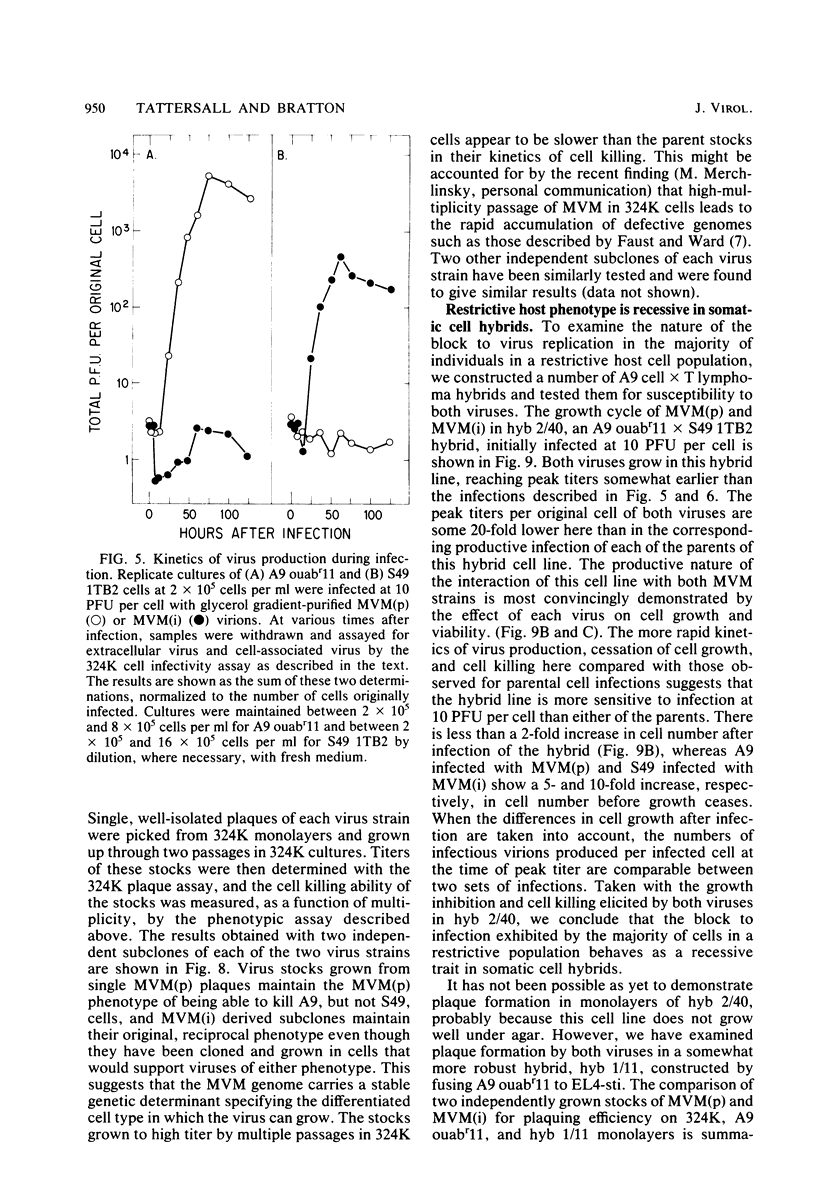
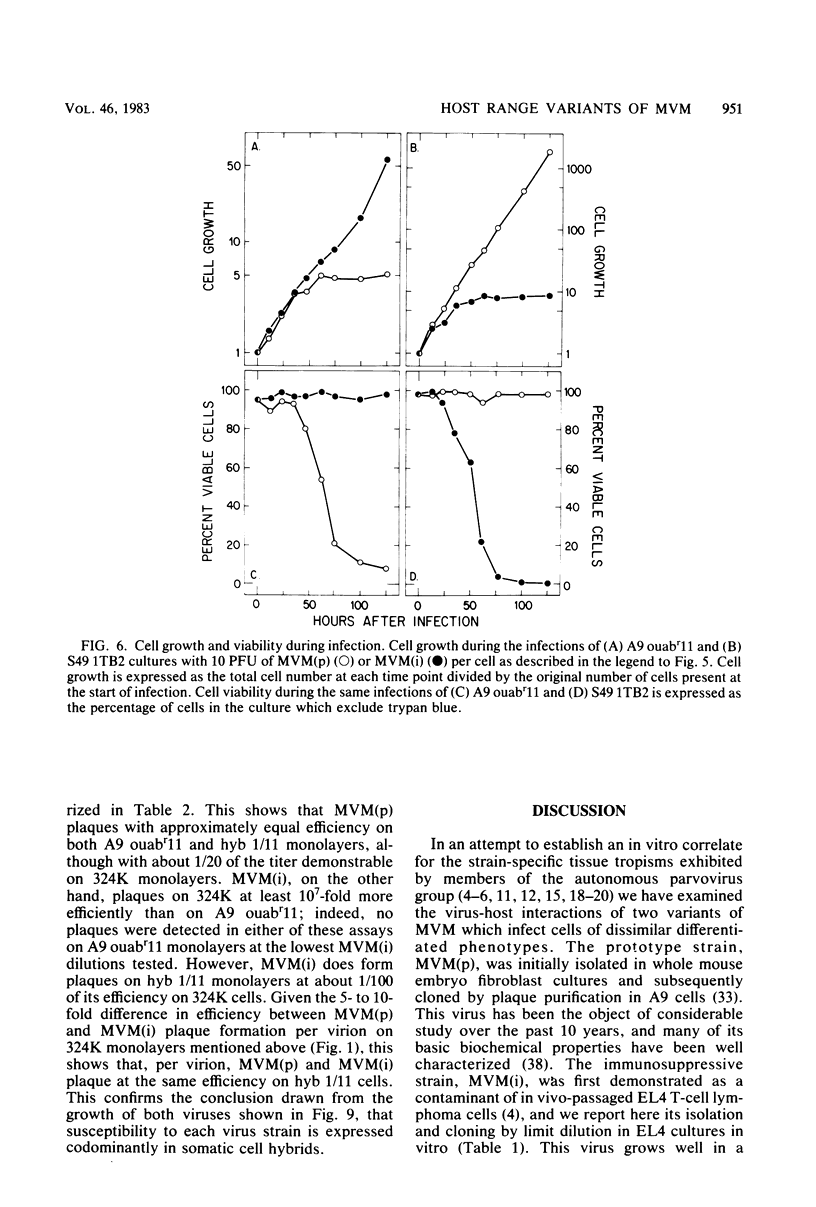
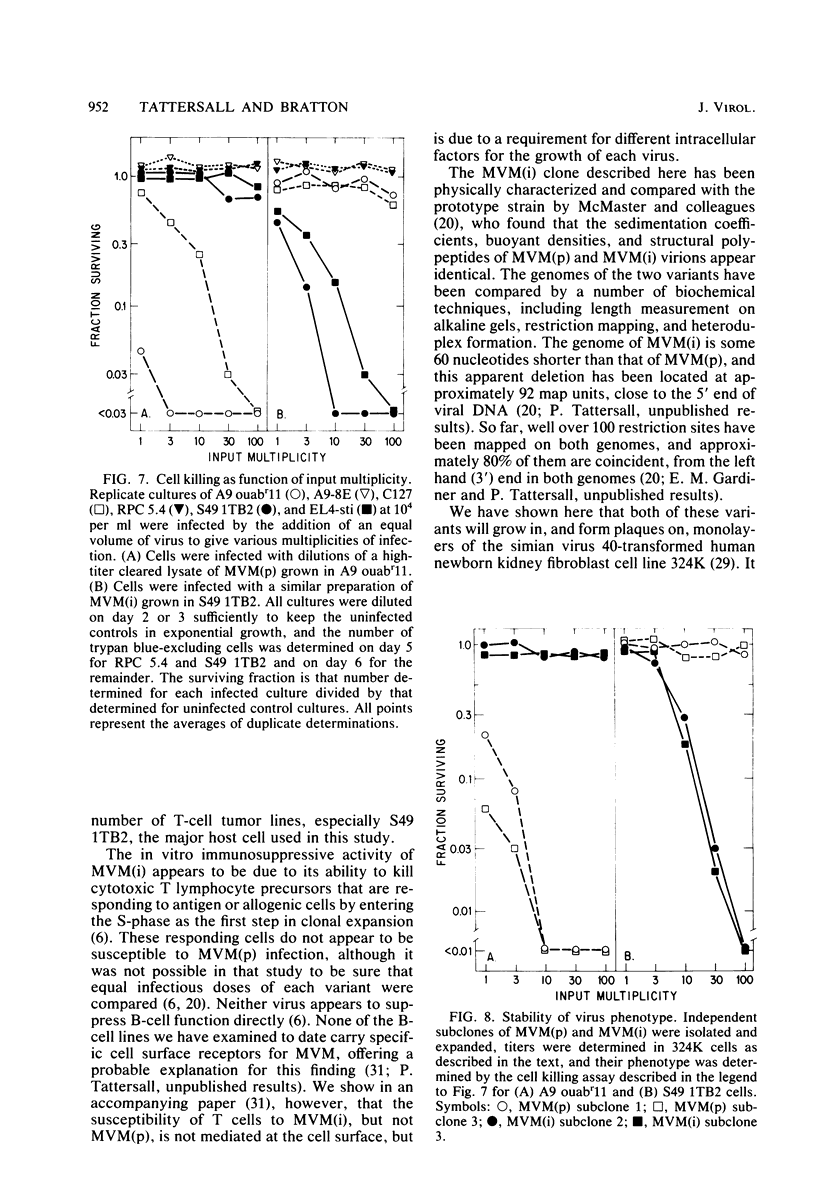
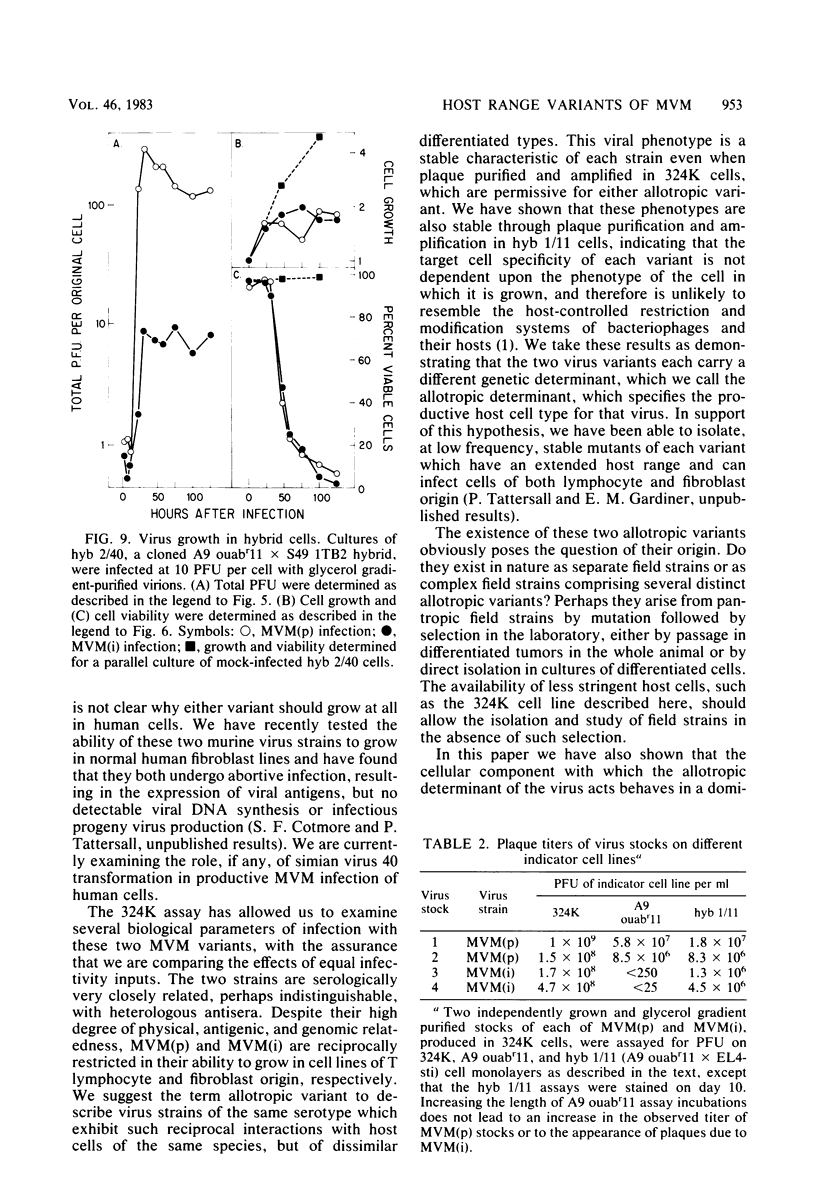
Abstract
Viral and cellular factors responsible for parvovirus target cell specificity have been examined for two serologically indistinguishable strains of the minute virus of mice which infect mouse cells of dissimilar differentiated phenotype. Both the prototype strain and the immunosuppressive strain grow in and form plaques on monolayers of simian virus 40-transformed human fibroblasts, a finding that has allowed the comparison of several aspects of their virus-host cell interactions. Although closely related by antigenic and genomic criteria, both the prototype strain and the immunosuppressive strain are restricted for lytic growth in each other's murine host cell, that is, in T cells and fibroblasts, respectively. The host range of each virus variant appears to be specified by a genetic determinant that is stably inherited in the absence of selection. In the restrictive virus-host interaction lytic growth is limited to a small or, in some cases, undetectable subset of the host cell population, and the majority of the infected cells remain viable, continuing to grow at the normal rate without expressing viral antigens. The susceptible host cell phenotype is dominant in T lymphocyte x fibroblast fusion hybrids, implying that different cell types express different developmentally regulated virus helper functions that can only be exploited by the virus variant that carries the appropriate strain-specific determinant.