Abstract
Consumer effects on prey are well known for cascading through food webs and producing dramatic top-down effects on community structure and ecosystem function. Bottom-up effects of prey (primary producer) biodiversity are also well known. However, the role of consumer diversity in affecting community structure or ecosystem function is not well understood. Here, we show that herbivore species richness can be critical for maintaining the structure and function of coral reefs. In two experiments over 2 years, we constructed large cages enclosing single herbivore species, equal densities of mixed species of herbivores, or excluding herbivores and assessed effects on both seaweeds and corals. When compared with single-herbivore treatments, mixed-herbivore treatments lowered macroalgal abundance by 54–76%, enhanced cover of crustose coralline algae (preferred recruitment sites for corals) by 52–64%, increased coral cover by 22%, and prevented coral mortality. Complementary feeding by herbivorous fishes drove the herbivore richness effects, because macroalgae were unable to effectively deter fishes with different feeding strategies. Maintaining herbivore species richness appears critical for preserving coral reefs, because complementary feeding by diverse herbivores produces positive, but indirect, effects on corals, the foundation species for the ecosystem.
Keywords: biodiversity, ecosystem function, functional diversity, overfishing, seaweed–herbivore–coral interactions
Experiments assessing the functional importance of biodiversity have advanced our understanding of how biodiversity impacts ecosystem function and have demonstrated links between plant diversity and increases in resource use, primary production, and plant biomass (1, 2). However, most empirical investigations have focused on diversity of primary producers; the links between consumer diversity and ecosystem function remain understudied (2, 3). This discrepancy is unfortunate, because consumers face higher threats of extinction than plants (4), and because consumers strongly impact community organization, often altering entire ecosystems when they are removed (5, 6). Humans are selectively impacting consumers world-wide, making it critical to understand how consumer identity and richness affect ecosystem function. Recent studies suggest that consumer diversity can both directly and indirectly impact ecosystems through facilitation of primary and secondary production (7) and modification of trophic cascades (8, 9). Marine ecosystems appear at special risk of degradation after loss of consumers (10, 11), with coral reefs being prime examples (5, 12).
On coral reefs, intense feeding by herbivorous fishes and sea urchins facilitates a coral-dominated community by removing upright macroalgae (13, 14) that negatively affect the recruitment, growth, reproduction, and survivorship of corals (15–17). Further, herbivores provide resilience to reefs, because they keep macroalgae at low levels after a disturbance and allow corals to recover (18). Yet, most experimental studies of how herbivory affects coral reefs have focused on the role of herbivores as a group (17–19) rather than on the role of particular species, or of herbivore diversity, in driving community patterns (20). Theory suggests that a greater diversity of herbivores will more effectively suppress macroalgae by making it unlikely that a seaweed will be defended against all consumers (3, 21). Thus, herbivore diversity could benefit coral reefs by more effectively removing macroalgae and thereby promoting coral settlement and growth.
Previous studies examining how different herbivores affect Caribbean reefs focused on the impacts of fishes vs. the sea urchin Diadema antillarum (22, 23), but the Caribbean-wide mortality of D. antillarum in the early 1980s made herbivorous fishes the single dominant herbivore group (24). Yet, we know little about how species richness and identity of herbivorous fishes affect the structure and function of Caribbean reefs. Previous observational studies show important among-species differences in foraging patterns (25–27) and bioerosion rates (28) for herbivorous Caribbean fishes. However, these observational studies cannot assess unambiguously the direct impact of herbivore richness or the more complex and longer-term indirect effects that may cascade from altering herbivore species richness. These processes can be evaluated, with limitations, using controlled experimentation.
Here, we describe two experiments, over 2 years, where we enclosed equivalent densities and similar biomasses of single- and mixed-species groups of herbivorous fishes in large replicate cages on a reef in the Florida Keys to assess how herbivore species richness and species identity affected reef structure. In year 1, we used the redband parrotfish Sparisoma aurofrenatum and the ocean surgeonfish Acanthurus bahianus to generate the treatments; in year 2, we used the redband parrotfish and the princess parrotfish Scarus taeniopterus. These three species are among the more common species of herbivorous fishes on Caribbean reefs and represent three dominant genera of herbivores (29). They have a range of adaptations for herbivory (30, 31) and are similar in size. Over the 7- to 10-month duration of the experiments, we monitored changes in macroalgal abundance, macroalgal species composition and coral survivorship and growth. Our results indicate that herbivore richness can have strong positive effects on coral reefs.
Results
Herbivore Effects on Algae.
