Abstract
During infection, Pseudomonas aeruginosa employs bacterial communication (quorum sensing [QS]) to coordinate the expression of tissue-damaging factors. QS-controlled gene expression plays a pivotal role in the virulence of P. aeruginosa, and QS-deficient mutants cause less severe infections in animal infection models. Treatment of cystic fibrosis (CF) patients chronically infected with P. aeruginosa with the macrolide antibiotic azithromycin (AZM) has been demonstrated to improve the clinical outcome. Several studies indicate that AZM may accomplish its beneficial action in CF patients by impeding QS, thereby reducing the pathogenicity of P. aeruginosa. This led us to investigate whether QS inhibition is a common feature of antibiotics. We present the results of a screening of 12 antibiotics for their QS-inhibitory activities using a previously described QS inhibitor selector 1 strain. Three of the antibiotics tested, AZM, ceftazidime (CFT), and ciprofloxacin (CPR), were very active in the assay and were further examined for their effects on QS-regulated virulence factor production in P. aeruginosa. The effects of the three antibiotics administered at subinhibitory concentrations were investigated by use of DNA microarrays. Consistent results from the virulence factor assays, reverse transcription-PCR, and the DNA microarrays support the finding that AZM, CFT, and CPR decrease the expression of a range of QS-regulated virulence factors. The data suggest that the underlying mechanism may be mediated by changes in membrane permeability, thereby influencing the flux of N-3-oxo-dodecanoyl-l-homoserine lactone.
The opportunistic pathogen Pseudomonas aeruginosa causes severe infections, particularly in immunocompromised individuals and patients with cystic fibrosis (CF). The progressive deterioration of the lungs caused by chronic P. aeruginosa infections and, thus, persistent inflammation are currently the main causes of morbidity and mortality in patients with CF (55). Intensive antipseudomonal treatment (maintenance therapy) has greatly improved the prospects for patients with CF (26, 29, 72). A serious side effect of antibiotic therapy is the development of resistance to the antibiotics used (3, 15, 28, 54). P. aeruginosa forms biofilms during the infection process, which adds to the difficulties of eradicating infections by antibiotic intervention, since bacterial cells living as biofilms are much more tolerant to antibiotics than their planktonic counterparts (4, 14, 17). When the infection enters a chronic state, the mucoid, alginate-overproducing phenotype dominates (22). Macrolides such as erythromycin, clarithromycin, and the erythromycin derivative azithromycin (AZM) have been shown to inhibit the enzymatic activity of guanosine diphosphomannose dehydrogenate in the alginate biosynthetic pathway of mucoid P. aeruginosa strains at concentrations well below the MIC (44, 49). Alginate induces a continuous antigen-antibody reaction locally in the small airways. Thus, a lower level of alginate production not only reduces the viscosity of the sputum but may also lead to less inflammation and, thus, improved lung function (8, 41, 49). Several clinical studies have shown that long-term treatment with AZM improves lung function and body weight in patients with CF (27, 43, 76). AZM is not usually considered to exhibit antipseudomonal activity due to its MIC for P. aeruginosa in the range of 128 to 512 μg/ml (43). The highest concentrations of AZM found in the sputum of patients receiving high-dose therapy (250 mg AZM daily) is 0.6 to 79.3 μg/ml, with the median AZM concentration being 9.5 μg/ml (6). It has been suggested that AZM treatment inhibits neutrophil recruitment to the lung by reducing the levels of expression of proinflammatory cytokines and inhibition of neutrophil migration, resulting in a significant reduction in airway-specific inflammation (88).
It has also been suggested that inhibition of cell-cell communication is the mode of action by which AZM exerts its activity in P. aeruginosa infections (60, 84). In gram-negative bacteria, cell-cell communication, also known as quorum sensing (QS), relies upon small diffusible signal molecules (N-acyl l-homoserine lactones [AHLs]), which interact with transcriptional activators to couple gene expression with cell population density. The QS system of P. aeruginosa is organized hierarchically, with the RhlI-RhlR components being subordinate to the LasI-LasR components. LasI directs the synthesis of N-3-oxo-dodecanoyl-l-homoserine lactone (OdDHL), whereas RhlI synthesizes N-butanoyl-L-homoserine lactone (BHL). P. aeruginosa cells are permeable to BHL, which freely diffuses across the cell membrane, whereas active efflux is required for the transportation of OdDHL (67). In addition to the AHL signal molecules BHL and OdDHL, a third intercellular signal, 2-heptyl-hydroxy-4-quinolone (designated the Pseudomonas quinolone signal [PQS]), has been found to be part of the QS regulon in P. aeruginosa (70). They all function in concert to control the expression of an array of genes, including genes encoding tissue-damaging exoproducts (31, 51, 62, 66). In addition to controlling the production of virulence factors, the QS signal molecules PQS and OdDHL have been reported to possess immunomodulatory effects, and in this way, they contribute directly to the pathogenesis of P. aeruginosa (24, 42, 85).