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- Baer P. N., Garrington G. E., Kilham L. Effect of age and H-1 virus on healing fractures in hamsters. J Gerontol. 1971 Jul;26(3):373–377. doi: 10.1093/geronj/26.3.373. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Harris A. W., Tomkins G. M., Cohn M. Glucocorticoid receptors in lymphoma cells in culture: relationship to glucocorticoid killing activity. Science. 1971 Jan 15;171(3967):189–191. doi: 10.1126/science.171.3967.189. [DOI] [PubMed] [Google Scholar]
- Bonnard G. D., Manders E. K., Campbell D. A., Jr, Herberman R. B., Collins M. J., Jr Immunosuppressive activity of a subline of the mouse EL-4 lymphoma. Evidence for minute virus of mice causing the inhibition. J Exp Med. 1976 Jan 1;143(1):187–205. doi: 10.1084/jem.143.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. A., Jr, Staal S. P., Manders E. K., Bonnard G. D., Oldham R. K., Salzman L. A., Herberman R. B. Inhibition of in vitro lymphoproliferative responses by in vivo passaged rat 13762 mammary adenocarcinoma cells. II. Evidenceth Kilham rat virus is responsible for the inhibitory effect. Cell Immunol. 1977 Oct;33(2):378–391. doi: 10.1016/0008-8749(77)90166-6. [DOI] [PubMed] [Google Scholar]
- Engers H. D., Louis J. A., Zubler R. H., Hirt B. Inhibition of T cell-mediated functions by MVM(i), a parvovirus closely related to minute virus of mice. J Immunol. 1981 Dec;127(6):2280–2285. [PubMed] [Google Scholar]
