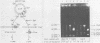Abstract
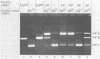
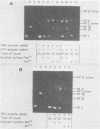
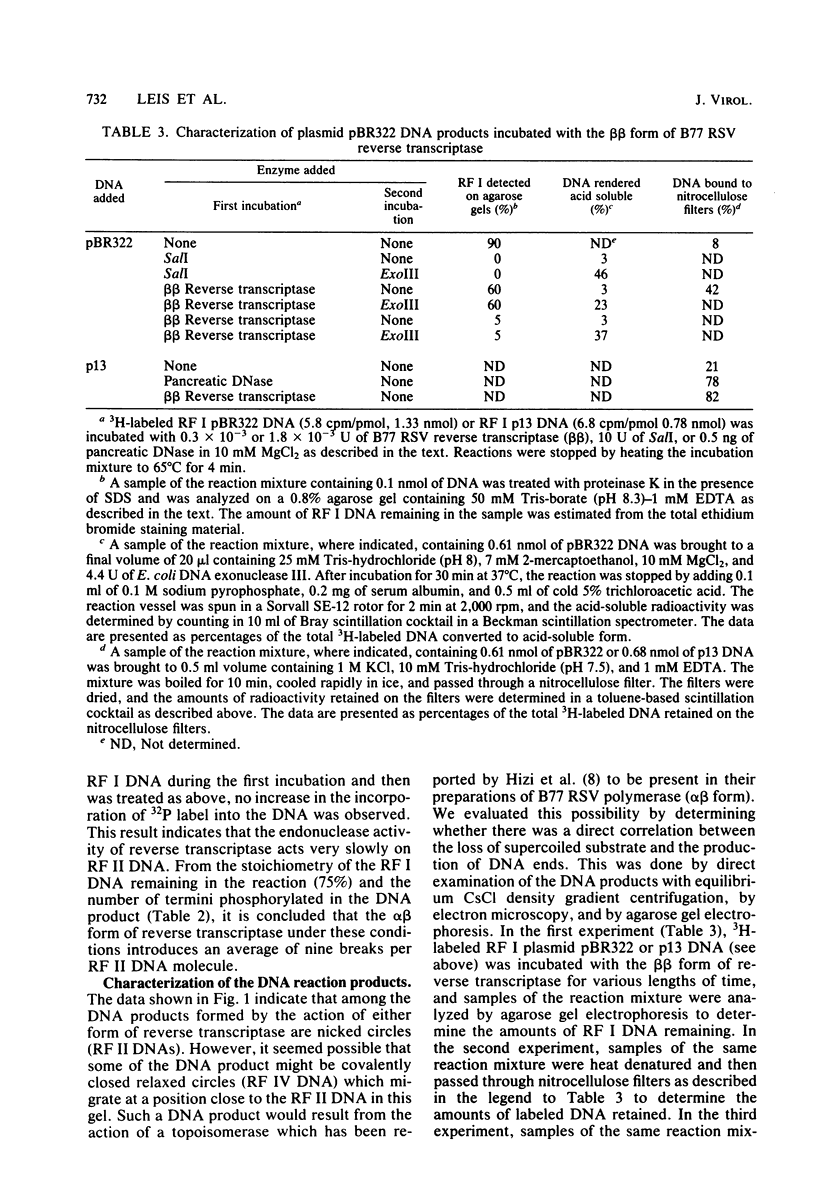
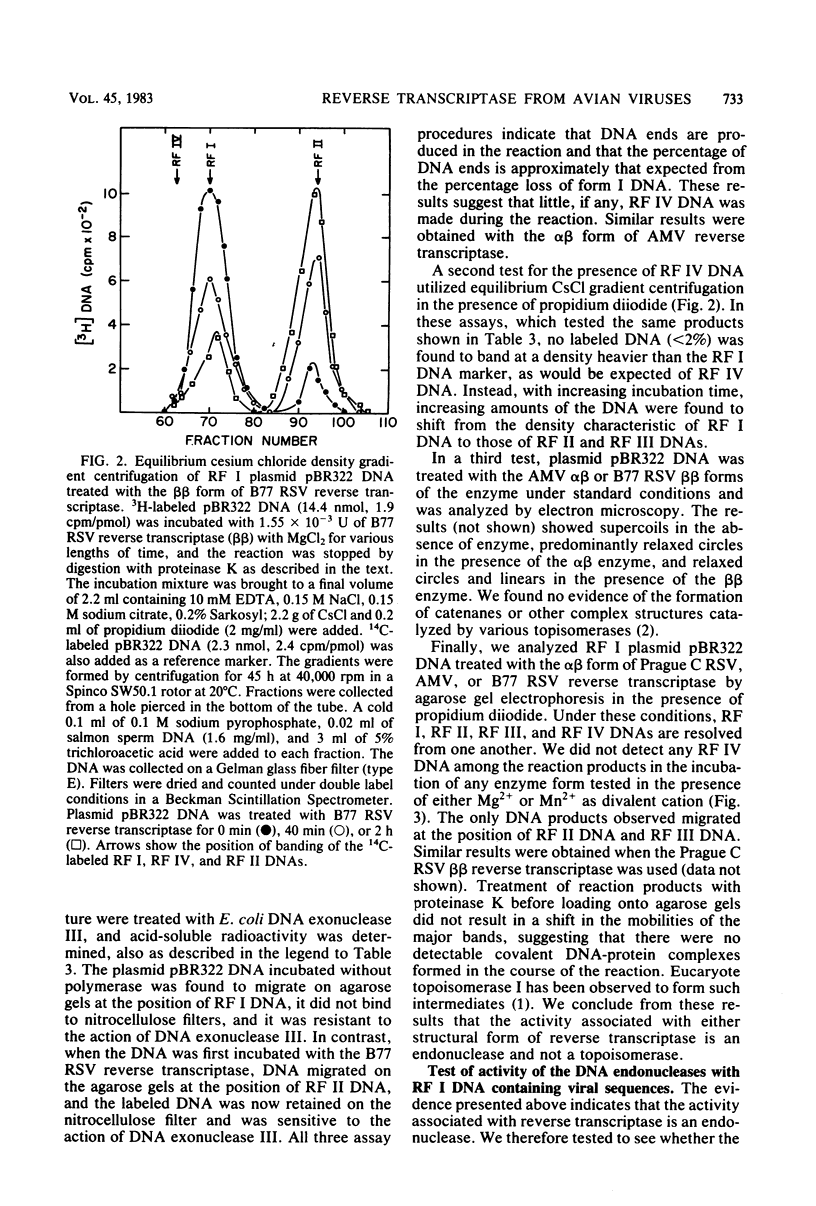
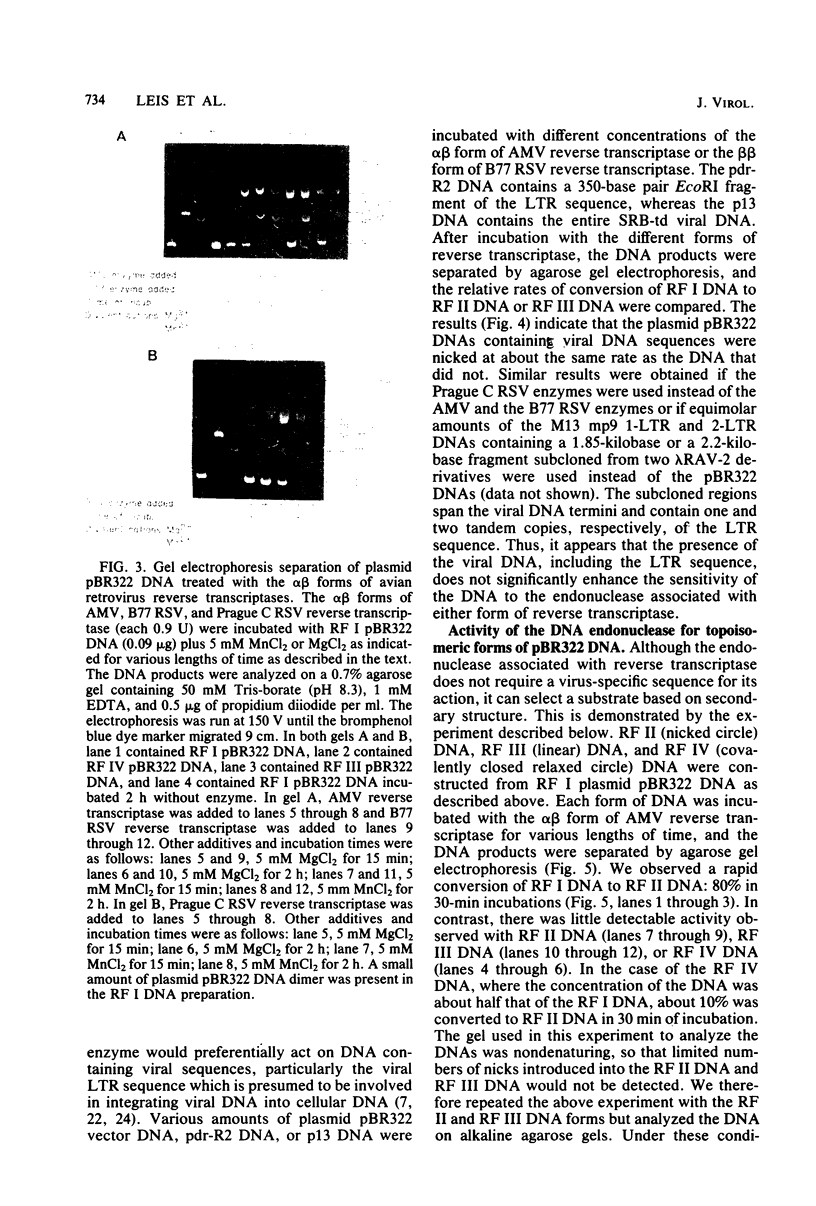
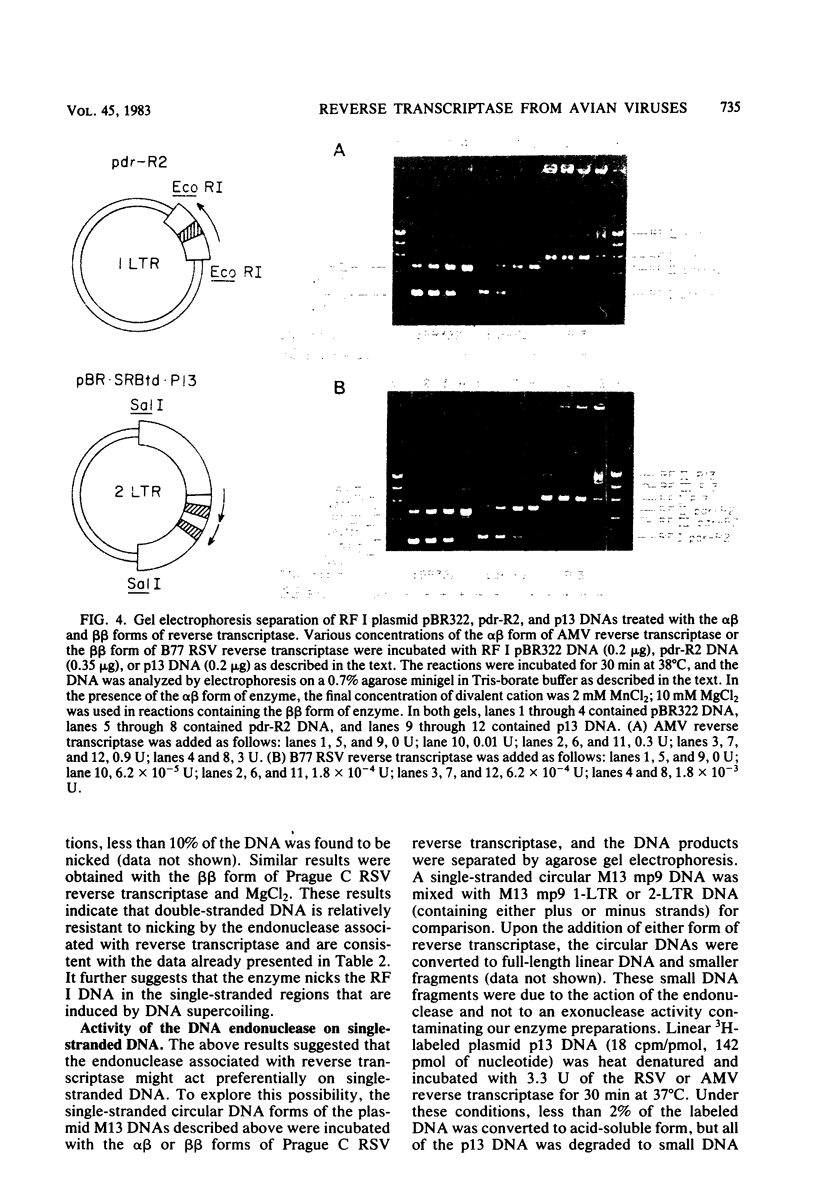
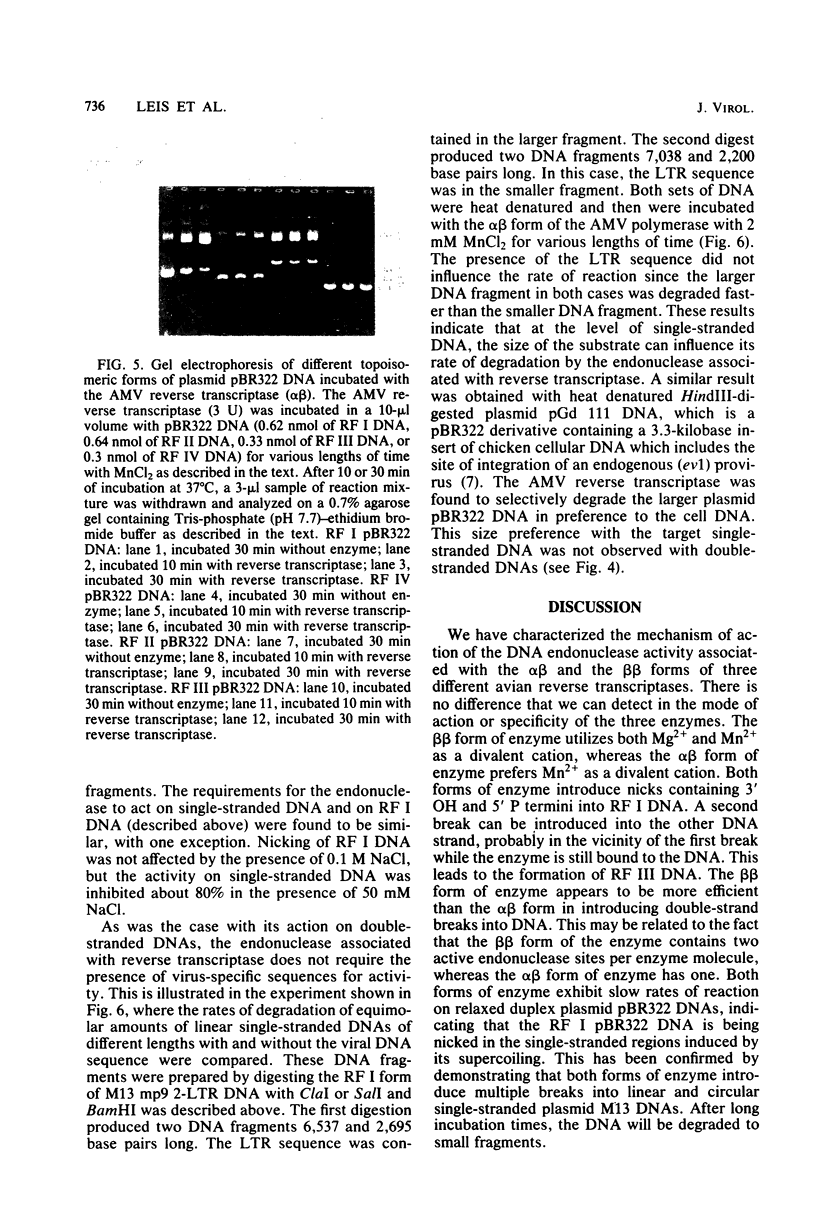
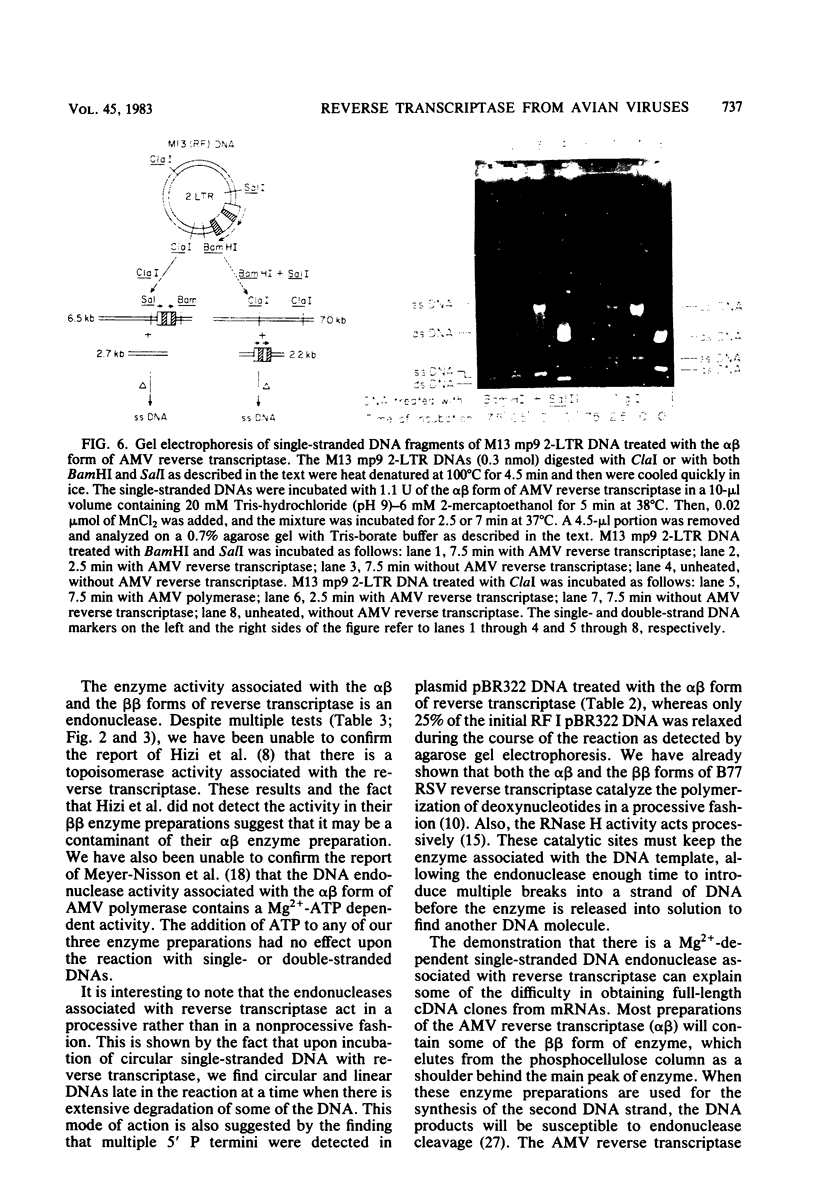
Preparations of the αβ and the ββ forms of reverse transcriptase from the Prague C strain of Rous sarcoma virus grown in chicken embryo fibroblasts, the αβ and the ββ forms of the enzyme from the B77 strain of Rous sarcoma virus grown in duck embryo fibroblasts, and the αβ form of reverse transcriptase from avian myeloblastosis virus have been analyzed. All these enzyme preparations contain a Mn2+ -activated endonuclease activity. The ββ form of enzyme, in addition, contains a Mg2+ -dependent endonuclease. Such an activity is barely detectable in the αβ form of enzymes. The endonuclease associated with reverse transcriptase introduces single- and double-strand breaks containing 3′ OH and 5′ P termini into RF I DNA. The conversion of RF I DNA to RF III DNA is more readily catalyzed by the ββ form of reverse transcriptase. In contrast to a recently