Abstract
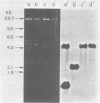
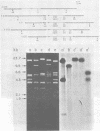
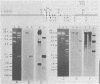
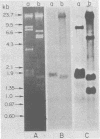
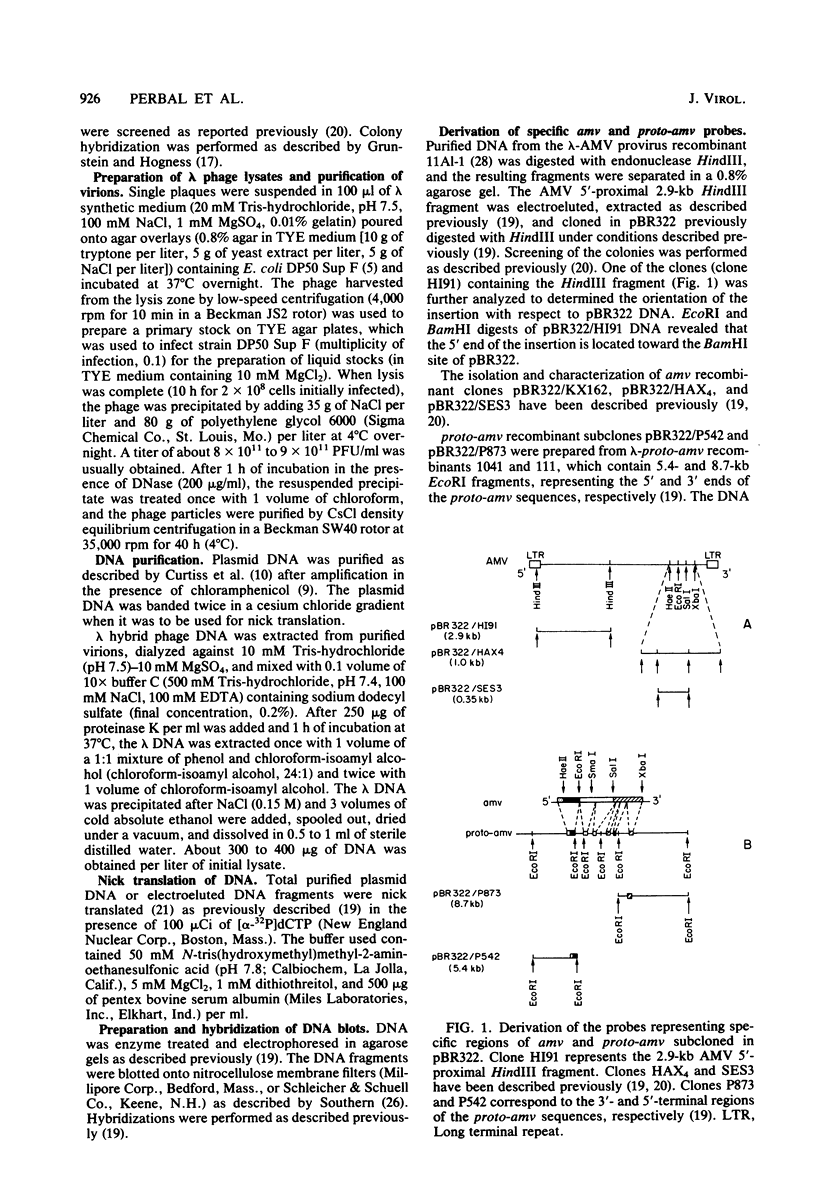
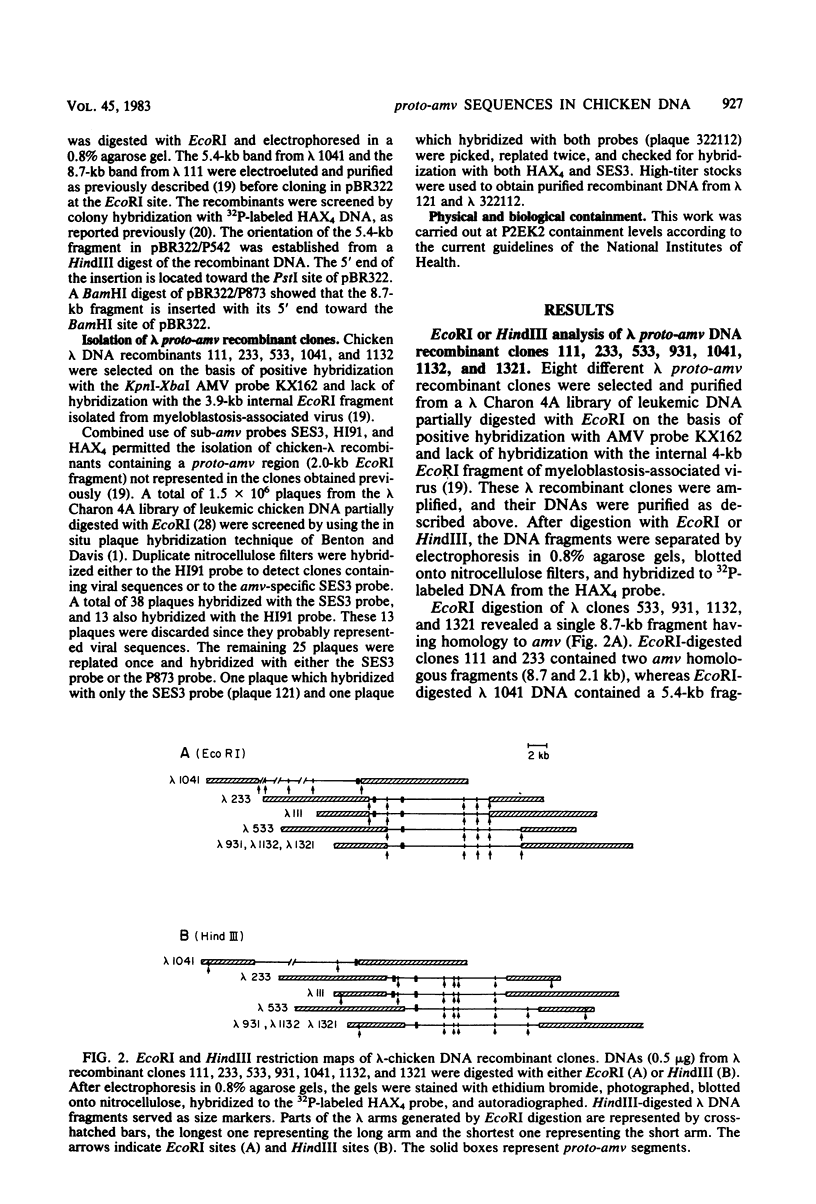
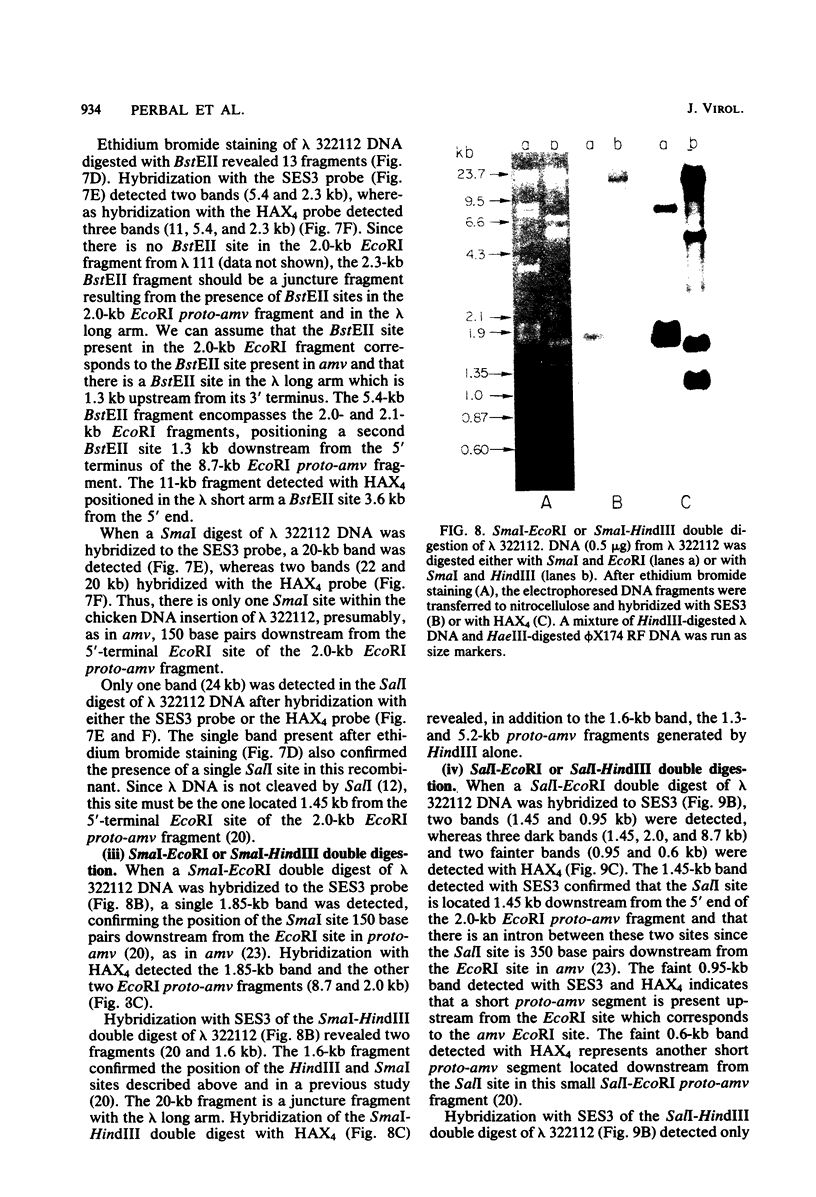
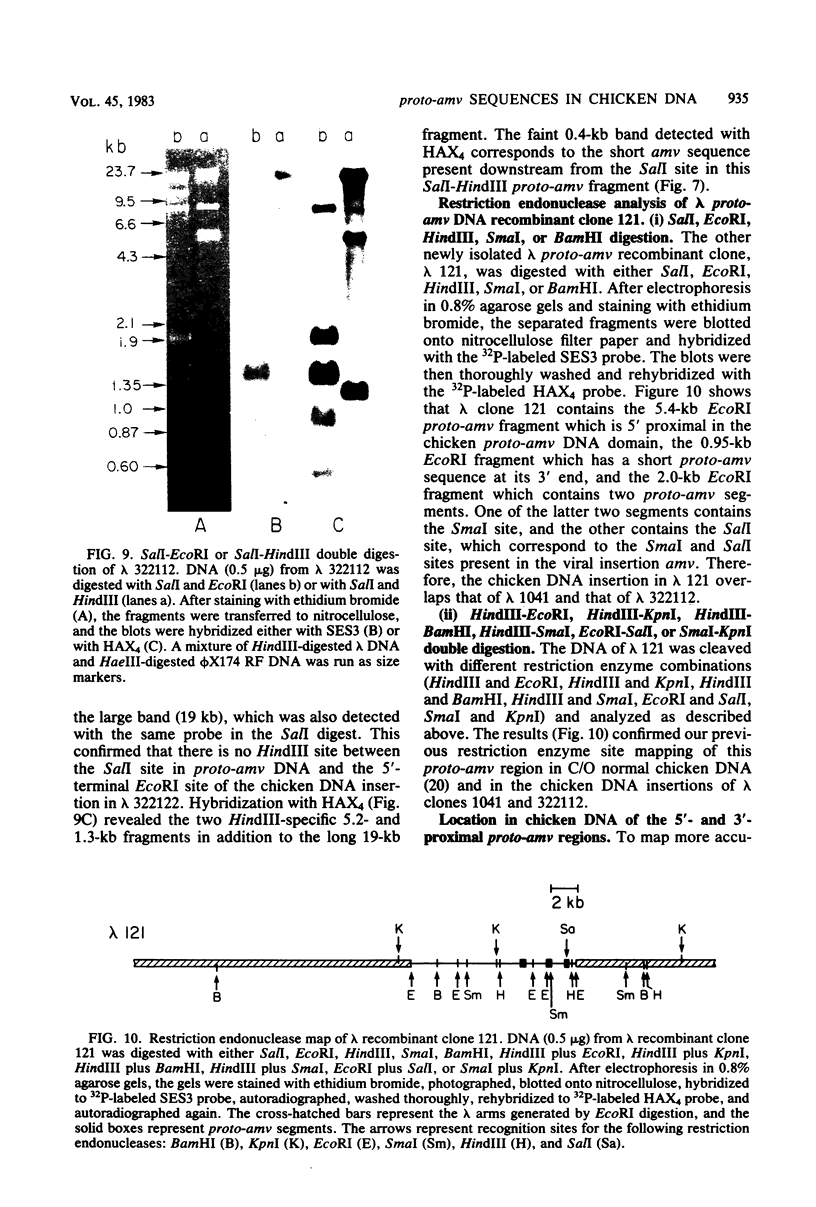
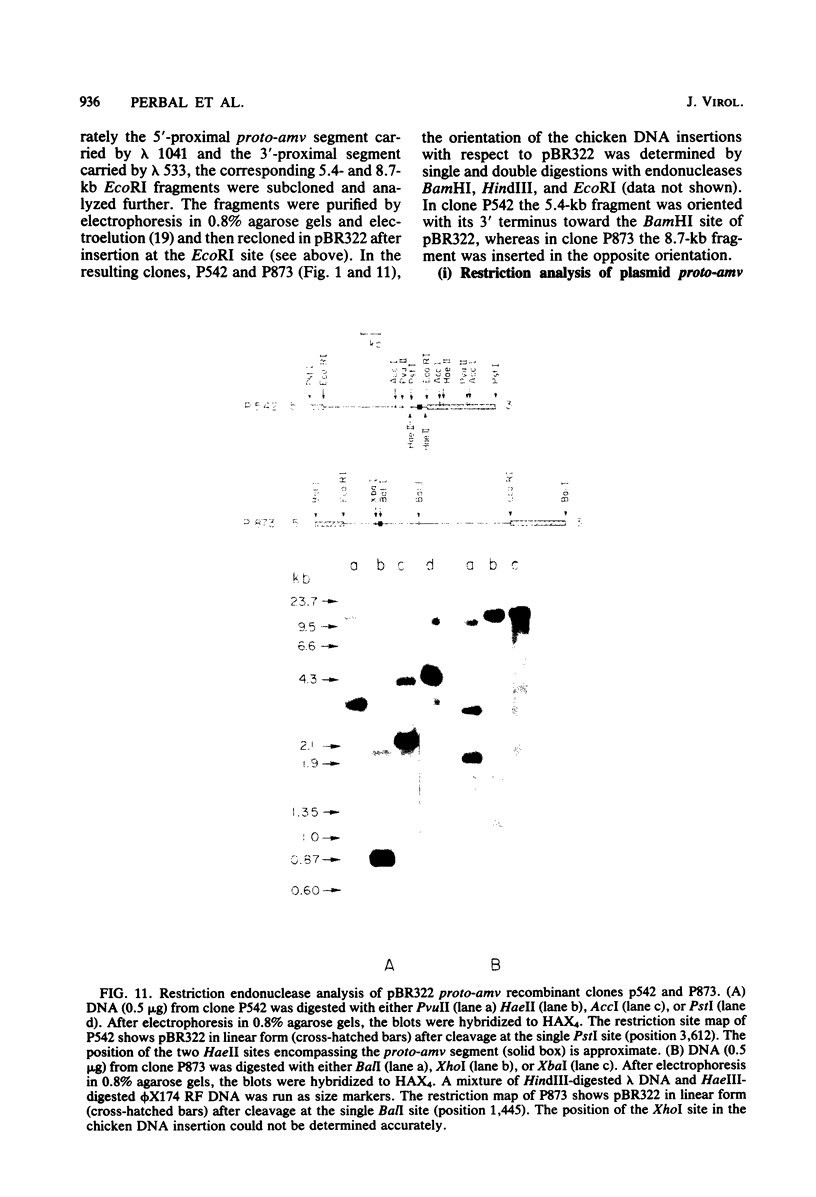
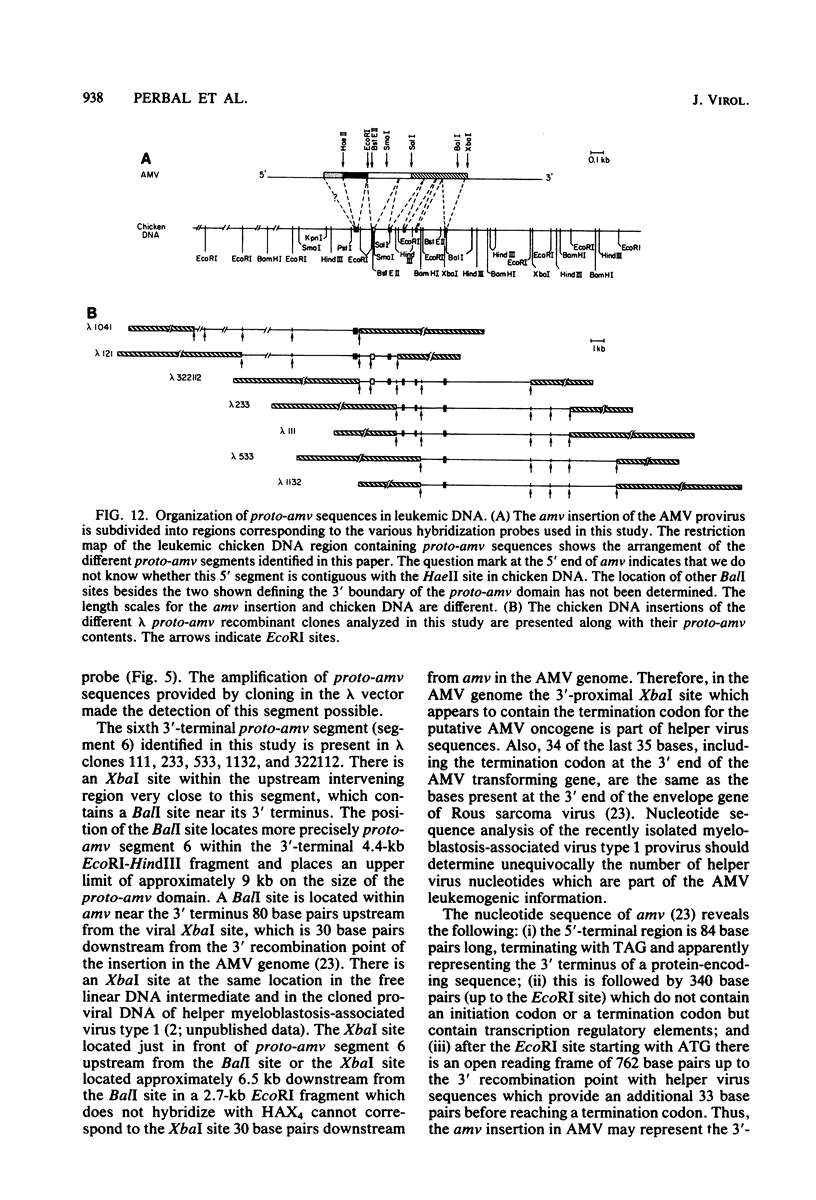
Several lambda proto-amv recombinants isolated from a lambda Charon 4A library of leukemic chicken DNA were analyzed by using various restriction endonucleases and hybridization with specific probes representing different regions of the transforming gene of avian myeloblastosis virus. The position of 30 sites for 11 different restriction endonucleases was established in the proto-amv region of chicken DNA. Identical restriction endonuclease maps were obtained for the normal and leukemic DNAs in the proto-amv domain, which covers 8 to 9 kilobases of DNA. The cellular genetic elements homologous to the cellular sequence (amv) inserted into the avian myeloblastosis virus genome are contained within six major proto-amv segments which are interrupted by at least five large DNA regions lacking homology with amv.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bergmann D. G., Souza L. M., Baluda M. A. Vertebrate DNAs contain nucleotide sequences related to the transforming gene of avian myeloblastosis virus. J Virol. 1981 Nov;40(2):450–455. doi: 10.1128/jvi.40.2.450-455.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M., Courtneidge S. A., Levinson A. D., Oppermann H., Quintrell N., Sheiness D. K., Weiss S. R., Varmus H. E. Origin and function of avian retrovirus transforming genes. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):919–930. doi: 10.1101/sqb.1980.044.01.099. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Enemies within: the genesis of retrovirus oncogenes. Cell. 1981 Jan;23(1):5–6. doi: 10.1016/0092-8674(81)90263-4. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Canaani E., Tronick S. R., Robbins K. C., Andersen P. R., Dunn C. Y., Aaronson S. A. Cellular origin of the transforming gene of Moloney murine sarcoma virus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):727–734. doi: 10.1101/sqb.1980.044.01.078. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Gallo R. C., Wong-Staal F. A human onc gene homologous to the transforming gene (v-sis) of simian sarcoma virus. Nature. 1981 Jul 2;292(5818):31–35. doi: 10.1038/292031a0. [DOI] [PubMed] [Google Scholar]
- DeFeo D., Gonda M. A., Young H. A., Chang E. H., Lowy D. R., Scolnick E. M., Ellis R. W. Analysis of two divergent rat genomic clones homologous to the transforming gene of Harvey murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3328–3332. doi: 10.1073/pnas.78.6.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Transforming genes of retroviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):13–29. doi: 10.1101/sqb.1980.044.01.005. [DOI] [PubMed] [Google Scholar]