- Faust E. A., Ward D. C. Incomplete genomes of the parvovirus minute virus of mice: selective conservation of genome termini, including the origin for DNA replication. J Virol. 1979 Oct;32(1):276–292. doi: 10.1128/jvi.32.1.276-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORER P. A. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950 Dec;4(4):372–379. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn D. E. Pathogenesis of feline panleukopenia. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):628–630. [PubMed] [Google Scholar]
- Kilham L., Margolis G., Colby E. D. Enhanced proliferation of H-1 virus in livers of rats infected with Cysticercus fasciolaris. J Infect Dis. 1970 Jun;121(6):648–652. doi: 10.1093/infdis/121.6.648. [DOI] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Problems of human concern arising from animal models of intrauterine and neonatal infections due to viruses: a review. I. Introduction and virologic studies. Prog Med Virol. 1975;20:113–143. [PubMed] [Google Scholar]
- Kilham L., Margolis G. Spontaneous hepatitis and cerebellar "hypoplasia" in suckling rats due to congenital infections with rat virus. Am J Pathol. 1966 Sep;49(3):457–475. [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THREE DEGREES OF GUANYLIC ACID--INOSINIC ACID PYROPHOSPHORYLASE DEFICIENCY IN MOUSE FIBROBLASTS. Nature. 1964 Sep 12;203:1142–1144. doi: 10.1038/2031142a0. [DOI] [PubMed] [Google Scholar]