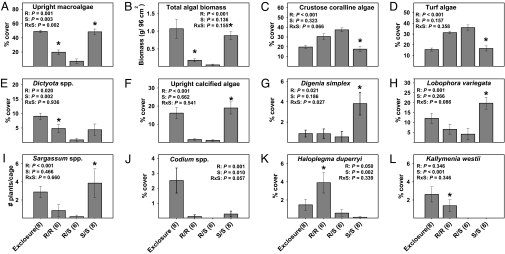
In year 1, redband parrotfish had significant effects on upright macroalgae and all common algal species or groups except for Kallymenia westii (Fig. 1). Ocean surgeonfish had significant effects on upright macroalgae and on Dictyota spp., Codium spp., Haloplegma duperryi, and K. westii. There was a significant herbivore richness effect on macroalgal abundance; in the single-herbivore treatments, cover was a significant 2.7–5.9× higher and biomass a significant 3.5–17.4× higher than in the mixed-herbivore treatment (Fig. 1). As with overall cover of upright macroalgae, herbivore richness also significantly suppressed macroalgal diversity [supporting information (SI) Fig. S1A]. The trends in cover of the different species of macroalgae developed early and strengthened over the duration of the study (Fig. S2). Our results may be conservative, because Hurricane Charley passed within 150 km of our field site 2 weeks before final data were gathered, and storm surge appeared to remove considerable quantities of larger poorly attached algae from the treatments where these were most abundant. For example, large-bladed algae like K. westii were visually estimated at ≈15% cover in many of the redband-only and exclosure cages before the storm but declined to ≈1–3% cover after the hurricane.
Fig. 1.
Year 1 abundance of algae (mean ± SE) at the end of the experiment. P values from two-factor ANOVA. * indicates that single- vs. mixed-herbivore treatments differ as determined via resampling statistics. R = redband parrotfish, S = ocean surgeonfish. n is in brackets below the x axis.
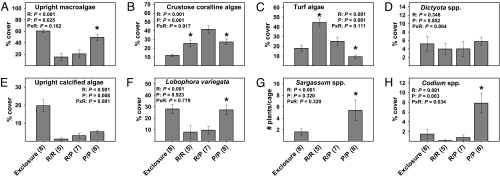
In year 2, redband parrotfish had significant effects on upright macroalgae and on all algal types except for Dictyota spp. (Fig. 2). Princess parrotfish had no significant effect on total cover of upright macroalgae but did affect the cover of crustose coralline algae, turf algae, upright calcified algae, and Codium spp. Significant interactions occurred for upright calcified algae and Codium spp. There was a significant effect of herbivore richness on crustose coralline algae with cover being 52–64% higher in the mixed- vs. the single-herbivore treatments (Fig. 2B). Only redband parrotfish affected macroalgal diversity in year 2 (Fig. S1B). As in year 1, the initial trajectory of change in the cover of macroalgae strengthened over the duration of the study (Fig. S2).
Fig. 2.
Year 2 algal abundance at the end of the experiment. Statistics and symbols as in Fig. 1. R = redband parrotfish, P = princess parrotfish.
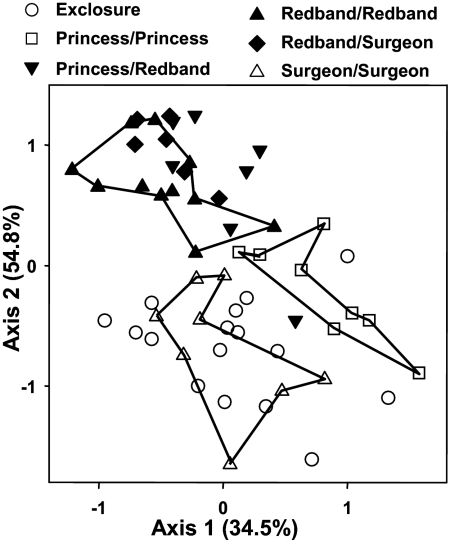
Nonmetric multidimensional scaling (NMDS) showed distinct separation among the different treatments (Fig. 3), with the three single-herbivore treatments each occupying separate axis space. All treatments that included redband parrotfish (redband-only and mixed-species treatments) clustered together, whereas the surgeonfish- and princess-only treatments clustered near the exclosure treatment.
Fig. 3.
Nonmetric multidimensional scaling showing similarity of macroalgal communities among the treatments from year 1 and 2. Lines delineate axis space occupied by redband- (filled triangles), princess- (open squares), and surgeonfish-only treatments (open triangles).
When macroalgae were offered to enclosed fishes in year 1, redband parrotfish consumed significant quantities of Dictyota menstrualis, Halimeda tuna, Lobophora variegata, Sargassum filipendula, and Codium taylori, whereas surgeonfish ate D. menstrualis, C. taylori, K. westii, and H. duperryi (Table 1). For year 2, redband parrotfish consumed the same species as in year 1, whereas princess parrotfish consumed significant quantities of only H. tuna (Table 1). Feeding patterns for enclosed fishes were similar to those for free-ranging fishes (Fig. S3).
Table 1.
