Abstract
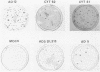
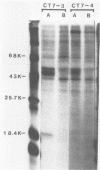
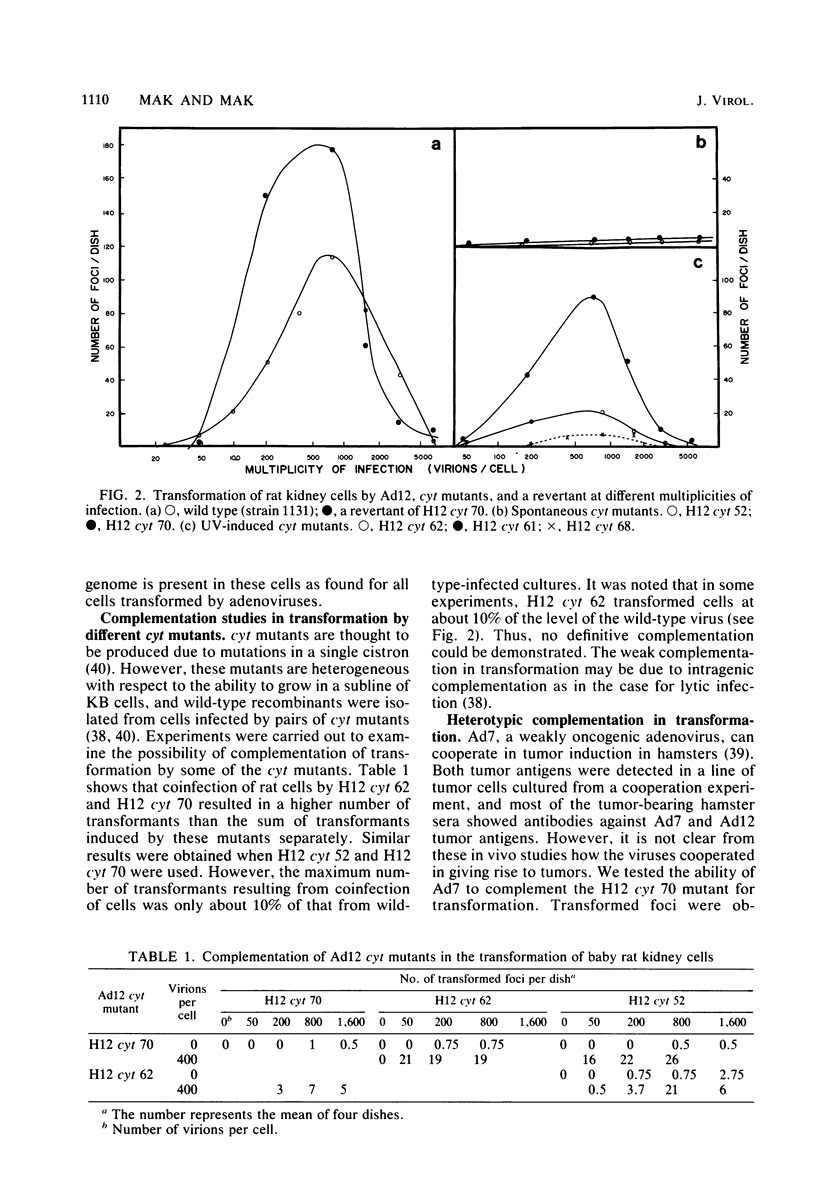
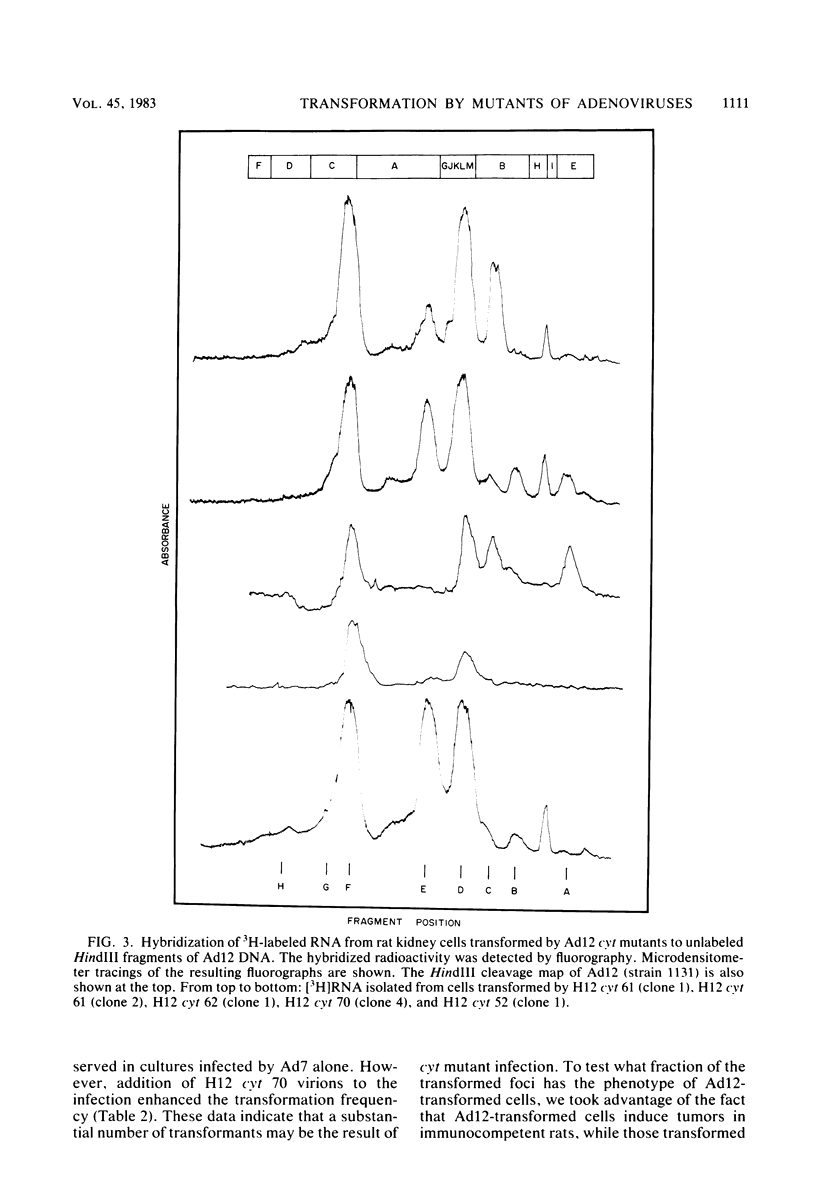
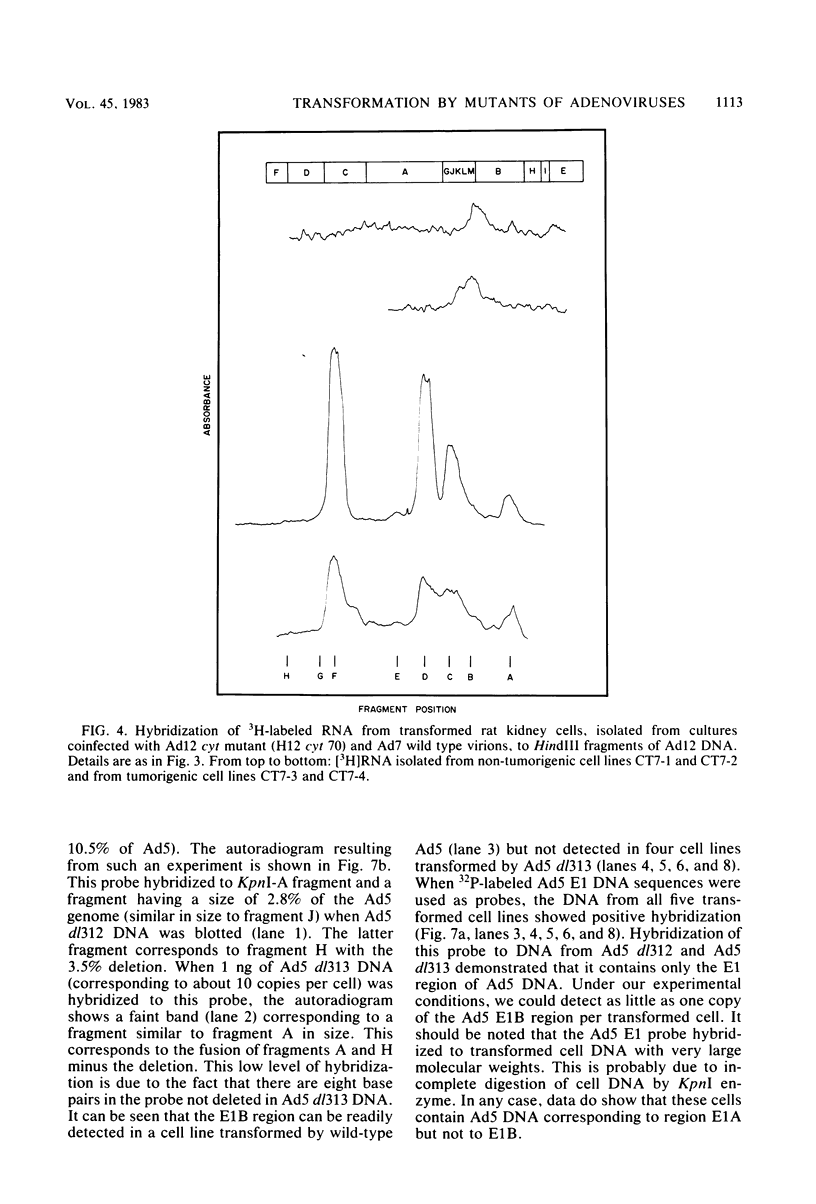
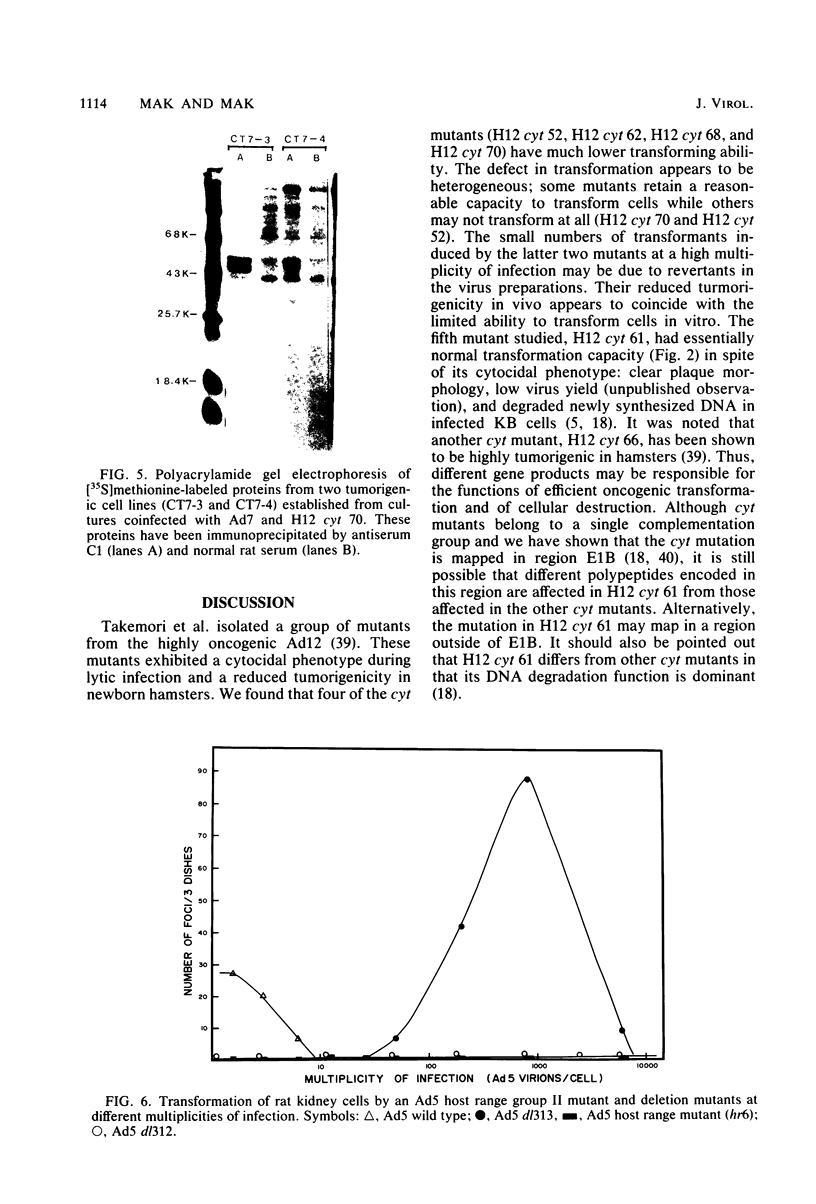
Several mutants with much reduced oncogenicity (spontaneous mutants H12 cyt 52 and H12 cyt 70 and UV-induced mutants H12 cyt 61, H12 cyt 62, and H12 cyt 68) of the highly oncogenic adenovirus type 12 (Ad12) were studied for their ability to transform primary baby rat kidney cells. Four of the mutants showed much reduced capacity to transform cells in vitro, while H12 cyt 61 transformed cells as efficiently as the wild-type virus. Viral gene expression in several cell lines established from cultures infected by cyt mutants was studied, and it was found that viral sequences belonging to the left 16% of Ad12 were always transcribed. These results suggest that the function of the transformed state is not defective in the cyt mutants studied. Heterotypic complementation studies showed that the defect(s) in a cyt mutant can be corrected by an Ad7 function. Ad5 dl 313, with a deletion between 3.5 and 10.5 map units, transformed rat cells only at high multiplicity. These results suggest that the region E1B of adenoviruses may be required for efficient transformation of rat cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Della Valle G., Fenton R. G., Basilico C. Polyoma large T antigen regulates the integration of viral DNA sequences into the genome of transformed cells. Cell. 1981 Feb;23(2):347–355. doi: 10.1016/0092-8674(81)90130-6. [DOI] [PubMed] [Google Scholar]
