Abstract
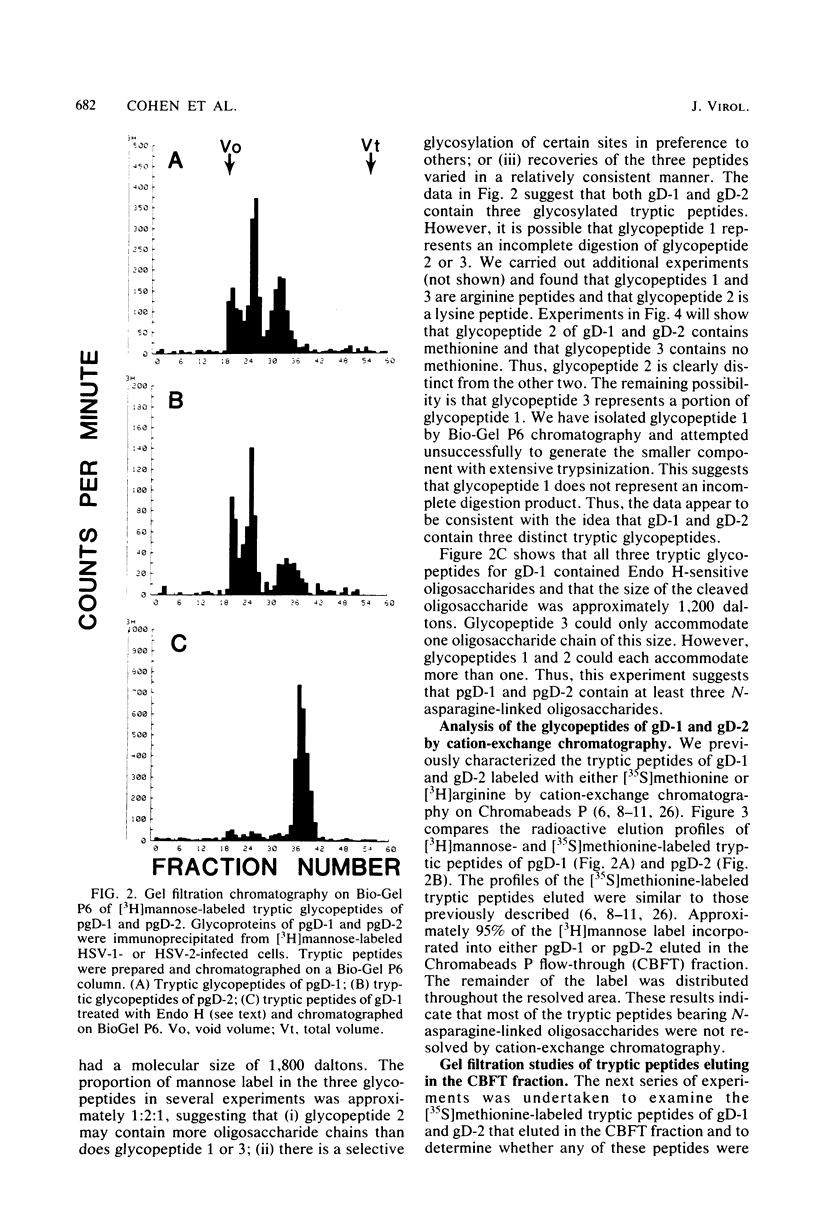
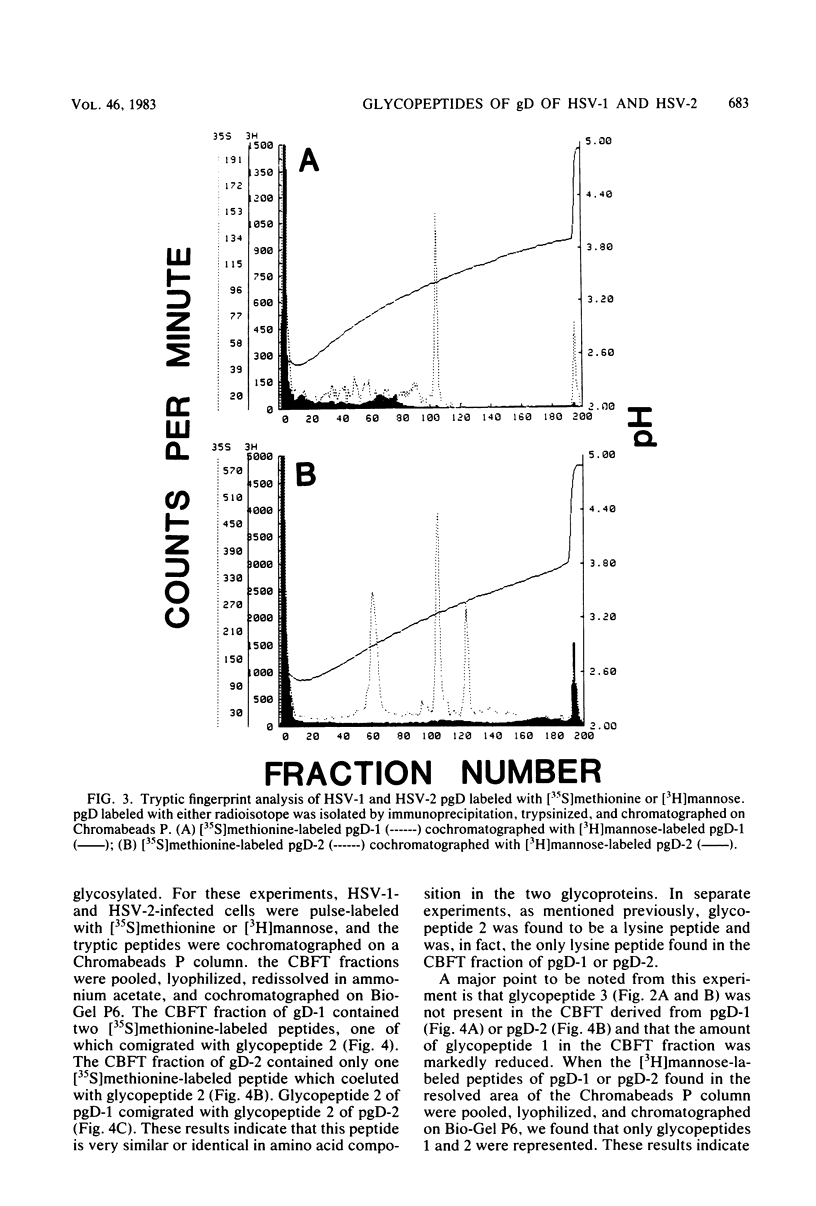
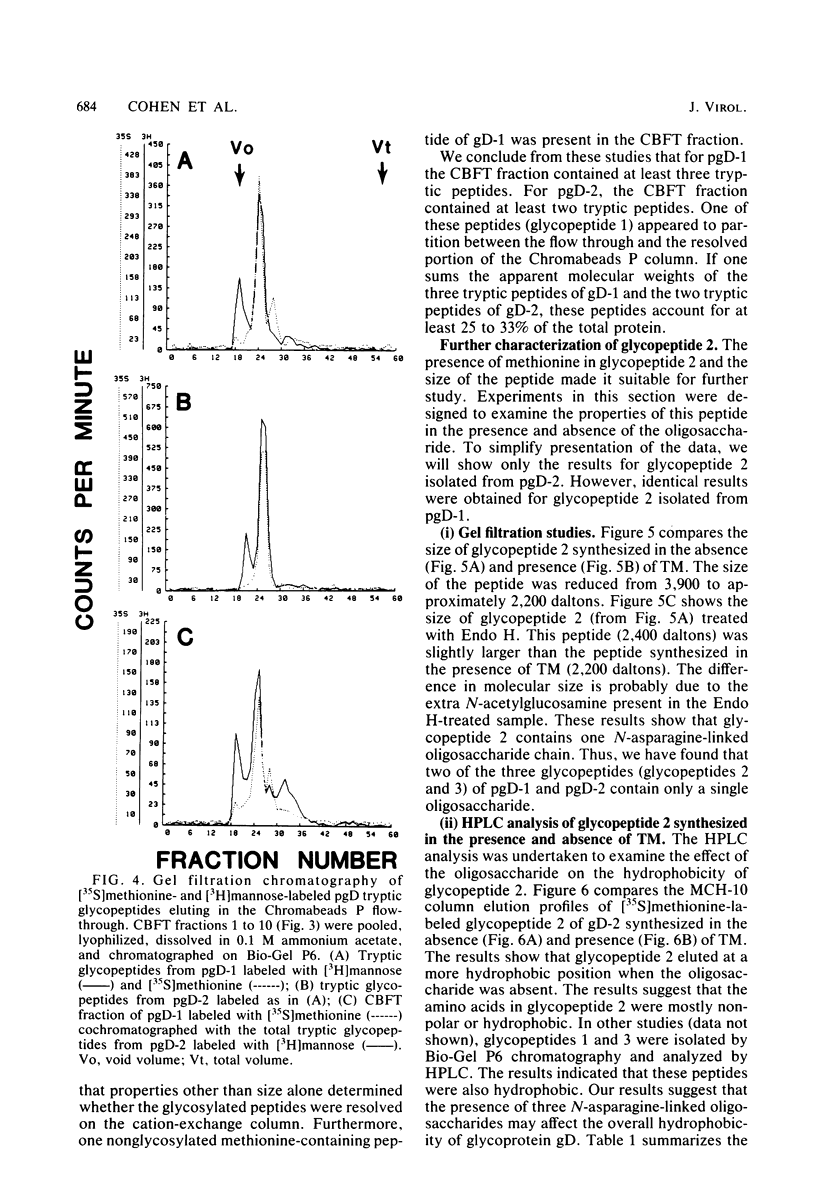
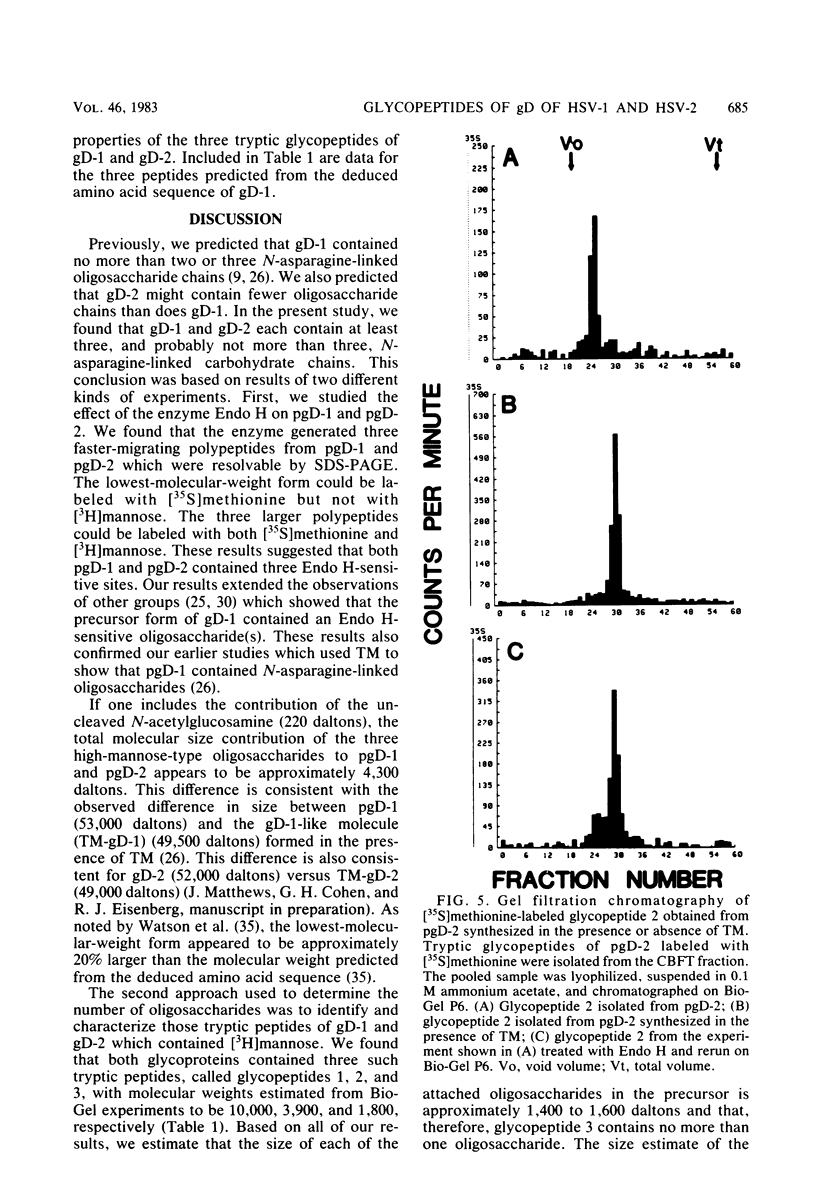
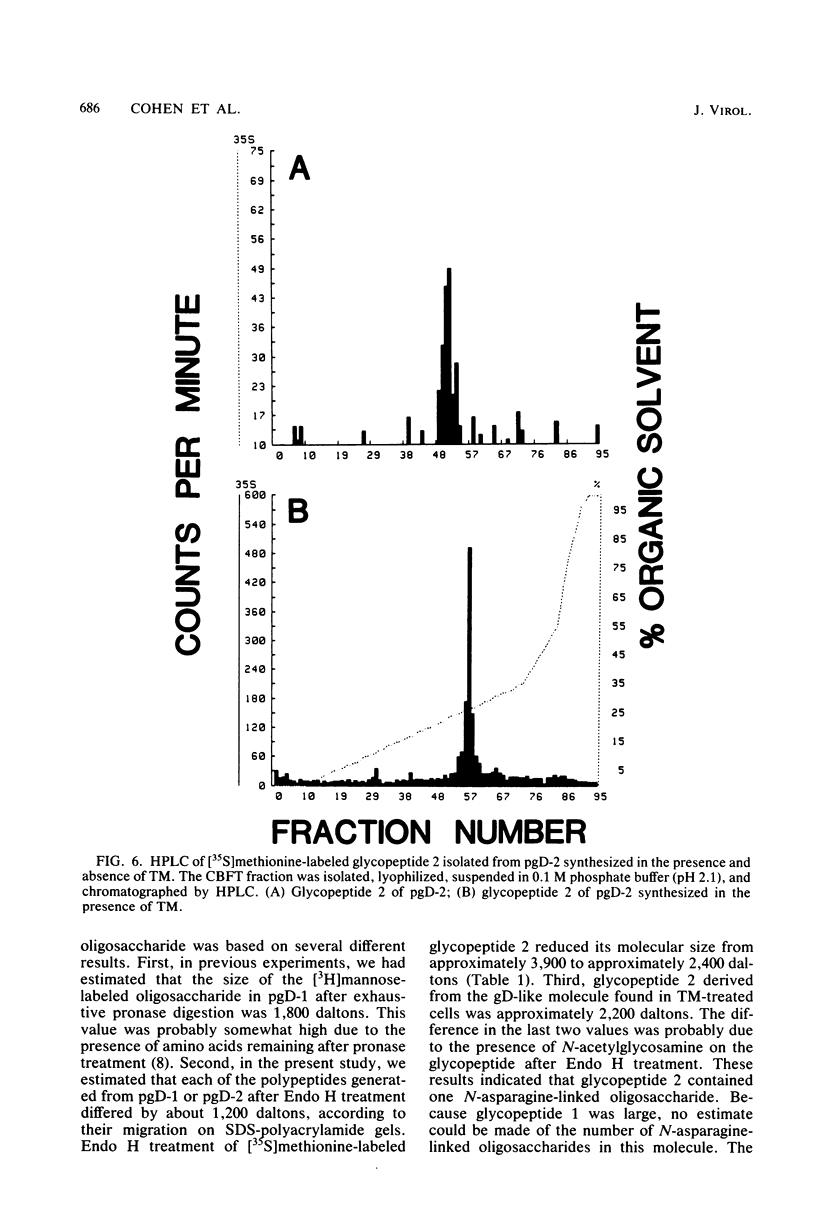
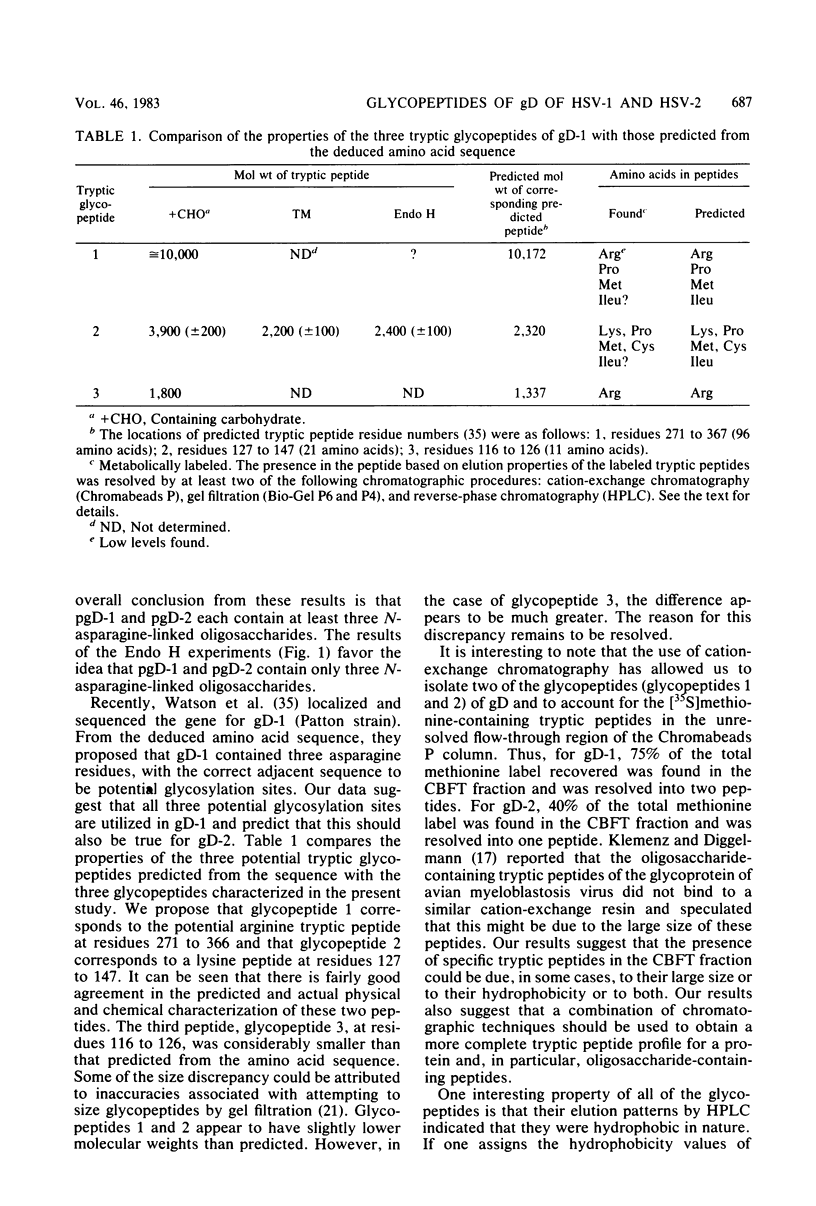
We have carried out detailed structural studies of the glycopeptides of glycoprotein gD of herpes simplex virus types 1 and 2. We first examined and compared the number of N-asparagine-linked oligosaccharides present in each glycoprotein. We found that treatment of either pgD-1 or pgD-2 with endo-β-N-acetylglucosaminidase H (Endo H) generated three polypeptides which migrated more rapidly than pgD on gradient sodium dodecyl sulfate-polyacrylamide gels. Two of the faster-migrating polypeptides were labeled with [3H]mannose, suggesting that both pgD-1 and pgD-2 contained three N-asparagine-linked oligosaccharides. Second, we characterized the [3H]mannose-labeled tryptic peptides of pgD-1 and pgD-2. We found that both glycoproteins contained three tryptic glycopeptides, termed glycopeptides 1, 2, and 3. Gel filtration studies indicated that the molecular weights of these three