Abstract
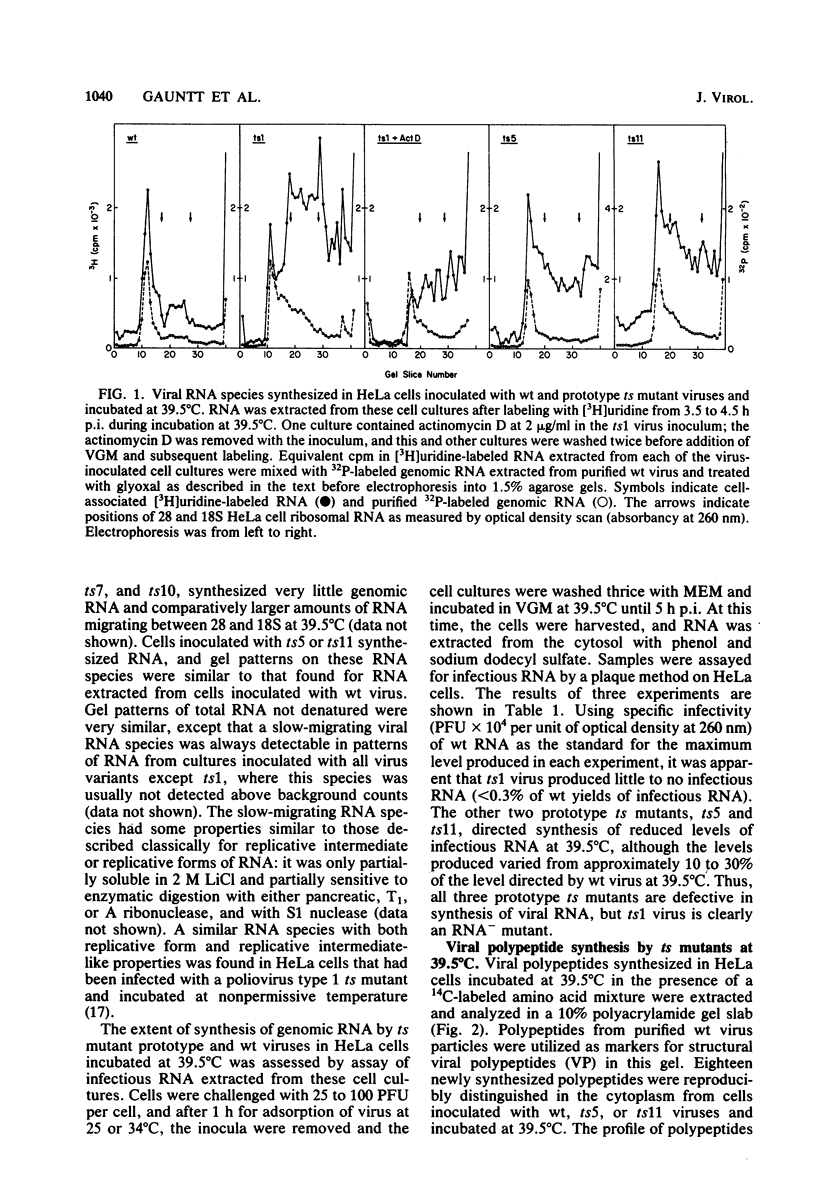
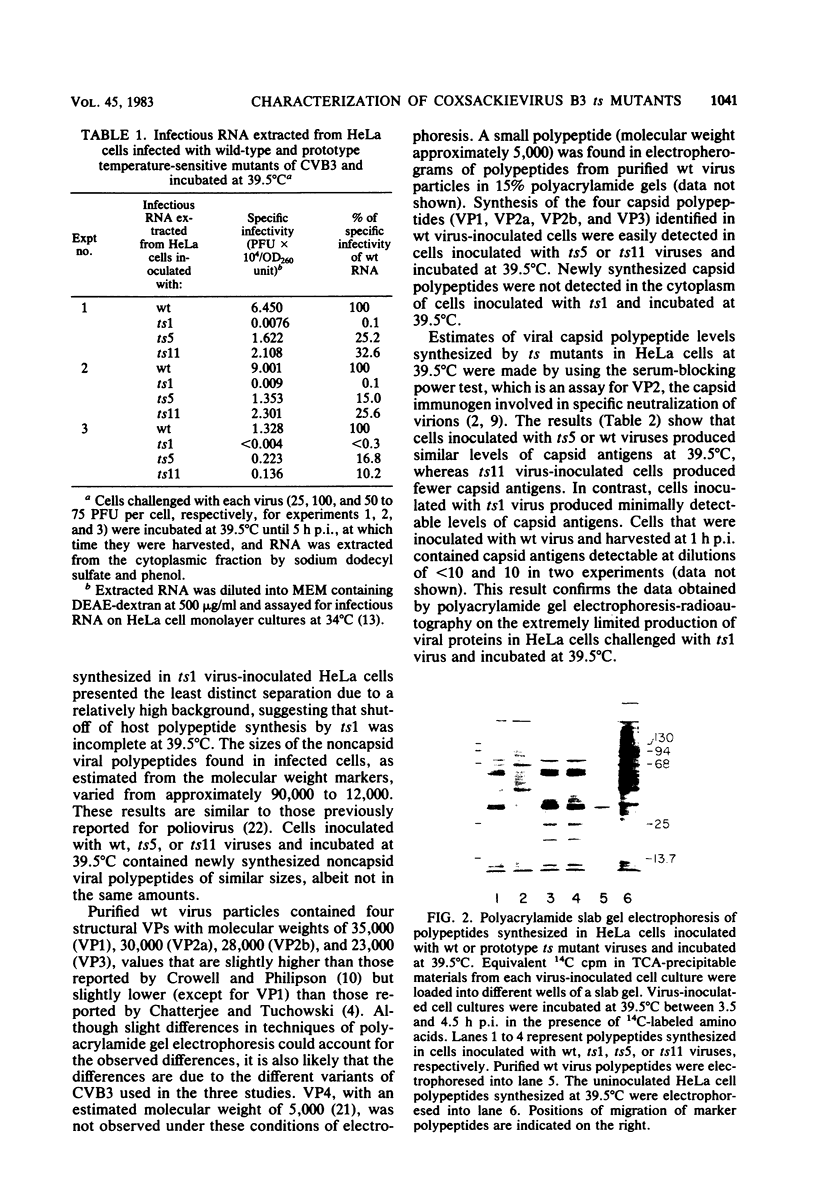
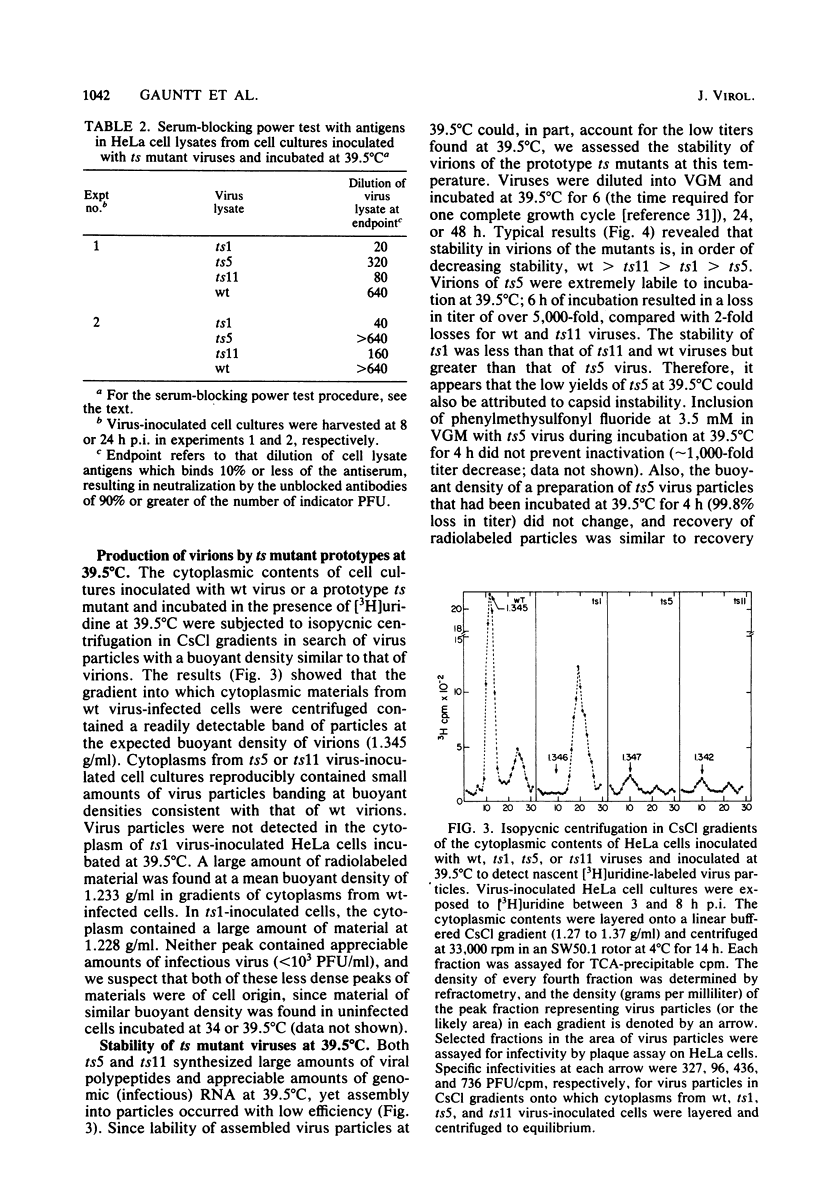
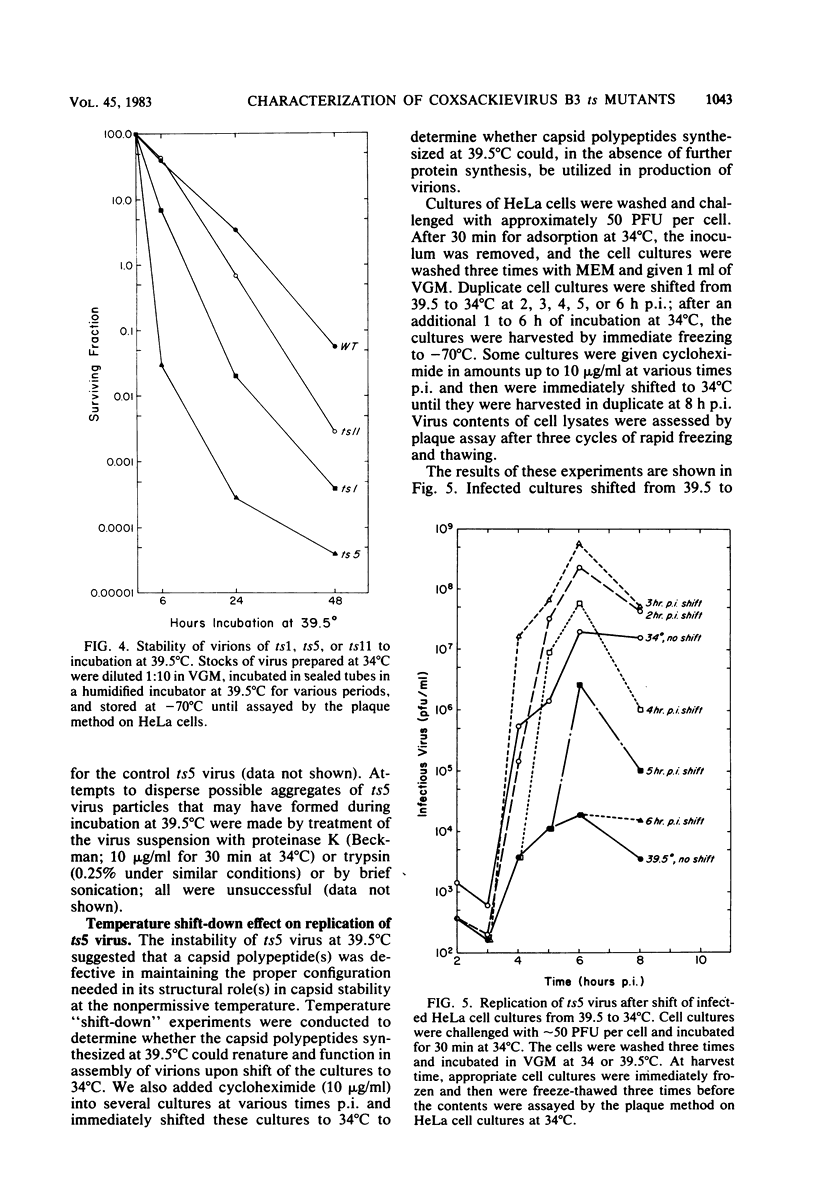
Prototype temperature-sensitive (ts) mutants of a coxsackievirus B3 parent virus capable of replication to similar levels at 34 or 39.5 degrees C were examined for the nature of the temperature-sensitive event restricting replication in HeLa cells at 39.5 degrees C. The ts mutant prototypes represented three different non-overlapping complementation groups. The ts1 mutant (complementation group III) synthesized less than 1% of the infectious genomic RNA synthesized by the coxsackievirus B3 parent virus at 39.5 degrees C and was designated an RNA- mutant. Agarose gel analysis of glyoxal-treated RNA from cells inoculated with ts1 virus revealed that cell RNA synthesis continued in the presence of synthesis of the small amount of viral RNA. This mutant was comparatively ineffective in inducing cell cytopathology and in directing synthesis of viral polypeptides, likely due to the paucity of nascent genomes for translation. The ts5 mutant (complementation group II) directed synthesis of appreciable quantities of both viral genomes (RNA+) and capsid polypeptides; however, assembly of these products into virions occurred at a low frequency, and virions assembled at 39.5 degrees C were highly unstable at that temperature. Shift-down experiments with ts5-inoculated cells showed that capsid precursor materials synthesized at 39.5 degrees C can, after shift to 34 degrees C, be