- Dijkema R., Dekker B. M., van der Feltz M. J., van der Eb A. J. Transformation of primary rat kidney cells by DNA fragments of weakly oncogenic adenoviruses. J Virol. 1979 Dec;32(3):943–950. doi: 10.1128/jvi.32.3.943-950.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezoe H., Fatt R. B., Mak S. Degradation of intracellular DNA in KB cells infected with cyt mutants of human adenovirus type 12. J Virol. 1981 Oct;40(1):20–27. doi: 10.1128/jvi.40.1.20-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezoe H., Mak S. Comparative studies on functions of human adenovirus type 12 and its low oncogenic mutant virions. J Virol. 1974 Oct;14(4):733–739. doi: 10.1128/jvi.14.4.733-739.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Girvitz S. C., Bacchetti S., Rainbow A. J., Graham F. L. A rapid and efficient procedure for the purification of DNA from agarose gels. Anal Biochem. 1980 Aug;106(2):492–496. doi: 10.1016/0003-2697(80)90553-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Harrison T., Williams J. Defective transforming capacity of adenovirus type 5 host-range mutants. Virology. 1978 May 1;86(1):10–21. doi: 10.1016/0042-6822(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Halbert D. N., Spector D. J., Raskas H. J. In vitro translation products specified by the transforming region of adenovirus type 2. J Virol. 1979 Sep;31(3):621–629. doi: 10.1128/jvi.31.3.621-629.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison T., Graham F., Williams J. Host-range mutants of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977 Mar;77(1):319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- Hassell J. A., Topp W. C., Rifkin D. B., Moreau P. E. Transformation of rat embryo fibroblasts by cloned polyoma virus DNA fragments containing only part of the early region. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3978–3982. doi: 10.1073/pnas.77.