Several studies have shown the effects of AZM on the expression of QS-regulated virulence genes in vitro (40, 60, 84). Applying a CF mouse model, we have recently demonstrated that AZM treatment significantly improved the clearance of pulmonary bacteria, reduced the extent of lung abscesses and decreased the severity of lung pathology, and reduced the level of alginate production in vivo and also in in vitro experiments, although it had no effect on the growth of infecting bacteria (40). Since QS seems to play a key role in the expression of virulence and the interaction with host protection, inhibitors of QS have been suggested to be important components of future antipseudomonal therapies (37). Examples of compounds that can block QS in P. aeruginosa are the halofuranones (32, 38), which originate from a benthic macroalgae, and the fungal metabolites patulin and penicillic acid (74). Furanone C-30 specifically inhibits QS over a concentration range from 1 to 10 μM; but when it is administered in excess, i.e., at concentrations 10-fold higher, the compound impairs growth and exhibits antibiotic properties. In addition to QS-inhibitory (QSI) activity, the bioactive halofuranones exhibit a multitude of activities, including antibiotic and antifouling properties, and can therefore be considered multifunctional compounds engaged in communication as well as competition (for a recent review, see reference 21). It was recently demonstrated that at sub-MICs antibiotics broadly affect the patterns of transcription in bacteria and seem to function as signaling mediators rather than function by targeting the growth of rivals (35). This raises the question of whether other compounds previously identified for their antibiotic properties may turn out to possess properties that interfere with communication and, potentially, QSI activity. To try to answer that question, we present the results of a screening of 12 antibiotics for their QSI activities. We identified three antibiotics, AZM, ciprofloxacin (CPR), and ceftazidime (CFT), that exhibit strong QSI activities in the screening assay. The effects of these three antibiotics were further investigated by means of transcriptome analysis and phenotyping assays.
MATERIALS AND METHODS
Bacterial strains applied in this study.
The sequenced P. aeruginosa PAO1 wild-type strain was obtained from the Pseudomonas Genetic Stock Center (strain PAO0001; www.pseudomonas.med.ecu.edu). The lasI-rhlI mutant was constructed by using a previously reported knockout system (7). The QSI selector strain QS inhibitor selector 1 (QSIS1) was described previously (73).
Bacterial screen for QS inhibition.
Strain QSIS1 has previously been described in detail (73). The QS element is derived from the Vibrio fischeri LuxR-regulated QS system and was cloned in Escherichia coli, where it responds to a broad range of AHLs and QS inhibitors (2, 73, 74). QSIS1 harbors plasmid pUC18Not-luxR-PluxI-RBSII-phlA T0-T1 Apr maintained in CSH37 (59), an E. coli strain that constitutively expresses β-galactosidase. The general concept of the selector system implies the presence of a QS regulator (luxR or a homologue) gene and a QS-controlled promoter fused to a gene encoding a toxic gene product. In the presence of signal molecules, the gene under QS control is expressed, causing cell death. However, in the presence of a QSI compound, production of the toxic gene product is switched off and the bacteria are rescued and able to grow normally. The toxic gene product of the QSIS1 selector system is cytolytic phospholipase A, which originates from Serratia liquefaciens MG1 (33, 34). Because signal molecules are added to the assay from an external source, QSIs targeting the AHL synthetase are not identified, whereas compounds that enhance the degradation of AHL, impede AHL uptake, or block the interaction of AHL with the luxR protein are found.
Briefly, the preparation of QSIS1 screens was performed as follows: BT medium (B medium [16] plus 2.5 mg thiamine per liter) containing 2% agar (wt/vol) was melted, 10% A10 buffer (16) was added, and the mixture was cooled to 45°C. To the resulting ABT agar were then added N-3-oxo-hexanoyl-l-homoserine lactone (Sigma-Aldrich, Germany), ampicillin (VepiDan, Denmark), 5-bromo-4-chloro-3-indoxyl-β-d-galactopyranoside (X-Gal; Apollo Scientific, United Kingdom), and 1-methyl-2-pyrrelidon (Merck, Germany) to final concentrations of 100 nM, 100 μg/ml, 40 μg/ml, and 0.2% (vol/vol), respectively; and the medium was supplemented with 0.5% (wt/vol) glucose and 0.5% (wt/vol) Casamino Acids. An overnight culture of QSIS1 (grown in ABT medium supplemented with 0.5% [wt/vol] glucose, 0.5% [wt/vol] Casamino Acids, and 100 μg/ml ampicillin) was added to a final concentration of 0.4%.
Plates for the screening assay were subsequently made by pouring the mixture into a box to give an agar thickness of 5 mm. Wells with diameters of 5 mm were made in the agar plates. Upon solidification, 50 μl of a 1- to 10-μg/ml solution of the antibiotic to be tested for QSI activity was added to the wells. The plate was then incubated at 30°C for 16 h. The appearance of a circular zone of growth (which appeared blue due to the presence of hydrolyzed X-Gal) indicated the presence of compounds with QSI activity in the sample tested.
Sample preparation for P. aeruginosa GeneChip analysis.