incorporated into ts5 virions. We suggest that the temperature-sensitive defect in this prototype is in the synthesis of one of the capsid polypeptides that cannot renature into the correct configuration required for stability in the capsid at 39.5 degrees C. The ts11 mutant (complementation group I) also synthesized appreciable amounts of viral genomes (RNA+) and viral polypeptides at 39.5 degrees C. Assembly of ts11 virions at 39.5 degrees C occurred at a low frequency, and the stability of these virions at 39.5 degrees C was similar to that of the parent coxsackievirus B3 virions. The temperature-sensitive defect in the ts11 prototype is apparently in assembly. The differences in biochemical properties of the three prototype ts mutants at temperatures above 34 degrees C may ultimately offer insight into the differences in pathogenicity observed in neonatal mice for the three prototype ts mutants.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balanian R. Structural and functional alterations in cultured cells infected with cytocidal viruses. Prog Med Virol. 1975;19:40–83. [PubMed] [Google Scholar]
- Beatrice S. T., Katze M. G., Zajac B. A., Crowell R. L. Induction of neutralizing antibodies by the coxsackievirus B3 virion polypeptide, VP2. Virology. 1980 Jul 30;104(2):426–438. doi: 10.1016/0042-6822(80)90345-1. [DOI] [PubMed] [Google Scholar]