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Jochemsen H., Daniels G. S., Lupker J. H., van der Eb A. J. Identification and mapping of the early gene products of adenovirus type 12. Virology. 1980 Sep;105(2):551–563. doi: 10.1016/0042-6822(80)90055-0. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai Fatt R. B., Mak S. Mapping of an adenovirus function involved in the inhibition of DNA degradation. J Virol. 1982 Jun;42(3):969–977. doi: 10.1128/jvi.42.3.969-977.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lassam N. J., Bayley S. T., Graham F. L. Synthesis of DNA, late polypeptides, and infectious virus by host-range mutants of adenovirus 5 in nonpermissive cells. Virology. 1978 Jun 15;87(2):463–467. doi: 10.1016/0042-6822(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Lassam N. J., Bayley S. T., Graham F. L. Tumor antigens of human Ad5 in transformed cells and in cells infected with transformation-defective host-range mutants. Cell. 1979 Nov;18(3):781–791. doi: 10.1016/0092-8674(79)90131-4. [DOI] [PubMed] [Google Scholar]
- MCBRIDE W. D., WIENER A. IN VITRO TRANSFORMATION OF HAMSTER KIDNEY CELLS BY HUMAN ADENOVIRUS TYPE 12. Proc Soc Exp Biol Med. 1964 Apr;115:870–874. doi: 10.3181/00379727-115-29060. [DOI] [PubMed] [Google Scholar]
- Mak I., Ezoe H., Mak S. Structure and function of adenovirus type 12 defective virions. J Virol. 1979 Oct;32(1):240–250. doi: 10.1128/jvi.32.1.240-250.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak I., Mak S. Synthesis and accumulation of polyadenylated and nonpolyadenylated virus-specific RNA in adenovirus-infected cells. Virology. 1977 Oct 15;82(2):380–391. doi: 10.1016/0042-6822(77)90013-7. [DOI] [PubMed] [Google Scholar]