Percentage of macroalgal mass (mean ± SE) removed when placed into cages with redband parrotfish (years 1 and 2), ocean surgeonfish (year 1), princess parrotfish (year 2), or into the exclosures (controls)
| Year | Ocean (Yr 1)/Princess (Yr 2) | Redband | Control |
|---|---|---|---|
| Year 1 (percentage of mass removed) | |||
| Dictyota | 37.0 ± 9.3* | 54.9 ± 16.3* | 15.7 ± 5.3 |
| Lobophora | 23.6 ± 6.4 | 53.2 ± 15.8* | 11.9 ± 6.1 |
| Halimeda | 2.9 ± 1.9 | 24.1 ± 11.0* | 3.6 ± 1.5 |
| Sargassum | −2.7 ± 1.3 | 92.8 ± 2.8** | −1.3 ± 2.9 |
| Kallymenia | 72.4 ± 14.1** | 6.5 ± 7.4 | 11.6 ± 8.9 |
| Haloplegma | 70.8 ± 19** | 5.0 ± 5.0 | 4.2 ± 4.2 |
| Codium | 30.7 ± 9.9** | 51.6 ± 18* | −2.9 ± 0.9 |
| Year 2 (percentage of mass removed) | |||
| Dictyota | 10.0 ± 1.9 | 46.5 ± 9.1* | 8.5 ± 2.8 |
| Lobophora | 9.7 ± 1.6 | 52.5 ± 9.3* | 7.9 ± 4.2 |
| Halimeda | 24.9 ± 7.9* | 81.7 ± 11.3** | 5.7 ± 0.15 |
| Sargassum | −3.8 ± 1.2 | 89.5 ± 2.3** | −0.5 ± 2.6 |
| Kallymenia | NA | NA | NA |
| Haloplegma | NA | NA | NA |
| Codium | −2.6 ± 0.5 | 57.6 ± 9.3* | −3.5 ± 0.9 |
*P < 0.05 and **P < 0.01 as determined by using one-tailed t tests comparing treatments and controls for that year. A significant difference between treatment and control indicates loss of more macroalgal mass from cages with vs. without herbivores (i.e., significant feeding). n = 5–8 for each treatment or control.
Herbivore Effects on Corals.
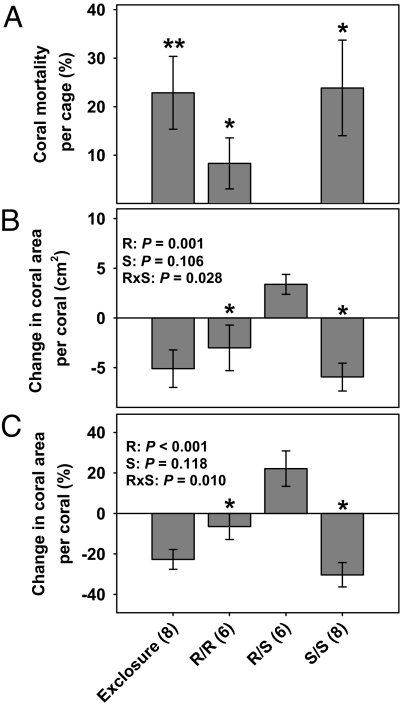
In year 1, coral mortality was 0% in the mixed-herbivore treatment but was significantly higher (8–24% in 10 months) in the single- or no-herbivore treatments (Fig. 4A). Patterns were similar for growth (Fig. 4 B and C). There was a significant herbivore richness effect as coral cover increased 22% in the mixed-herbivore treatment but declined by 6–30% in all other treatments. Both absolute and relative change in coral area was negatively correlated with cover of upright macroalgae in our treatments (slope = −0.119, r2 = 0.150, P = 0.011; slope = −0.742, r2 = 0.278, P < 0.001, respectively), suggesting that coral decline was driven by treatment effects on macroalgal cover. For year 2, unexpected termination of the experiment by Hurricane Dennis prevented evaluations of treatment effects on coral survivorship and growth.
Fig. 4.
Treatment effects (year 1) on coral mortality (A), absolute change (cm2) (B), or proportional change (%) (C) in colony area per coral (mean ± SE). Statistics are via (A) t tests (*, P < 0.05; **, P < 0.01) of mortality against an expected of 0% (i.e., did significant mortality occur?), and (B and C) two-factor ANOVA. In B and C, * indicates a difference between single- and mixed-herbivore treatments as in Fig. 1. R = redband parrotfish, S = ocean surgeonfish.
Potential Confounding Factors.
There was no difference in biomass between the average redband parrotfish and ocean surgeonfish used for experiments in year 1 [142.7 ± 11 g vs. 137.4 ± 4.6 g, respectively [mean ± SE]; F1,26 = 0.03, P = 0.863]. Thus, between-species contrasts were not biased due to among-treatment differences in fish biomass. Weight:length ratios of caged vs. free-ranging fishes at the end of year 1 also did not differ (redband parrotfish: F1,26 = 2.36, P = 0.137; ocean surgeonfish: F1,36 = 0.02, P = 0.885). Thus, fishes in cages were neither feasting nor starving relative to free-ranging fishes. For year 2, hurricane damage to cages precluded weighing caged fishes at the end of the experiment; however, a sample of free-ranging fishes in the size classes that we used suggested minimal differences in biomass between redband and princess parrotfishes [143.6 ± 10.3 g vs. 166.5 ± 17.7 g, respectively (mean ± SE); F1,20 = 1.30, P = 0.268].
