Abstract
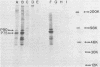
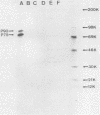
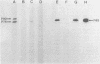
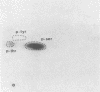
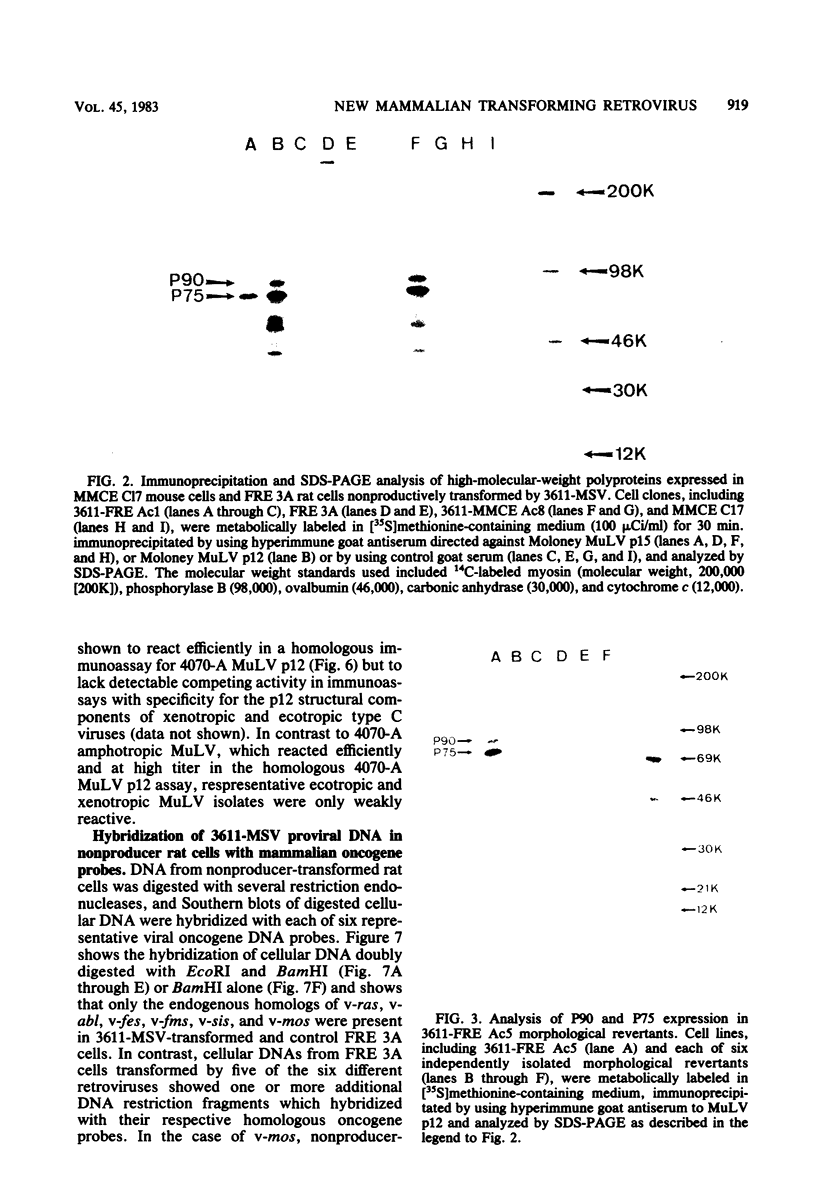
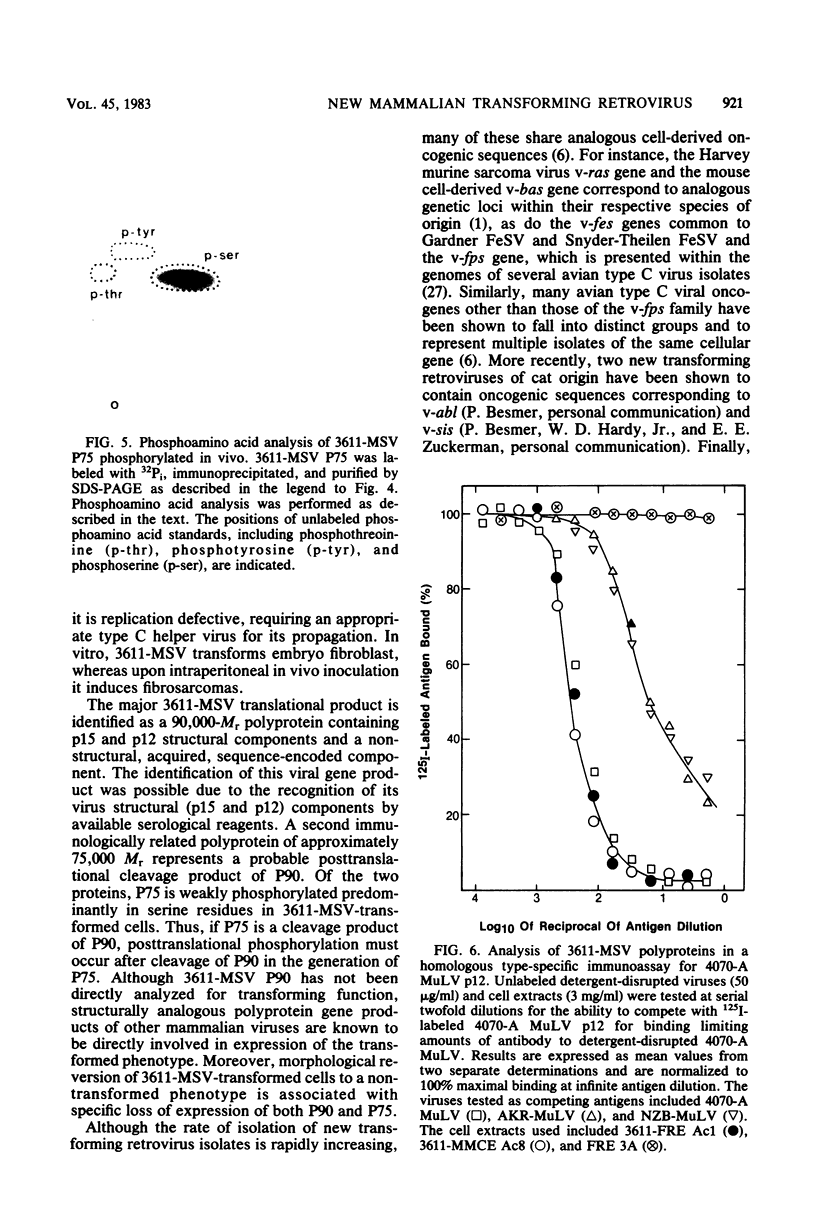
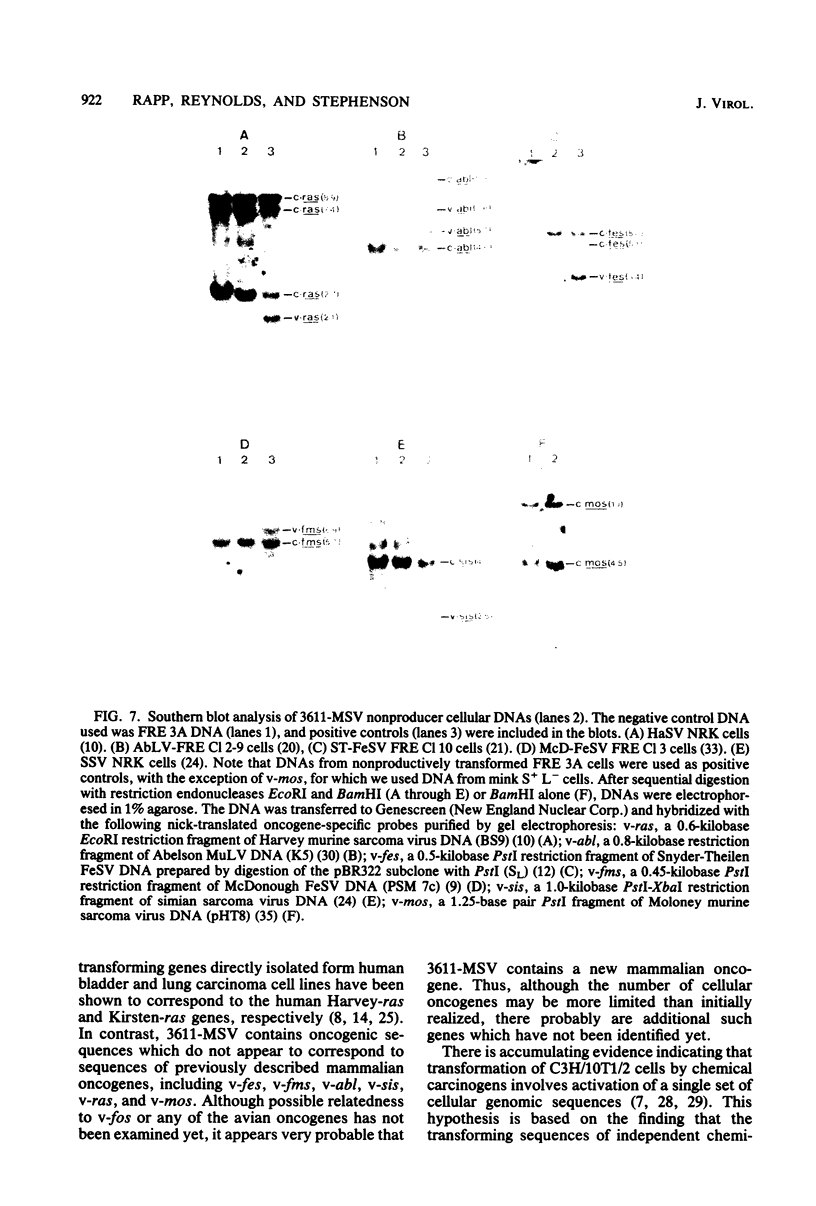
A new acute transforming type C retrovirus was isolated from mice inoculated with a virus stock obtained by iododeoxyuridine induction of methylcholanthrene-transformed C3H/10T1/2 mouse cells. This virus, designated 3611-MSV, transforms embryo fibroblasts and epithelial cells in culture and induces fibrosarcomas in vivo. 3611-MSV is replication defective, requiring a type C helper virus for propagation both in vitro and in vivo. By using endpoint transmission of 3611-MSV to MMCE C17 mouse and FRE 3A rat cells, several nonproductively transformed clonal cell lines have been derived. Pseudotype virus stocks obtained from such clones transform cells in vitro, are highly oncogenic in vivo, and exhibit host range and serological properties that are characteristic of their helper virus component. Analysis of viral antigen expression in 3611-MSV-transformed cells has led to the demonstration of a 90,000-molecular-weight (Mr) polyprotein and a 75,000-Mr probable cleavage product, both containing the amino-terminal murine leukemia virus gag gene proteins p15 and p12. In contrast to gene products of many previously described mammalian transforming viruses, 3611-MSV-encoded polyproteins lack detectable protein kinase activity, and 3611-MSV-transformed cells resemble chemically transformed cell line C3H/MCA-5, from which 3611-MuLV was originally derived, in that they do not exhibit elevated levels of phosphotyrosine. By using molecular hybridization the 3611-MSV transforming gene was found to be distinct from previously described mammalian cellular oncogenic sequences, including c-ras, c-abl, c-fes, c-fms, c-sis, and c-mos.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. R., Devare S. G., Tronick S. R., Ellis R. W., Aaronson S. A., Scolnick E. M. Generation of BALB-MuSV and Ha-MuSC by type C virus transduction of homologous transforming genes from different species. Cell. 1981 Oct;26(1 Pt 1):129–134. doi: 10.1016/0092-8674(81)90041-6. