Abstract
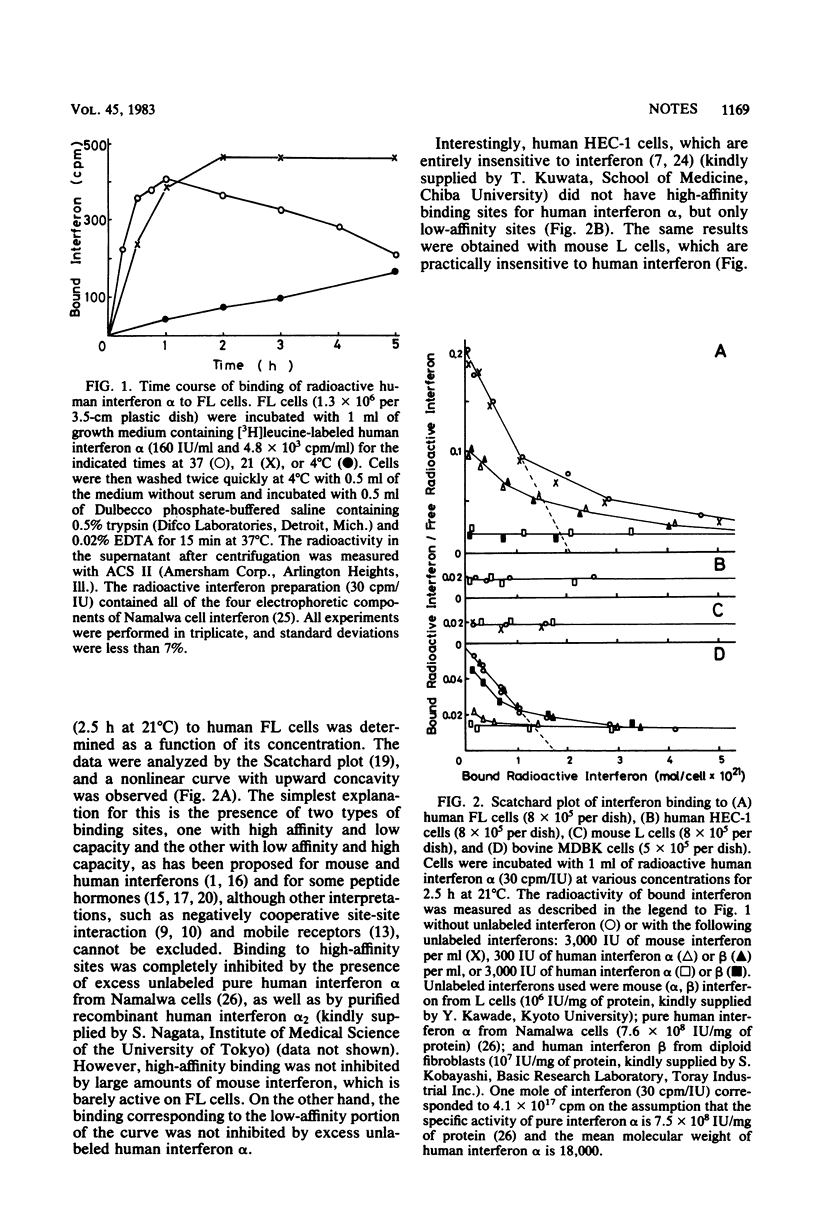
The characteristics of interferon binding to various cells with different interferon sensitivity were studied by using [3H]leucine-labeled, pure human interferon alpha from Namalwa cells. Scatchard analysis of the binding data on cells sensitive to interferon alpha (human FL and fibroblasts and bovine MDBK) indicated the presence of two kinds of binding sites with high and low affinities. The binding constants of the high-affinity sites in these cells were similar (4 X 10(10) to 11 X 10(10) M-1). Cells insensitive to human interferon alpha (human HEC-1 and mouse L cells) were shown to have only low-affinity sites, suggesting that high-affinity binding sites are indispensable for interferon sensitivity and represent interferon receptors. However, the number of sites in three human diploid fibroblast strains and one strain trisomic for chromosome 21 were not proportionally correlated to the interferon sensitivity of the cells. The high-affinity binding to human cells was completely inhibited by both nonradioactive human interferons alpha and beta in a similar manner, but binding to bovine MDBK cells, on which human interferon beta is practically inactive, was inhibited effectively only by interferon alpha and not by beta. These results suggest that the receptor for human interferon alpha is common to human interferon beta in human cells, whereas the receptor on bovine cells binds only human interferon alpha.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Belardelli F., Blanchard B., Marcucci F., Gresser I. High-affinity binding of 125I-labeled mouse interferon to a specific cell surface receptor. IV. Mouse gamma interferon and cholera toxin do not compete for the common receptor site of alpha / beta interferon. Virology. 1982 Mar;117(2):541–544. doi: 10.1016/0042-6822(82)90497-4. [DOI] [PubMed] [Google Scholar]
- Aguet M., Blanchard B. High affinity binding of 125I-Labeled mouse interferon to a specific cell surface receptor. II. Analysis of binding properties. Virology. 1981 Dec;115(2):249–261. doi: 10.1016/0042-6822(81)90108-2. [DOI] [PubMed] [Google Scholar]
- Aguet M. High-affinity binding of 125I-labelled mouse interferon to a specific cell surface receptor. Nature. 1980 Apr 3;284(5755):459–461. doi: 10.1038/284459a0. [DOI] [PubMed] [Google Scholar]
- Amir S. M., Carraway T. F., Jr, Kohn L. D., Winand R. J. The binding of thyrotropin to isolated bovine thyroid plasma membranes. J Biol Chem. 1973 Jun 10;248(11):4092–4100. [PubMed] [Google Scholar]
- Ankel H., Chany C., Galliot B., Chevalier M. J., Robert M. Antiviral effect of interferon covalently bound to sepharose. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2360–2363. doi: 10.1073/pnas.70.8.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branca A. A., Baglioni C. Evidence that types I and II interferons have different receptors. Nature. 1981 Dec 24;294(5843):768–770. doi: 10.1038/294768a0. [DOI] [PubMed] [Google Scholar]
- Chen H. Y., Sato T., Fuse A., Kuwata T., Content J. Resistance to interferon of a human adenocarcinoma cell line, HEC-1, and its sensitivity to natural killer cell action. J Gen Virol. 1981 Jan;52(Pt 1):177–181. doi: 10.1099/0022-1317-52-1-177. [DOI] [PubMed] [Google Scholar]