Feeding rates for individuals enclosed in single- vs. mixed-species treatments also did not differ in either year (Fig. S4). Thus, differences in macroalgal communities between single- and mixed-herbivore treatments should not have been influenced by differences in intra- vs. interspecific interactions altering feeding behavior within our treatments. Bite rates of free-ranging princess parrotfish and ocean surgeonfish were 2.9× and 3.6× higher, respectively, than those of redband parrotfish (Fig. S4). Contrasts among caged fishes were similar; princess- and surgeonfish-only treatments exhibited bite rates that were 2.7× and 2.9× higher, respectively, than the redband-only treatments (Fig. S4). The mixed-species treatments were intermediate between the single-species treatments. Thus, the differences in macroalgal abundance between single- and mixed-species treatments were not explained by increased bite rates in the mixed-species treatments. Additionally, bite rates for caged fishes were similar to those of free-ranging fishes (Fig. S5), suggesting that caging the fishes did not significantly alter bite rates. Overall bite rates (bites/hr per m2) in the redband-only and mixed-species treatments were comparable to bite rates by the natural assemblage of herbivorous fishes on natural areas of Conch reef, whereas bite rates in the surgeonfish- and princess-only treatments were considerably higher than rates outside of cages (Fig. S6), because these species take many small bites rather that a few larger ones. Given that these fishes take smaller bites, the higher bite rate does not translate into greater rates of algal removal (Figs. 1A and 2A).
Discussion
Increased herbivore richness strongly reduced the cover, biomass, and diversity of algal prey, although facilitating coral survivorship and growth. This effect of transgressive overyielding, where the mixed-species treatment performs better than any of the single-species treatments, has been elusive in previous experiments on consumer diversity (7–9) and experiments assessing biodiversity effects on ecosystem function in general (1). Further, complementary resource use, a mechanism often cited as driving the positive effect of plant species diversity on ecosystem function (32) but that is less commonly addressed in studies of consumer diversity (10), drove the strong direct and indirect effects of herbivore richness in our study. In year 1, feeding complementarity between redband parrotfish and ocean surgeonfish (Table 1) suppressed the cover and biomass of upright macroalgae (Fig. 1), which led to a 22% increase in coral surface area and prevented coral mortality in the mixed-herbivore treatment. In contrast, corals in single-herbivore treatments declined in cover by 6–30% and experienced 8–23% mortality (Fig. 4). In year 2, Hurricane Dennis disrupted our experiment at month 7 and prevented an assessment of treatment effects on coral growth and survivorship. However, before this disturbance, redband and princess parrotfish showed complementary feeding on different functional groups of algae; redband parrotfish suppressed upright macroalgae, whereas princess parrotfish suppressed turf algae (Fig. 2). This facilitated crustose coralline algae in the mixed-herbivore treatment. These algae enhance reefs by reinforcing their structural integrity (33) and serving as preferred recruitment sites for corals (34). Additionally, both turf algae and upright macroalgae impair coral growth and survivorship (35), so their joint suppression in the mixed-herbivore treatment should have facilitated corals; however, cage destruction by Hurricane Dennis prevented a direct assessment of this. Complementary feeding driven by differential tolerance of macroalgal traits also occurs for parrotfishes and surgeonfishes in the tropical Pacific (36), suggesting that complementarity may help control macroalgae in multiple geographic regions.
Despite different species of parrotfish in the Caribbean having different feeding behaviors, bioerosion rates, and preferred diets (26, 28, 37), parrotfishes are often considered as a unified functional group when inferring their effects on community structure (13, 38–40). However, we found that redband and princess parrotfish had considerably different effects on communities, suggesting that grouping all parrotfishes may blur important distinctions among species. Further, effects of a critical species can drive the relationship between diversity and ecosystem function (i.e., the sampling or species identity effect) (1). Even though we show strong effects of herbivore richness in our study, redband parrotfish appear to have disproportionately strong effects on macroalgal community structure, because all treatments containing redband parrotfish clustered regardless of the presence of other herbivore species (Fig. 3). Other Caribbean studies also show strong effects of Sparisoma parrotfishes on reef structure and function (12, 26, 28), suggesting that this genus is important for reef health. In contrast, surgeonfish appear to play a smaller role in regulating overall cover and biomass of macroalgae but are important for preventing blooms of rare macroalgae that are avoided by other herbivores (Fig. 1, Table 1). Despite their different feeding morphologies, ocean surgeonfish and princess parrotfish generated similar macroalgal communities dominated by upright brown macroalgae (e.g., L. variegata and Sargassum spp.). In contrast, despite their more similar jaw morphology, the communities generated by redband and princess parrotfish differed considerably in the abundance of upright macroalgae. Similar to the work of Bellwood et al. (41), these results show that fishes with different feeding morphologies can have similar effects on community structure, suggesting that relying primarily on jaw functional morphology to construct functional groups or infer a species' impact may be unreliable. Additional research to determine the functional diversity or redundancy within Sparisoma, Scarus, and Acanthurus species would facilitate a better understanding of how consumer species richness impacts community organization and ecosystem function and of how representative our data are for these genera.