published report by Hizi et al. (J. Virol 41:974-981, 1982), we have failed to detect the conversion of RF I DNA to covalently closed relaxed circles (RF IV DNA) by any of the αβ form of enzymes tested. RF IV DNA was not produced by the ββ form of reverse transcriptase either. We conclude that topoisomerization is not an intrinsic activity of reverse transcriptase. Although the conversion of RF I DNA to RF II DNA was found to be rapid, the endonuclease associated with reverse transcriptase acted slowly on RF II, RF III, and RF IV DNAs. Circular and linear single-stranded DNAs were also susceptible to cleavage by the endonuclease at a rate comparable to nicking of RF I DNA. This pattern of activity suggests that the endonuclease cleaves the RF I DNA in the single-stranded regions of the DNA induced by its supercoiling. The preference of the αβ and the ββ forms of the endonuclease for viral DNA was tested with Rous-associated virus type 2 and Rous sarcoma virus transformation-defective Schmidt-Ruppin B strain DNA molecularly cloned in plasmid pBR322 and M13 DNA vectors, respectively. The rate of nicking of RF I DNA containing viral DNA or partial sequences of viral DNA with one or two tandem long terminal repeats was the same as when these sequences were not present in the host vectors. A similar lack of preference was observed with single-stranded M13 DNAs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Been M. D., Champoux J. J. DNA breakage and closure by rat liver type 1 topoisomerase: separation of the half-reactions by using a single-stranded DNA substrate. Proc Natl Acad Sci U S A. 1981 May;78(5):2883–2887. doi: 10.1073/pnas.78.5.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA topoisomerases. Cell. 1980 Nov;22(2 Pt 2):327–328. doi: 10.1016/0092-8674(80)90341-4. [DOI] [PubMed] [Google Scholar]
- Gibson W., Verma I. M. Studies on the reverse transcriptase of RNA tumor viruses. Structural relatedness of two subunits of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4991–4994. doi: 10.1073/pnas.71.12.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M., Grandgenett D. P. Endonuclease activity of purified RNA-directed DNA polymerase from avian myeloblastosis virus. J Biol Chem. 1979 Mar 10;254(5):1606–1613. [PubMed] [Google Scholar]