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Lauver A. V., Devare S. G. Biochemical and immunological characterization of polyproteins coded for by the McDonough, Gardner-Arnstein, and Snyder-Theilen strains of feline sarcoma virus. J Virol. 1980 Jan;33(1):196–207. doi: 10.1128/jvi.33.1.196-207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battula N., Todaro G. J. Physical map of infectious baboon type C viral DNA and sites of integration in infected cells. J Virol. 1980 Dec;36(3):709–718. doi: 10.1128/jvi.36.3.709-718.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Varmus H. E., Bishop J. M., Essex M., Hardy W. D., Jr, Martin G. S., Rosenberg N. E., Scolnick E. M., Weinberg R. A., Vogt P. K. Proposal for naming host cell-derived inserts in retrovirus genomes. J Virol. 1981 Dec;40(3):953–957. doi: 10.1128/jvi.40.3.953-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Okenquist S., Silverman L. Transforming activity of DNA of chemically transformed and normal cells. Nature. 1980 Apr 3;284(5755):418–421. doi: 10.1038/284418a0. [DOI] [PubMed] [Google Scholar]
- Der C. J., Krontiris T. G., Cooper G. M. Transforming genes of human bladder and lung carcinoma cell lines are homologous to the ras genes of Harvey and Kirsten sarcoma viruses. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3637–3640. doi: 10.1073/pnas.79.11.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner L., Fedele L. A., Garon C. F., Anderson S. J., Sherr C. J. McDonough feline sarcoma virus: characterization of the molecularly cloned provirus and its feline oncogene (v-fms). J Virol. 1982 Feb;41(2):489–500. doi: 10.1128/jvi.41.2.489-500.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Franchini G., Even J., Sherr C. J., Wong-Staal F. onc sequences (v-fes) of Snyder-Theilen feline sarcoma virus are derived from noncontiguous regions of a cat cellular gene (c-fes). Nature. 1981 Mar 12;290(5802):154–157. doi: 10.1038/290154a0. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada L. F., Tabin C. J., Shih C., Weinberg R. A. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982 Jun 10;297(5866):474–478. doi: 10.1038/297474a0. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Birkenmeier E., Bonner T. I., Gonda M. A., Gunnell M. Genome structure of mink cell focus-forming murine leukemia virus in epithelial mink lung cells transformed vitro by iododeoxyuridine-induced C3H/MuLV cells. J Virol. 1983 Feb;45(2):740–754. doi: 10.1128/jvi.45.2.740-754.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Keski-Oja J., Heine U. I. Establishment and characterization of the epithelial mouse embryo cell line MMC-E. Cancer Res. 1979 Oct;39(10):4111–4118. [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C., Reznikoff C. A., Heidelberger C. Endogenous oncornaviruses in chemically induced transformation. I. Transformation independent of virus production. Virology. 1975 Jun;65(2):392–409. doi: 10.1016/0042-6822(75)90045-8. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Todaro C. Generation of new mouse sarcoma viruses in cell culture. Science. 1978 Sep 1;201(4358):821–824. doi: 10.1126/science.210501. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Todaro G. J. Generation of oncogenic mouse type C viruses: in vitro selection of carcinoma-inducing variants. Proc Natl Acad Sci U S A. 1980 Jan;77(1):624–628. doi: 10.1073/pnas.77.1.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Sacks T. L., Deobagkar D. N., Stephenson J. R. Cells nonproductively transformed by Abelson murine leukemia virus express a high molecular weight polyprotein containing structural and nonstructural components. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3974–3978. doi: 10.1073/pnas.75.8.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Van de Ven W. J., Stephenson J. R. Feline sarcoma virus P115-associated protein kinase phosphorylates tyrosine. Identification of a cellular substrate conserved during evolution. J Biol Chem. 1980 Nov 25;255(22):11040–11047. [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Robbins K. C., Devare S. G., Aaronson S. A. Molecular cloning of integrated simian sarcoma virus: genome organization of infectious DNA clones. Proc Natl Acad Sci U S A. 1981 May;78(5):2918–2922. doi: 10.1073/pnas.78.5.