- Linser P., Bruning H., Armentrout R. W. Specific binding sites for a parvovirus, minute virus of mice, on cultured mouse cells. J Virol. 1977 Oct;24(1):211–221. doi: 10.1128/jvi.24.1.211-221.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Johnson R. T. The pathogenesis of rat virus infections in the newborn hamster. Lab Invest. 1972 Nov;27(5):508–513. [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum G. S. Serological studies of rat viruses in relation to tumors. Oncology. 1970;24(5):335–343. doi: 10.1159/000224534. [DOI] [PubMed] [Google Scholar]
- Margolis G., Kilham L. Problems of human concern arising from animal models of intrauterine and neonatal infections due to viruses: a review. II. Pathologic studies. Prog Med Virol. 1975;20:144–179. [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier P. C., Glickman L. T., Appel M. J., Shin S. J. Canine parvovirus in a commercial kennel: epidemiologic and pathologic findings. Cornell Vet. 1981 Jan;71(1):96–110. [PubMed] [Google Scholar]
- Miller R. A., Ward D. C., Ruddle F. H. Embryonal carcinoma cells (and their somatic cell hybrids) are resistant to infection by the murine parvovirus MVM, which does infect other teratocarcinoma-derived cell lines. J Cell Physiol. 1977 Jun;91(3):393–401. doi: 10.1002/jcp.1040910309. [DOI] [PubMed] [Google Scholar]
