. Structural proteins of HADEN virus. Virology. 1973 Jan;51(1):129–137. doi: 10.1016/0042-6822(73)90373-5. [DOI] [PubMed] [Google Scholar]
- Johnson F. B., Ozer H. L., Hoggan M. D. Structural proteins of adenovirus-associated virus type 3. J Virol. 1971 Dec;8(6):860–863. doi: 10.1128/jvi.8.6.860-863.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollek R., Tseng B. Y., Goulian M. DNA polymerase requirements for parvovirus H-1 DNA replication in vitro. J Virol. 1982 Mar;41(3):982–989. doi: 10.1128/jvi.41.3.982-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsvik J. R., Gierthy J. F., Rhode S. L., 3rd Replication process of the parvovirus H-1. IV. H-1-specific proteins synthesized in synchronized human NB kidney cells. J Virol. 1974 Dec;14(6):1600–1603. doi: 10.1128/jvi.14.6.1600-1603.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Matsunaga Y., Matsuno S., Mukoyama J. Isolation and characterization of a parvovirus of rabbits. Infect Immun. 1977 Nov;18(2):495–500. doi: 10.1128/iai.18.2.495-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard C., Bates R. C., Stout E. R. Levels of cellular DNA polymerases in synchronized bovine paravovirus-infected cells. J Virol. 1978 Jul;27(1):258–261. [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Maizel J. V., Jr, Inman J. K., Shatkin A. J. Structural proteins of adenovirus-associated viruses. J Virol. 1971 Nov;8(5):766–770. doi: 10.1128/jvi.8.5.766-770.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R. J., Mayor H. D. Structural polypeptides of parvoviruses. Virology. 1977 May 1;78(1):340–345. doi: 10.1016/0042-6822(77)90107-6. [DOI] [PubMed] [Google Scholar]
- Salzman L. A., White W. L., McKerlie L. Growth characteristics of Kilham rat virus and its effect on cellular cellular macromolecular synthesis. J Virol. 1972 Oct;10(4):573–577. doi: 10.1128/jvi.10.4.573-577.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A., White W. L. Structural proteins of Kilham rat virus. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1551–1556. doi: 10.1016/0006-291x(70)90564-4. [DOI] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. II. Biochemical characteristics of virus replication. Arch Gesamte Virusforsch. 1973;40(1):119–127. doi: 10.1007/BF01242643. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]