Previous studies document significant among-species differences in diet selection, spatial impact, and contribution to grazing and bioerosion rates for herbivorous fishes (25, 26, 28, 29, 37, 42). However, it is not clear that observations of feeding patterns or measurements of grazing rates will be predictive of community-level effects, because rates of grazing need to be scaled to in situ rates of prey production (which are rarely measured), and because indirect effects within a complex community can alter, or even reverse, the short-term direct effects that are observed between sets of species (43). Additionally, our findings indicate that herbivores are selectively consuming or avoiding species at sizes that are not visually detectable by humans. For example, Kallymenia westii and Haloplegma duperryi became abundant in the absence of ocean surgeonfish, but these species were rare to absent in treatments that included ocean surgeonfish (Fig. 1 H and I) and were never seen on the open reef. It appears that surgeonfish eliminate them and redband parrotfish avoid them in the germling stage. Similarly, redband parrotfish suppressed Sargassum spp. and ocean surgeonfish enhanced Digenia simplex abundance; both genera are rare to absent on the reef slope, suggesting that observational studies alone will underestimate the role of specific herbivores in controlling many prey species. Our manipulative experiments could detect these more cryptic or indirect effects, but such manipulations are limited in the spatial scale and duration over which they can be conducted. Thus, both observational and experimental studies are needed to better understand the role of consumer identity and diversity in ecosystem function.
Our experiment addressed the role of species richness in affecting the health of coral reefs, but the scale of our enclosures (4 m2) limited our treatments to only 0 vs. 1 vs. 2 herbivore species during each year. This limitation restricts our ability to predict the effects of herbivore species richness across larger numbers of consumer types. However, the positive effects of increasing herbivore richness suggest that herbivore richness may be critical to reef community function. Our design necessitated a minimum fish density of 0.5 fish/m2, because it required two fish per cage for the mixed-species treatments; this produced a herbivore biomass of ≈75–100 g/m2. This density and biomass are within the range but above the mean, seen on present-day Caribbean reefs at depths of 10–20 m (density: mean of 0.22 fish/m2 with range up to 0.731 fish/m2; biomass: mean of 24 g/m2 with range up to 124 g/m2) (44). Natural densities and masses on unfished reefs of the past would have been considerably higher (5, 45). Thus, our density of consumers was higher than on many present-day reefs but may be similar to reefs of the past; however, our enclosures were considerably smaller than the typical home ranges for these fishes (46). This constrained foraging to a smaller area than normal and could have forced less-selective feeding as favored foods were depleted. However, comparisons of feeding by enclosed (Table 1) and free-ranging individuals (Fig. S2 and Fig. S5) does not suggest noticeable alteration of feeding rate or selectivity, and if enclosed fishes were less selective, this should have biased against the strong effects of herbivore richness that we demonstrated.
Harvesting of marine consumers has impacted the function of many marine ecosystems through the removal of disproportionately important species and the reduction of functional diversity at all trophic levels (10). Herbivores are critical drivers of ecosystem function on coral reefs, because they suppress macroalgae (13, 14) and facilitate the recruitment, growth, survival, and resilience of corals (18, 47). Although herbivore biomass is often emphasized as an important determinant of macroalgal abundance (12, 40), we show that herbivore richness and identity also play important roles in the regulation of reef community structure. Understanding the importance of herbivore identity and richness may be especially important for Caribbean reefs, because they are species-poor compared with coral reefs in other regions, and their ecosystem function may be more susceptible to the loss of particular species that lack ecological equivalents within the system (20). Additionally, species may differ in their ability to prevent and reverse phase shifts within communities (48), further underscoring the importance of maintaining consumer diversity as a means of protecting ecosystem health. A better understanding of how combinations of Caribbean herbivores affect reef function could facilitate the development of conservation strategies that enhance critical species, or critical mixtures, of herbivorous fishes to facilitate recovery of coral reefs. Because coral reefs face global threats, such as increasing sea surface temperatures and coral bleaching, that are beyond the scope of local or regional management, scientists and managers need to identify biotic and abiotic processes that can be preserved or managed to promote healthy ecosystem function and resilience in the face of these global stressors (18, 49). Our findings suggest that a combination of (i) marine protected areas that help restore fish stocks and (ii) proactive management for critical components of herbivorous fish diversity could hasten recovery of coral reefs. A better appreciation for the functional role of consumer diversity in marine ecosystems might facilitate their protection, conservation, and restoration and our ability to serve as wise stewards of these threatened resources.
Materials and Methods
Experimental Setup and Maintenance.
In November 2003, at a depth of 16–18 m on Conch Reef (24°57′N/80°27′W) in the Florida Keys, we constructed 32 cages that were 2 × 2 × 1 m tall from 0.6-cm steel bar supporting PVC-coated galvanized wire (2.5-cm mesh). This mesh creates no effects on algal community development (50). A 30-cm flange of mesh was conformed to the reef and affixed by using galvanized fencing nails. Cages enclosed natural assemblages of algae and benthic invertebrates.