- Franchini G., Even J., Sherr C. J., Wong-Staal F. onc sequences (v-fes) of Snyder-Theilen feline sarcoma virus are derived from noncontiguous regions of a cat cellular gene (c-fes). Nature. 1981 Mar 12;290(5802):154–157. doi: 10.1038/290154a0. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Perbal B., Baluda M. A. Avian myeloblastosis virus transforming gene is related to unique chicken DNA regions separated by at least one intervening sequence. J Virol. 1982 Jan;41(1):250–257. doi: 10.1128/jvi.41.1.250-257.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B., Baluda M. A. Organization of chicken DNA sequences homologous to the transforming gene of avian myeloblastosis virus. I. Restriction enzyme analysis of total DNA from normal and leukemic cells. J Virol. 1982 Nov;44(2):586–594. doi: 10.1128/jvi.44.2.586-594.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Rushlow K. E., Lautenberger J. A., Papas T. S., Baluda M. A., Perbal B., Chirikjian J. G., Reddy E. P. Nucleotide sequence of the transforming gene of avian myeloblastosis virus. Science. 1982 Jun 25;216(4553):1421–1423. doi: 10.1126/science.6283631. [DOI] [PubMed] [Google Scholar]
- Shalloway D., Zelenetz A. D., Cooper G. M. Molecular cloning and characterization of the chicken gene homologous to the transforming gene of Rous sarcoma virus. Cell. 1981 May;24(2):531–541. doi: 10.1016/0092-8674(81)90344-5. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Bishop J. M. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol. 1979 Aug;31(2):514–521. doi: 10.1128/jvi.31.2.514-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Briskin M. J., Hillyard R. L., Baluda M. A. Identification of the avian myeloblastosis virus genome. II. Restriction endonuclease analysis of DNA from lambda proviral recombinants and leukemic myeoblast clones. J Virol. 1980 Nov;36(2):325–336. doi: 10.1128/jvi.36.2.325-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Komaromy M. C., Baluda M. A. Identification of a proviral genome associated with avian myeloblastic leukemia. Proc Natl Acad Sci U S A. 1980 May;77(5):3004–3008. doi: 10.1073/pnas.77.5.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Strommer J. N., Hillyard R. L., Komaromy M. C., Baluda M. A. Cellular sequences are present in the presumptive avian myeloblastosis virus genome. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5177–5181. doi: 10.1073/pnas.77.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Varmus H. E., Bishop J. M. Nucleotide sequences related to the transforming gene of avian sarcoma virus are present in DNA of uninfected vertebrates. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4102–4106. doi: 10.1073/pnas.75.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Vennstrom B., Sheiness D., Zabielski J., Bishop J. M. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J Virol. 1982 Jun;42(3):773–779. doi: 10.1128/jvi.42.3.773-779.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Daniels D. L., Schroeder J. L., Williams B. G., Denniston-Thompson K., Moore D. D., Blattner F. R. Restriction maps for twenty-one Charon vector phages. J Virol. 1980 Jan;33(1):401–410. doi: 10.1128/jvi.33.1.401-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]