- Bond C. W., Swim H. E. Physiological characterization of temperature-sensitive mutants of mengovirus. J Virol. 1975 Feb;15(2):288–296. doi: 10.1128/jvi.15.2.288-296.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER P. D. RESCUE OF ONE PHENOTYPE IN MIXED INFECTIONS WITH HEAT-DEFECTIVE MUTANTS OF TYPE 1 POLIOVIRUS. Virology. 1965 Mar;25:431–438. doi: 10.1016/0042-6822(65)90064-4. [DOI] [PubMed] [Google Scholar]
- Chatterjee N. K., Tuchowski C. Comparison of capsid polypeptides of group B coxsackie-viruses and polypeptide synthesis in infected cells. Arch Virol. 1981;70(3):255–269. doi: 10.1007/BF01315132. [DOI] [PubMed] [Google Scholar]
- Cole C. N. Defective interfering (di) particles of poliovirus. Prog Med Virol. 1975;20:180–207. [PubMed] [Google Scholar]
- Courtney R. J., Steiner S. M., Benyesh-Melnick M. Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology. 1973 Apr;52(2):447–455. doi: 10.1016/0042-6822(73)90340-1. [DOI] [PubMed] [Google Scholar]
- Crowell R. L., Philipson L. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971 Oct;8(4):509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer D. N., Sunderland S., Colter J. S. Isolation and partial characterization of temperature-sensitive mutants of Mengo virus. Virology. 1976 Mar;70(1):190–194. doi: 10.1016/0042-6822(76)90250-6. [DOI] [PubMed] [Google Scholar]
- Garwes D. J., Wright P. J., Cooper P. D. Poliovirus temperature-sensitivie mutants defective in cytopathic effects are also defective in synthesis of double-stranded RNA. J Gen Virol. 1975 Apr;27(1):45–59. doi: 10.1099/0022-1317-27-1-45. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Lockart R. Z., Jr Destruction of L cells by Mengo virus: use of interferon to study the mechanism. J Virol. 