- Golomb M., Grandgenett D. P., Mason W. Virus-coded DNA endonuclease from avian retrovirus. J Virol. 1981 May;38(2):548–555. doi: 10.1128/jvi.38.2.548-555.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshey R. M., Bukhari A. I. A mechanism of DNA transposition. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1090–1094. doi: 10.1073/pnas.78.2.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishinuma F., DeBona P. J., Astrin S., Skalka A. M. Nucleotide sequence of acceptor site and termini of integrated avian endogenous provirus ev1: integration creates a 6 bp repeat of host DNA. Cell. 1981 Jan;23(1):155–164. doi: 10.1016/0092-8674(81)90280-4. [DOI] [PubMed] [Google Scholar]
- Hizi A., Gazit A., Guthmann D., Yaniv A. DNA-processing activities associated with the purified alpha, beta 2, and alpha beta molecular forms of avian sarcoma virus RNA-dependent DNA polymerase. J Virol. 1982 Mar;41(3):974–981. doi: 10.1128/jvi.41.3.974-981.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizi A., Joklik W. K. RNA-dependent DNA polymerase of avian sarcoma virus B77. I. Isolation and partial characterization of the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Apr 10;252(7):2281–2289. [PubMed] [Google Scholar]
- Hizi A., Leis J. P., Joklik W. K. RNA-dependent DNA polymerase of avian sarcoma virus B77. II. Comparison of the catalytic properties of the alpha, beta2, and alphabeta enzyme forms. J Biol Chem. 1977 Apr 10;252(7):2290–2295. [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Ju G., Boone L., Skalka A. M. Isolation and characterization of recombinant DNA clones of avian retroviruses: size heterogeneity and instability of the direct repeat. J Virol. 1980 Mar;33(3):1026–1033. doi: 10.1128/jvi.33.3.1026-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Berkower I., Hurwitz J. Mechanism of action of ribonuclease H isolated from avian myeloblastosis virus and Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):466–470. doi: 10.1073/pnas.70.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor virus. VI. Processive mode of action of avian myeloblastosis virus polymerase. J Virol. 1976 Sep;19(3):932–939. doi: 10.1128/jvi.19.3.932-939.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Scheible P., Smith R. E. Correlation of RNA binding affinity of avian oncornavirus p19 proteins with the extent of processing of virus genome RNA in cells. J Virol. 1980 Sep;35(3):722–731. doi: 10.1128/jvi.35.3.722-731.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Smith R. E., Dierks P., Parsons J. T., Collett M. S., Faras A. J. In vitro transcription of reconstituted 35s RNA.tRNAtrp template.primer complexes by the avian oncornavirus DNA polymerase. Effect of temperature on the size of the DNA transcripts. Virology. 