peptides were approximately 10,000, 3,900, and 1,800, respectively, for both pgD-1 and pgD-2. Three methods were employed to determine the size of the attached oligosaccharides. First, the [3H]mannose-labeled glycopeptides were treated with Endo H, and the released oligosaccharide was chromatographed on Bio-Gel P6. The size of this molecule was estimated to be approximately 1,200 daltons. Second, Endo H treatment of [35S]methionine-labeled glycopeptide 2 reduced the molecular size of this peptide from approximately 3,900 to approximately 2,400 daltons. Third, glycopeptide 2 isolated from the gD-like molecule formed in the presence of tunicamycin was approximately 2,200 daltons. From these experiments, the size of each N-asparagine-linked oligosaccharide was estimated to be approximately 1,400 to 1,600 daltons. Our experiments indicated that glycopeptides 2 and 3 each contained one N-asparagine-linked oligosaccharide chain. Although glycopeptide 1 was large enough to accommodate more than one oligosaccharide chain, the experiments with Endo H treatment of the glycoprotein indicated that there were only three N-asparagine-linked oligosaccharides present in pgD-1 and pgD-2. Further studies of the tryptic glycopeptides by reverse-phase high-performance liquid chromatography indicated that all of the glycopeptides were hydrophobic in nature. In the case of glycopeptide 2, we observed that when the carbohydrate was not present, the hydrophobicity of the peptide increased. The properties of the tryptic glycopeptides of pgD-1 were compared with the properties predicted from the deduced amino acid sequence of gD-1. The size and amino acid composition compared favorably for glycopeptides 1 and 2. Glycopeptide 3 appeared to be somewhat smaller than would be predicted from the deduced sequence of gD-1. It appears that all three potential glycosylation sites predicted by the amino acid sequence are utilized in gD-1 and that a similar number of glycosylation sites are present in gD-2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. Biosynthesis of the erythrocyte anion transport protein. J Biol Chem. 1981 Nov 10;256(21):11337–11344. [PubMed] [Google Scholar]