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa T., Hanafusa H., Stephenson J. R. Homology exists among the transforming sequences of avian and feline sarcoma viruses. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6536–6540. doi: 10.1073/pnas.77.11.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Shilo B. Z., Goldfarb M. P., Dannenberg A., Weinberg R. A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo B. Z., Weinberg R. A. Unique transforming gene in carcinogen-transformed mouse cells. Nature. 1981 Feb 12;289(5798):607–609. doi: 10.1038/289607a0. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Reddy E. P., Aaronson S. A. Abelson murine leukemia virus: molecular cloning of infectious integrated proviral DNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2077–2081. doi: 10.1073/pnas.78.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Khan A. S., Sliski A. H., Essex M. Feline oncornavirus-associated cell membrane antigen: evidence for an immunologically crossreactive feline sarcoma virus-coded protein. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5608–5612. doi: 10.1073/pnas.74.12.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Ven W. J., Reynolds F. H., Jr, Nalewaik R. P., Stephenson J. R. Characterization of a 170,000-dalton polyprotein encoded by the McDonough strain of feline sarcoma virus. J Virol. 1980 Jul;35(1):165–175. doi: 10.1128/jvi.35.1.165-175.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Ven W. J., Reynolds F. H., Jr, Stephenson J. R. The nonstructural components of polyproteins encoded by replication-defective mammalian transforming retroviruses are phosphorylated and have associated protein kinase activity. Virology. 1980 Feb;101(1):185–197. doi: 10.1016/0042-6822(80)90495-x. [DOI] [PubMed] [Google Scholar]
- Vande Woude G. F., Oskarsson M., McClements W. L., Enquist L. W., Blair D. G., Fischinger P. J., Maizel J. V., Sullivan M. Characterization of integrated Moloney Sarcoma proviruses and flanking host sequences cloned in bacteriophage lambda. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):735–745. doi: 10.1101/sqb.1980.044.01.079. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]