Abstract
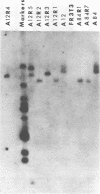
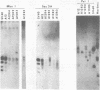
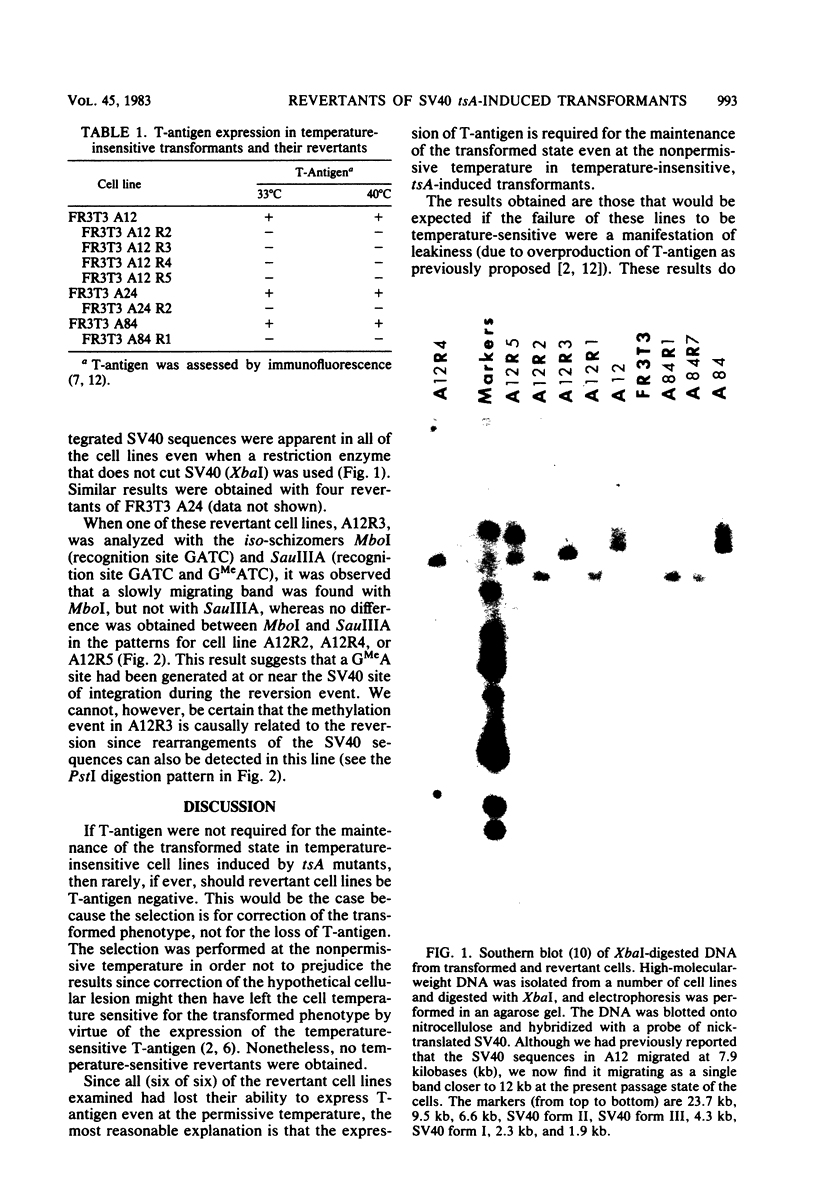
Temperature-insensitive transformants that contained simian virus 40 sequences at only one or a few sites in the rat chromosome and that were induced by a temperature-sensitive A gene mutant of simian virus 40 were used to select flat revertants (revertants that had lost the transformed phenotype). The isolation was performed at the nonpermissive temperature so as not to select against temperature-sensitive transformants. Nonetheless, all of the revertants examined had lost their ability to express the T-antigen at both temperatures, and all contained rearrangements of the integrated simian virus 40 sequences. These results are most compatible with the hypothesis that the T-antigen of simian virus 40 is required for the maintenance of the transformed state even in temperature-insensitive cell lines.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Reed S. I., Stark G. R. Characterization of the autoregulation of simian virus 40 gene A. J Virol. 1977 Oct;24(1):22–27. doi: 10.1128/jvi.24.1.22-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepelinsky A. B., Seif R., Martin R. G. Integration of the simian virus 40 genome into cellular DNA in temperature-sensitive (N) and temperature-insensitive (A) transformants of 3T3 rat and Chinese hamster lung cells. J Virol. 1980 Jul;35(1):184–193. doi: 10.1128/jvi.35.1.184-193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley S., Bender M. A., Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: effect of transformation technique on the transformed phenotype. J Virol. 1980 Jan;33(1):550–552. doi: 10.1128/jvi.33.1.550-552.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G. The transformation of cell growth and transmogrification of DNA synthesis by simian virus 40. Adv Cancer Res. 1981;34:1–68. doi: 10.1016/s0065-230x(08)60238-9. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cuzin F. Transformation of rat fibroblast cells with early mutants of polyoma (tsa) and simian virus 40 (tsA30): occurrence of either A or N transformants depends on the multiplicity of infection. J Virol. 1980 Feb;33(2):909–911. doi: 10.1128/jvi.33.2.909-911.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Growth state of the cell early after infection with simian virus 40 determines whether the maintenance of transformation will be A-gene dependent or independent. J Virol. 1979 Aug;31(2):350–359. doi: 10.1128/jvi.31.2.350-359.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinberg B., Pollack R., Topp W., Botchan M. Isolation and characterization of T antigen-negative revertants from a line of transformed rat cells containing one copy of the SV40 genome. Cell. 1978 Jan;13(1):19–32. doi: 10.1016/0092-8674(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Tenen D. G., Martin R. G., Anderson J., Livingston D. M. Biological and biochemical studies of cells transformed by simian virus 40 temperature-sensitive gene A mutants and A mutant revertants. J Virol. 1977 Apr;22(1):210–218. doi: 10.1128/jvi.22.1.210-218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]