- Mohanty S. B., Bachmann P. A. Susceptibility of fertilized mouse eggs to minute virus of mice. Infect Immun. 1974 Apr;9(4):762–763. doi: 10.1128/iai.9.4.762-763.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohit B., Fan K. Hybrid cell line from a cloned immunoglobulin-producing mouse myeloma and a nonproducing mouse lymphoma. Science. 1971 Jan 8;171(3966):75–77. doi: 10.1126/science.171.3966.75. [DOI] [PubMed] [Google Scholar]
- O'Malley K. A., Davidson R. L. A new dimension in suspension fusion techniques with polyethylene glycol. Somatic Cell Genet. 1977 Jul;3(4):441–448. doi: 10.1007/BF01542972. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo P. R., Margolis G., Kilham L. The induction of hepatitis by prior partial hepatectomy in resistant adult rats injected with H-1 virus. Light and electron microscopy and virologic studies. Am J Pathol. 1966 Nov;49(5):795–824. [PMC free article] [PubMed] [Google Scholar]
- SHEIN H. M., ENDERS J. F. Multiplication and cytopathogenicity of Simian vacuolating virus 40 in cultures of human tissues. Proc Soc Exp Biol Med. 1962 Mar;109:495–500. doi: 10.3181/00379727-109-27246. [DOI] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. The multiplication of parvovirus Lu3 in a synchronized culture system. I. Optimum conditions for virus replication. Arch Gesamte Virusforsch. 1973;40(1):105–118. doi: 10.1007/BF01242642. [DOI] [PubMed] [Google Scholar]
- Spalholz B. A., Tattersall P. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J Virol. 1983 Jun;46(3):937–943. doi: 10.1128/jvi.46.3.937-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]