For year 1, we enclosed redband parrotfish (S. aurofrenatum) and ocean surgeonfish (A. bahianus) to create treatments of: (i) two redband parrotfish, (ii) two ocean surgeonfish, (iii) one redband parrotfish and one ocean surgeonfish, or (iv) no enclosed fishes. Density of enclosed herbivorous fishes was within the range seen on present-day Caribbean reefs (48). Fishes were 14- to 18-cm standard length for redband parrotfishes and 12- to 16-cm standard length for ocean surgeonfishes. Four cages were blocked spatially (typically within 3–4 m of each other), treatments were allocated randomly, and there were eight such blocks. Every 4–6 weeks, we scrubbed the cages, surveyed fishes inside the cages, and replaced missing fishes (Table S1). In year 1, the experiment ran for 10 months (November 2003–August 2004). The mesh was then removed and grazing allowed for 3 months to remove treatment effects.
In November 2004, we set up year 2 using the same design, except we used redband and princess parrotfish (S. taeniopterus). Fishes were intermediate-phase and were 14- to 19-cm standard length for redband parrotfishes and 15- to 22-cm standard length for princess parrotfishes. The experiment ran from November 2004 until July 2005 (7 months), when surge from Hurricane Dennis destroyed the cages. However, all data presented for year 2 were collected in June 2005, while the treatments were intact.
Measuring Herbivore Affects on the Algal Community.
Every 6–10 weeks, we used a 1.5 × 0.75-m quadrant containing 50 points to sample two areas within each cage, identifying organisms under each point. For Sargassum spp., we also counted individuals. At the end of year 1 (August 2004), we assessed macroalgal biomass by coring (3.5-cm diameter) at 10 random locations within each cage and determining macroalgal dry mass. Destruction from Hurricane Dennis prevented measurement of biomass for year 2. At the end of each experiment, among-treatment differences in algal species, types, or functional groups (51) were assessed via two-factor ANOVAs, following data transformations when necessary. In year 1, we excluded four replicates due to persistent loss of fishes from these cages, resulting in n = 6–8 for each treatment. Similar occurrences in year 2 resulted in n = 5–8. We noted moray eels in these cages, suggesting a reason for fish loss. There were no among-treatment differences in macroalgal abundance at the beginning of year 1 or 2 (Table S2).
We assessed the effect of herbivore richness on macroalgal abundance using resampling statistics (resampling 10,000 times with replacement) to compare the mixed- to each single-species treatment (see ref. 7). We controlled the error rate using the Bonferroni correction, i.e., α = 0.025 for each test. To assess similarity in algal communities across both years, we performed ordination using NMDS with PC-ORD for Windows, Version 4 (52). NMDS is an iterative technique that ordinates based on rank distances between sampling entities (i.e., replicates of the herbivore treatments). We used Sørensen (Bray–Curtis) distance to generate the distance matrix for the analysis.
In both years, we evaluated feeding patterns of treatment fishes by placing weighed portions of various algae in each single-herbivore treatment and in the herbivore exclosures. We offered five to seven algal species common during the experiments. Pieces of a single algal species (3.0 ± 0.3 g for H. tuna and 2.0 ± 0.2 g for other macroalgae) were blotted with a paper towel, weighed to the nearest milligram, and entwined in a three-strand rope. Algae were placed in each single herbivore and exclosure cage for 24–30 h (n = 5–8 separate cages), then reweighed. Change in mass was calculated for each alga, and this value was compared between herbivore and exclosure treatments using a one-tailed t test.
Coral Survivorship and Growth.
The most common corals in treatments were Siderastrea siderea (45% of all coral individuals), Porites astreoides (21%), Agaricia spp. (13%), and Stephanocoenia michelini (11%). To monitor corals, we took digital photographs of each coral colony within each replicate at the beginning (November 2003) and end (August 2004) of the experiment. The camera was mounted on a quadrapod frame to allow consistent positioning directly above each coral. Change in live tissue area was measured by using the computer program Image J (http://rsbweb.nih.gov/ij) to outline each coral and calculate colony area at the beginning and end of the experiment. We calculated absolute change (in cm2) and percentage change for each coral. This method is inaccurate for branching corals such as Porites porites, but these were <5% of all corals, so we excluded these from the analysis. Coral mortality was assessed by calculating the percentage of individuals that died in each replicate and testing whether the mean for each treatment differed from zero using a one-tailed t test. Change in coral area was analyzed by a two-factor ANOVA. Effects of herbivore richness on coral area were evaluated by using resampling statistics as discussed for macroalgal abundance. We used linear least-squares regression to investigate the relationship between the abundance of upright macroalgae and change in coral area by regressing the average change in coral area for each cage against macroalgal abundance. At the beginning of the experiment (November 2003), mean coral colony size was 26.6 ± 3.0 cm2 (mean ± SE), and the size distribution of corals did not differ across treatments (Table S3). There were 6.0 ± 0.4 coral colonies per cage with no differences among treatments for the number of corals present (Table S3). Hurricane Dennis prevented an assessment of treatment effects on corals during year 2.