1968 Jun;2(6):567–575. doi: 10.1128/jvi.2.6.567-575.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J. Synthesis of ribonucleic acids in KB cells infected with rhinovirus type 14. J Gen Virol. 1973 Nov;21(2):253–267. doi: 10.1099/0022-1317-21-2-253. [DOI] [PubMed] [Google Scholar]
- Hewlett M. J., Axelrod J. H., Antinoro N., Feld R. Isolation and preliminary characterization of temperature-sensitive mutants of poliovirus type 1. J Virol. 1982 Mar;41(3):1089–1094. doi: 10.1128/jvi.41.3.1089-1094.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. M., Newman J. W. Temperature-sensitive mutants of foot-and-mouth disease virus with altered structural polypeptides. I. Identification by electrofocusing. J Virol. 1980 Apr;34(1):59–66. doi: 10.1128/jvi.34.1.59-66.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J., Mackenzie J. S. Improved technique for the isolation of temperature-sensitive mutants of foot-and-mouth disease virus. J Virol. 1973 Sep;12(3):665–668. doi: 10.1128/jvi.12.3.665-668.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Summers D. F. Evidence for differences in size and composition of the poliovirus-specific polypeptides in infected HeLa cells. Virology. 1968 Sep;36(1):48–54. doi: 10.1016/0042-6822(68)90115-3. [DOI] [PubMed] [Google Scholar]
- McCahon D., Cooper P. D. Identification of poliovirus temperature-sensitive mutants having defects in virus structural protein. J Gen Virol. 1970 Jan;6(1):51–62. doi: 10.1099/0022-1317-6-1-51. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeve C. W., Trent D. W. Identification of Saint Louis encephalitis virus mRNA. J Virol. 