- Creagan R. P., Tan Y. H., Chen S., Ruddle F. H. Somatic cell genetic analysis of the interferon system. Fed Proc. 1975 Dec;34(13):2222–2226. [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Frazier W. A., Boyd L. F., Bradshaw R. A. Properties of the specific binding of 125I-nerve growth factor to responsive peripheral neurons. J Biol Chem. 1974 Sep 10;249(17):5513–5519. [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-boye D., Tovey M. Pronounced antiviral activity of human interferon on bovine and porcine cells. Nature. 1974 Oct 11;251(5475):543–545. doi: 10.1038/251543a0. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. The mobile receptor hypothesis and "cooperativity" of hormone binding. Application to insulin. Biochim Biophys Acta. 1976 May 21;433(3):482–495. doi: 10.1016/0005-2736(76)90275-3. [DOI] [PubMed] [Google Scholar]
- Krupp M. N., Livingston J. N. Insulin binding to solubilized material from fat cell membranes: evidence for two binding species. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2593–2597. doi: 10.1073/pnas.75.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen K. E., Bandu M. T., Vignaux F., Aguet M., Gressner I. Binding of 125I-labelled human alpha interferon to human lymphoid cells. Int J Cancer. 1981 Nov 15;28(5):575–582. doi: 10.1002/ijc.2910280508. [DOI] [PubMed] [Google Scholar]
- Poe M., Hoogsteen K. 5,6,7,8-Tetrahydrofolic acid. Conformation of the tetrahydropyrazine ring. J Biol Chem. 1978 Jan 25;253(2):543–546. [PubMed] [Google Scholar]
- Revel M., Bash D., Ruddle F. H. Antibodies to a cell-surface component coded by human chromosome 21 inhibit action of interferon. Nature. 1976 Mar 11;260(5547):139–141. doi: 10.1038/260139a0. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Schneider E. L., Tischfield J., Epstein C. J., Ruddle F. H. Human chromosome 21 dosage: effect on the expression of the interferon induced antiviral state. Science. 1974 Oct 4;186(4158):61–63. doi: 10.1126/science.186.4158.61. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Tischfield J., Ruddle F. H. The linkage of genes for the human interferon-induced antiviral protein and indophenol oxidase-B traits to chromosome G-21. J Exp Med. 1973 Feb 1;137(2):317–330. doi: 10.1084/jem.137.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengris V. E., Stollar B. D., Pitha P. M. Interferon externalization by producing cell before induction of antiviral state. Virology. 1975 Jun;65(2):410–417. doi: 10.1016/0042-6822(75)90046-x. [DOI] [PubMed] [Google Scholar]
- Verhaegen M., Divizia M., Vandenbussche P., Kuwata T., Content J. Abnormal behavior of interferon-induced enzymatic activities in an interferon-resistant cell line. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4479–4483. doi: 10.1073/pnas.77.8.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara S. Radioactive human lymphoblastoid interferon. One-step purification, regulation of heterogeneous species production and its use for radioimmunoassay. Eur J Biochem. 1982 Jul;125(3):529–533. doi: 10.1111/j.1432-1033.1982.tb06714.x. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Yanase Y., Sano T., Imai M., Nakasawa S., Mori H. Purification of human lymphoblastoid interferon by a simple procedure with high yields. J Biol Chem. 1981 Apr 25;256(8):3770–3775. [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]