Evaluation of Possible Confounding Factors.
To determine whether our experimental design resulted in artifacts that confounded our results, we evaluated: (i) feeding preferences for enclosed vs. free-ranging fishes, (ii) bite rates of individuals enclosed in single vs. mixed-species treatments, (iii) differences in bite rates among treatments, (iv) bite rates of enclosed vs. free-ranging fishes, and (v) how bite rates in our treatments compared with the natural environment. (See SI Text for detailed methods of these tests.)
Supplementary Material
Acknowledgments.
Personnel at the University of North Carolina, Wilmington, National Undersea Research Center (UNCW-NURC) in Key Largo, FL, facilitated this research. We thank the many people who helped this project succeed. A. Chequer was particularly indispensable. Support was provided by UNCW-NURC Project #SEGM-2003-19A and National Science Foundation IGERT Grant DGE 0114400 (to M.E.H.), a National Science Foundation Graduate Fellowship (to D.E.B.), and the Teasley Endowment to Georgia Institute of Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801946105/DCSupplemental.
References
- 1.Cardinale BJ, et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- 2.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 3.Duffy JE. Biodiversity and ecosystem function: The consumer connection. Oikos. 2002;99:201–219. [Google Scholar]
- 4.Duffy JE. Biodiversity loss, trophic skew and ecosystem functioning. Ecol Lett. 2003;6:680–687. [Google Scholar]
- 5.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 6.Steneck RS, Vavrinec J, Leland AV. Accelerating trophic-level dysfunction in kelp forest ecosystems of the western North Atlantic. Ecosystems. 2004;7:323–332. [Google Scholar]
- 7.Duffy JE, Richardson JP, Canuel EA. Grazer diversity effects on ecosystem functioning in seagrass beds. Ecol Lett. 2003;6:637–645. [Google Scholar]
- 8.Bruno JF, O'Connor MI. Cascading effects of predator diversity and omnivory in a marine food web. Ecol Lett. 2005;8:1048–1056. [Google Scholar]
- 9.Byrnes J, et al. Predator diversity strengthens trophic cascades in kelp forests by modifying herbivore behaviour. Ecol Lett. 2006;9:61–71. doi: 10.1111/j.1461-0248.2005.00842.x. [DOI] [PubMed] [Google Scholar]
- 10.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 11.Shurin JB, et al. A cross-ecosystem comparison of the strength of trophic cascades. Ecol Lett. 2002;5:785–791. [Google Scholar]
- 12.Mumby PJ, et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science. 2006;311:98–101. doi: 10.1126/science.1121129. [DOI] [PubMed] [Google Scholar]
- 13.Steneck RS. Herbivory on coral reefs: A synthesis. Proc 6th Int Coral Reef Symp Austr. 1988;1:37–49. [Google Scholar]
- 14.Burkepile DE, Hay ME. Herbivore vs. nutrient control of marine primary producers: Context-dependent effects. Ecology. 2006;87:3128–3139. doi: 10.1890/0012-9658(2006)87[3128:hvncom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Kuffner IB, et al. Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser. 2006;323:107–117. [Google Scholar]
- 16.Tanner JE. Competition between scleractinian corals and macroalgae—an experimental investigation of coral growth, survival and reproduction. J Exp Mar Biol Ecol. 1995;190:151–168. [Google Scholar]
- 17.Lewis SM. The role of herbivorous fishes in the organization of a Caribbean reef community. Ecol Monogr. 1986;56:183–200. [Google Scholar]
- 18.Hughes TP, et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol. 2007;17:360–365. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 19.Hatcher BG, Larkum AWD. An experimental analysis of factors controlling the standing crop of the epilithic algal community on a coral reef. J Exp Mar Biol Ecol. 1983;69:61–84. [Google Scholar]
- 20.Bellwood DR, Hughes TP, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 21.Lubchenco J, Gaines SD. A unified approach to marine plant-herbivore interactions. I. Populations and communities. Annu Rev Ecol Syst. 1981;12:405–437. [Google Scholar]
- 22.Carpenter RC. Partitioning herbivory and its effects on coral reef algal communities. Ecol Monogr. 1986;56:345–363. [Google Scholar]
- 23.Morrison D. Comparing fish and urchin grazing in shallow and deeper coral reef algal communities. Ecology. 1988;69:1367–1382. [Google Scholar]
- 24.Carpenter RC. Mass mortality of Diadema antillarum II: Effects on population densities and grazing intensity of parrotfishes and surgeonfishes. Mar Biol. 1990;104:79–86. [Google Scholar]
- 25.McAfee ST, Morgan SG. Resource use by five sympatric parrotfishes in the San Blas Archipelago, Panama. Mar Biol. 1996;125:427–437. [Google Scholar]