1978 Feb;25(2):535–545. doi: 10.1128/jvi.25.2.535-545.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R., Slade W. R., Elworthy P., O'Sullivan M. Properties of temperature-sensitive mutants of the KENYA 3/57 strain of foot-and-mouth disease virus. J Gen Virol. 1970 Feb;6(2):213–220. doi: 10.1099/0022-1317-6-2-213. [DOI] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R. J. Isolation and preliminary characterization of temperature-sensitive mutants of encephalomyocarditis virus. J Virol. 1978 Jul;27(1):182–192. doi: 10.1128/jvi.27.1.182-192.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrom M., Bablanian R. Altered cellular morphology resulting from cytocidal virus infection. Arch Virol. 1981;70(3):173–187. doi: 10.1007/BF01315124. [DOI] [PubMed] [Google Scholar]
- Teisner B., Haahr S. Poikilothermia and susceptibility of suckling mice to Coxsackie B1 virus. Nature. 1974 Feb 22;247(5442):568–568. doi: 10.1038/247568a0. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Gauntt C. J. Isolation of Coxsackievirus B3 temperture-sensitive mutants and their assignment to complementation groups. Biochem Biophys Res Commun. 1976 May 23;76(2):368–375. doi: 10.1016/0006-291x(77)90734-3. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Nealon T., Gauntt C. J. Assessment of coxsackievirus B3 ts mutants for induction of myocarditis in a murine model. Infect Immun. 1979 Feb;23(2):486–495. doi: 10.1128/iai.23.2.486-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