- Carter V. C., Rice P. L., Tevethia S. S. Intratypic and intertypic specificity of lymphocytes involved in the recognition of herpes simplex virus glycoproteins. Infect Immun. 1982 Jul;37(1):116–126. doi: 10.1128/iai.37.1.116-126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Eisenberg R. J. Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J Virol. 1980 Nov;36(2):429–439. doi: 10.1128/jvi.36.2.429-439.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Hydrean-Stern C., Cohen G. H. Structural analysis of precursor and product forms of type-common envelope glycoprotein D (CP-1 antigen) of herpes simplex virus type 1. J Virol. 1979 Sep;31(3):608–620. doi: 10.1128/jvi.31.3.608-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Pereira L., Hampar B., Zweig M., Cohen G. H. Effect of monoclonal antibodies on limited proteolysis of native glycoprotein gD of herpes simplex virus type 1. J Virol. 1982 Feb;41(2):478–488. doi: 10.1128/jvi.41.2.478-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Cohen G. H. Comparative structural analysis of glycoprotein gD of herpes simplex virus types 1 and 2. J Virol. 1980 Aug;35(2):428–435. doi: 10.1128/jvi.35.2.428-435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Pereira L., Long D., Cohen G. H. Purification of glycoprotein gD of herpes simplex virus types 1 and 2 by use of monoclonal antibody. J Virol. 1982 Mar;41(3):1099–1104. doi: 10.1128/jvi.41.3.1099-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J. C., Levine M., Holland T. C., Szczesiul M. S. Mutant analysis of herpes simplex virus-induced cell surface antigens: resistance to complement-mediated immune cytolysis. J Virol. 1980 Sep;35(3):672–681. doi: 10.1128/jvi.35.3.672-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarr L., Marsden H. S. Two-dimensional gel analysis of HSV type 1-induced polypeptides and glycoprotein processing. J Gen Virol. 1981 Jan;52(Pt 1):77–92. doi: 10.1099/0022-1317-52-1-77. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J Virol. 1975 Nov;16(5):1308–1326. doi: 10.1128/jvi.16.5.1308-1326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. The generation of the two envelope glycoproteins of Rous sarcoma virus from a common precursor polypeptide. Virology. 1978 Mar;85(1):63–74. doi: 10.1016/0042-6822(78)90411-7. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Wold W. S. Structures of the oligosaccharides of the glycoprotein coded by early region E3 of adenovirus 2. J Virol. 