- Mak S., Mak I., Smiley J. R., Graham F. L. Tumorigenicity and viral gene expression in rat cells transformed by Ad 12 virions or by the EcoRI c fragment of Ad 12 DNA. Virology. 1979 Oct 30;98(2):456–460. doi: 10.1016/0042-6822(79)90568-3. [DOI] [PubMed] [Google Scholar]
- Moore J. L., Chowdhury K., Martin M. A., Israel M. A. Polyoma large tumor antigen is not required for tumorigenesis mediated by viral DNA. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1336–1340. doi: 10.1073/pnas.77.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin J., Scheidtmann K. H., Greenberg R., Westphal M., Doerfler W. Transcription of the genome of adenovirus type 12. III. Maps of stable RNA from productively infected human cells and abortively infected and transformed hamster cells. J Virol. 1976 Nov;20(2):355–372. doi: 10.1128/jvi.20.2.355-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ross S. R., Flint S. J., Levine A. J. Identification of the adenovirus early proteins and their genomic map positions. Virology. 1980 Jan 30;100(2):419–432. doi: 10.1016/0042-6822(80)90533-4. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Silver J. E., Benjamin T. L. Tumor antigen(s) in cell productively infected by wild-type polyoma virus and mutant NG-18. Proc Natl Acad Sci U S A. 1978 Jan;75(1):79–83. doi: 10.1073/pnas.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekikawa K., Shiroki K., Shimojo H., Ojima S., Fujinaga K. Transformation of a rat cell line by an adenovirus 7 DNA fragment. Virology. 1978 Jul 1;88(1):1–7. doi: 10.1016/0042-6822(78)90103-4. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Handa H., Shimojo H., Yano S., Ojima S., Fujinaga K. Establishment and characterization of rat cell lines transformed by restriction endonuclease fragments of adenovirus 12 DNA. Virology. 1977 Oct 15;82(2):462–471. doi: 10.1016/0042-6822(77)90019-8. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Maruyama K., Saito I., Fukui Y., Shimojo H. Incomplete transformation of rat cells by a deletion mutant of adenovirus type 5. J Virol. 1981 Jun;38(3):1048–1054. doi: 10.1128/jvi.38.3.1048-1054.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H., Sawada Y., Uemizu Y., Fujinaga K. Incomplete transformation of rat cells by a small fragment of adenovirus 12 DNA. Virology. 1979 May;95(1):127–136. doi: 10.1016/0042-6822(79)90407-0. [DOI] [PubMed] [Google Scholar]
- Smiley J. R., Mak S. Transcription map for adenovirus type 12 DNA. J Virol. 1978 Oct;28(1):227–239. doi: 10.1128/jvi.28.1.227-239.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnick D., Anderson M. A. Transformation-deficient adenovirus mutant defective in expression of region 1A but not region 1B. J Virol. 1982 Apr;42(1):106–113. doi: 10.1128/jvi.42.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takemori N. Genetic studies with tumorigenic adenoviruses. 3. Recombination in adenovirus type 12. Virology. 1972 Jan;47(1):157–167. doi: 10.1016/0042-6822(72)90249-8. [DOI] [PubMed] [Google Scholar]
- Takemori N., Riggs J. L., Aldrich C. D. Genetic studies with tumorigenic adenoviruses. II. Heterogeneity of cyt mutants of adenovirus type 12. Virology. 1969 May;38(1):8–15. doi: 10.1016/0042-6822(69)90122-6. [DOI] [PubMed] [Google Scholar]
- Takemori N., Riggs J. L., Aldrich C. Genetic studies with tumorigenic adenoviruses. I. Isolation of cytocidal (cyt) mutants of adenovirus type 12. Virology. 1968 Dec;36(4):575–586. doi: 10.1016/0042-6822(68)90189-x. [DOI] [PubMed] [Google Scholar]
- Tibbetts C. Physical organization of subgroup B human adenovirus genomes. J Virol. 1977 Nov;24(2):564–579. doi: 10.1128/jvi.24.2.564-579.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]