- 26.Paddack MJ, Cowen RK, Sponaugle S. Grazing pressure of herbivorous coral reef fishes on low coral-cover reefs. Coral Reefs. 2006;25:461–472. [Google Scholar]
- 27.Lewis SM. Herbivory on coral reefs: Algal susceptibility to herbivorous fishes. Oecologia. 1985;65:370–375. doi: 10.1007/BF00378911. [DOI] [PubMed] [Google Scholar]
- 28.Bruggemann JH, van Kessel AM, van Rooij JM, Breeman AM. Bioerosion and sediment ingestion by the Caribbean parrotfish Scarus vetula and Sparisoma viride: Implications of fish size, feeding mode and habitat use. Mar Ecol Prog Ser. 1996;134:59–71. [Google Scholar]
- 29.Lewis SM, Wainwright PC. Herbivore abundance and grazing intensity on a Caribbean coral reef. J Exp Mar Biol Ecol. 1985;87:215–228. [Google Scholar]
- 30.Horn MH. Biology of marine herbivorous fishes. Oceanogr Mar Biol. 1989;27:167–272. [Google Scholar]
- 31.Bellwood DR. A phylogenetic study of the parrotfishes family Scaridae (Pisces: Labroidei), with a revision of genera. Rec Aust Mus Suppl. 1994;20:1–84. [Google Scholar]
- 32.Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- 33.Littler MM, Doty MS. Ecological components structuring seaward edges of tropical Pacific reefs—distribution, communities and productivity of Porolithon. J Ecol. 1975;63:117–129. [Google Scholar]
- 34.Heyward AJ, Negri AP. Natural inducers for coral larval metamorphosis. Coral Reefs. 1999;18:273–279. [Google Scholar]
- 35.Nugues MM, Roberts CM. Coral mortality and interaction with algae in relation to sedimentation. Coral Reefs. 2003;22:507–516. [Google Scholar]
- 36.Schupp PJ, Paul VJ. Calcium carbonate and secondary metabolites in tropical seaweeds—variable effects on herbivorous fishes. Ecology. 1994;75:1172–1185. [Google Scholar]
- 37.Bruggemann JH, Kuyper MWM, Breeman AM. Comparative analysis of foraging and habitat use by the sympatric Caribbean parrotfish Scarus vetula and Sparisoma viride (Scaridae) Mar Ecol Prog Ser. 1994;112:51–66. [Google Scholar]
- 38.Mumby PJ. The impact of exploiting grazers (scaridae) on the dynamics of Caribbean coral reefs. Ecol Appl. 2006;16:747–769. doi: 10.1890/1051-0761(2006)016[0747:tioegs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007b;450:98–101. doi: 10.1038/nature06252. [DOI] [PubMed] [Google Scholar]
- 40.Williams ID, Polunin NVC. Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs. 2001;19:358–366. [Google Scholar]
- 41.Bellwood DR, Wainwright PC, Fulton CJ, Hoey AS. Functional versatility supports coral reef biodiversity. Proc R Soc B. 2006;273:101–107. doi: 10.1098/rspb.2005.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellwood DR, Hoey AS, Choat JH. Limited functional redundancy in high diversity systems: Resilience and ecosystem function on coral reefs. Ecol Lett. 2003;6:281–285. [Google Scholar]
- 43.Hay ME, et al. Mutualisms and aquatic community structure: The enemy of my enemy is my friend. Annu Rev Ecol Evol Syst. 2004;35:175–197. [Google Scholar]
- 44.Marks KW, Lang JC. AGRRA Summary Products, version (08/2005) 2005 http://www.agrra.org.
- 45.Knowlton N, Jackson JBC. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol. 2008;6:215–220. doi: 10.1371/journal.pbio.0060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mumby PJ, Wabnitz CCC. Spatial patterns of aggression, territory size, and harem size in five sympatric Caribbean parrotfish species. Environ Biol Fishes. 2002;63:265–279. [Google Scholar]
- 47.Mumby PJ, et al. Trophic cascade facilitates coral recruitment in a marine reserve. Proc Natl Acad Sci USA. 2007;104:8362–8367. doi: 10.1073/pnas.0702602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellwood DR, Hughes TP, Hoey AS. Sleeping functional group drives coral reef recovery. Curr Biol. 2006;16:2434–2439. doi: 10.1016/j.cub.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 49.Hughes TP, Bellwood DR, Folke C, Steneck RS, Wilson J. New paradigms for supporting the resilience of marine ecosystems. Trends Ecol Evol. 2005;20:380–386. doi: 10.1016/j.tree.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 50.Miller MW, et al. Effects of nutrients versus herbivores on reef algae: A new method for manipulating nutrients on coral reefs. Limnol Oceanogr. 1999;44:1847–1861. [Google Scholar]
- 51.Steneck RS, Dethier MN. A functional group approach to the structure of algal-dominated communities. Oikos. 1994;69:476–498. [Google Scholar]
- 52.McCune B, Mefford MJ. PC-ORD: Multivariate Analysis of Ecological Data. Gleneden Beach, OR: MjM Software Design; 1999. Ver 4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.