1981 Nov;40(2):440–449. doi: 10.1128/jvi.40.2.440-449.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan M., Cecka J. M., Hood L., Murphy D. B., McDevitt H. O. Peptide map analyses of murine Ia antigens of the I-E subregion using HPLC. Nature. 1979 Feb 22;277(5698):663–665. doi: 10.1038/277663a0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. The cellular site of sulfation of influenza viral glycoproteins. Virology. 1977 Jun 15;79(2):381–392. doi: 10.1016/0042-6822(77)90365-8. [DOI] [PubMed] [Google Scholar]
- Norrild B., Bjerrum O. J., Ludwig H., Vestergaard B. F. Analysis of herpes simplex virus type 1 antigens exposed on the surface of infected tissue culture cells. Virology. 1978 Jun 15;87(2):307–316. doi: 10.1016/0042-6822(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Norrild B. Immunochemistry of herpes simplex virus glycoproteins. Curr Top Microbiol Immunol. 1980;90:67–106. doi: 10.1007/978-3-642-67717-5_4. [DOI] [PubMed] [Google Scholar]
- Person S., Kousoulas K. G., Knowles R. W., Read G. S., Holland T. C., Keller P. M., Warner S. C. Glycoprotein processing in mutants of HSV-1 that induce cell fusion. Virology. 1982 Mar;117(2):293–306. doi: 10.1016/0042-6822(82)90470-6. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector J. T., Lausch R. N., Oakes J. E. Use of monoclonal antibodies for analysis of antibody-dependent immunity to ocular herpes simplex virus type 1 infection. Infect Immun. 1982 Oct;38(1):168–174. doi: 10.1128/iai.38.1.168-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Katz F. N., Lodish H. F. Glycosylation of a membrane protein is restricted to the growing polypeptide chain but is not necessary for insertion as a transmembrane protein. Cell. 1978 Dec;15(4):1447–1454. doi: 10.1016/0092-8674(78)90068-5. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Cessi F., Campadelli-Fiume G. Studies on benzhydrazone, a specific inhibitor of herpesvirus glycoprotein synthesis. Size distribution of glycopeptides and endo-beta-N-acetylglucosaminidase-H treatment. Arch Virol. 1981;70(4):331–343. doi: 10.1007/BF01320248. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982 Oct 22;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]