1978 Mar;85(1):28–42. doi: 10.1016/0042-6822(78)90409-9. [DOI] [PubMed] [Google Scholar]
- Linial M., Mason W. S. Characterization of two conditional early mutants of Rous sarcoma virus. Virology. 1973 May;53(1):258–273. doi: 10.1016/0042-6822(73)90484-4. [DOI] [PubMed] [Google Scholar]
- Muster C. J., Lee Y. S., Newbold J. E., Leis J. Physical mapping of adeno-associated virus serotype 4 DNA. J Virol. 1980 Sep;35(3):653–661. doi: 10.1128/jvi.35.3.653-661.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen-Meyer J., Raae A. J., Nes I. F. Effect of ATP on the Friend Murine leukemia virus-associated endonuclease activity and the endonuclease activity of the avian myeloblastosis virus RNA-directed DNA polymerase. J Biol Chem. 1981 Aug 10;256(15):7985–7989. [PubMed] [Google Scholar]
- Rho H. M., Grandgenett D. P., Green M. Sequence relatedness between the subunits of avian myeloblastosis virus reverse transcriptase. J Biol Chem. 1975 Jul 10;250(13):5278–5280. [PubMed] [Google Scholar]
- Schiff R. D., Grandgenett D. P. Virus-coded origin of a 32,000-dalton protein from avian retrovirus cores: structural relatedness of p32 and the beta polypeptide of the avian retrovirus DNA polymerase. J Virol. 1978 Oct;28(1):279–291. doi: 10.1128/jvi.28.1.279-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Nebes S., Leis J. Production of large amounts of 35S RNA and complementary DNA from avian RNA tumor viruses. Anal Biochem. 1977 Jan;77(1):226–234. doi: 10.1016/0003-2697(77)90308-6. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., DeLorbe W. J., Bishop J. M., Varmus H. E. Nucleotide sequence of cloned unintegrated avian sarcoma virus DNA: viral DNA contains direct and inverted repeats similar to those in transposable elements. Proc Natl Acad Sci U S A. 1981 Jan;78(1):124–128. doi: 10.1073/pnas.78.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M. The reverse transcriptase. Biochim Biophys Acta. 1977 Mar 21;473(1):1–38. doi: 10.1016/0304-419x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Yoo-Warren H., Cimbala M. A., Felz K., Monahan J. E., Leis J. P., Hanson R. W. Identification of a DNA clone to phosphoenolpyruvate carboxykinase (GTP) from rat cytosol. Alterations in phosphoenolpyruvate carboxykinase RNA levels detectable by hybridization. J Biol Chem. 1981 Oct 25;256(20):10224–10227. [PubMed] [Google Scholar]