ABT medium supplemented with 0.5% Casamino Acids was inoculated with exponentially growing P. aeruginosa PAO1 cells to an optical density at 600 nm (OD600) of 0.05, and the cells were grown at 37°C and 200 rpm in five 500-ml flasks containing 100 ml each. When the OD600 was 0.3, AZM (8 and 2 μg/ml; Zithromax; Pfizer), CPR (0.04 μg/ml; Fluka), or CFT (0.25 μg/ml; Fortum; GlaxoSmithKline) was added. These concentrations of antibiotics did not affect the growth of P. aeruginosa. Samples were retrieved when the OD600 reached 2.0, immediately transferred to 2 volumes of RNAlater (Ambion), and stored at −80°C. RNA was isolated with an RNeasy mini purification kit (Qiagen), according to the protocol provided by the manufacturer, including the on-column DNase treatment. The synthesis of cDNA was performed with 12 μg RNA, 300 ng/μl random primers (Invitrogen), 1,500 U SuperScript III reverse transcriptase (Invitrogen), and 30 U SUPERase·In RNase inhibitor (Ambion), according to the Affymetrix expression analysis protocol. The cDNA synthesized was purified with a QIAquick PCR purification kit (Qiagen), and 3 to 4 μg cDNA was fragmented with 0.2 U FPLC pure DNase I (Amersham Bioscience) per μg cDNA. The fragmented cDNA was labeled at the terminus with biotin-ddUTP (Enzo Bioarray terminal labeling kit) and hybridized for 18 h at 55°C to a P. aeruginosa genome microarray GeneChip (Affymetrix). The chips were washed and stained according to the Affymetrix protocol. The microarray hybridization signal intensity was scaled to an overall signal average of 500; and the microarray was evaluated for the presence, marginal presence, or absence of probe sets (representing different genes or open reading frames) by the use of Affymetrix GeneChip operating system (version 1.4) software. The expression values only for probe sets estimated as being “present” were included in the further analyses. The microarray experiments were conducted twice for the antibiotic-treated cultures (two arrays for 2 μg/ml AZM, two arrays for 8 μg/ml AZM, two arrays for 0.04 μg/ml CPR, and two arrays for 0.25 μg/ml CFT), while the untreated controls (references) were tested in four replicates. The data presented here are based on the average of each set.
Quantification of mRNA by real-time PCR. (i) Primer design.
The primers used for mRNA amplification (Table 1) were designed by the use of Integrated DNA Technologies Primer Quest software (http://www.idtdna.com). Briefly, the sizes of the amplicon designed ranged from 80 to 200 bp, and the primer melting temperatures were designed for 60°C, with a melting temperature difference of less than 2°C for each primer pair. The primer sequences were also subjected to a BLAST analysis against the P. aeruginosa PAO1 genome sequence to eliminate the possibility of nonspecific binding.
TABLE 1.
Primers used for real-time PCR
| Gene | Forward | Reverse |
|---|---|---|
| rhlA | 5′-TCT GTT GGT ATC GGT TTG CAA GGG-3′ | 5′-ACA GCA CCA CGT TGA AAT GTT CGG-3′ |
| pprA | 5′-TGC TGT TGG GCA TGG ACA TCT-3′ | 5′-AGA CGC GAA AGG ATC AGC TT-3′ |
| pprB | 5′-TCA AGT ACG AGG TAC ACG GCA ACA-3′ | 5′-TAT CTG GTA GTT GGT CAG GCC CTT-3′ |
| rpoD | 5′-ACA TGC GTG AAA TGG GTA CCG T-3′ | 5′-ATG CGC TTG GCG ATT TCG ATC T-3′ |
(ii) Real-time PCR.
Each real-time PCR mixture (final volume, 20 μl) contained 9 μl of cDNA, 10 μl of 2×SYBR green quantitative PCR master mix (Fermentas), and 0.5 μM of each forward and reverse primer. Real-time PCR was performed with a Chromo4 real-time detector (Bio-Rad [formerly MJ Research]) by using the following cycling parameters: an initial denaturation at 95°C for 10 min before 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. The data were analyzed by the efficiency-corrected relative quantification method (71). Expression of the target genes was normalized to the expression of reference gene rpoD, as the DNA microarray analysis showed that the expression of this gene was stable under all conditions. Furthermore, Savli et al. (77) have found that among the housekeeping genes, this gene has the most stable expression. To control for variations between runs, the housekeeping gene and the various target genes for each individual treatment were amplified at the same time on a 96-well plate. The changes in the levels of expression of the target genes in the treated samples were calculated relative to the average level of expression of the respective gene in the nontreated sample. The efficiency of the reverse transcription-PCR (RT-PCR) for the gene pairs (under treated versus untreated conditions) varied less than 5%.
Accordingly, the average efficiencies of each gene in this study were very similar for the conditions compared, allowing accurate analysis.
Supernatants for virulence factor assays.
P. aeruginosa PAO1 cultures were grown either for 16 to 20 h (for hemolysis assay) or to an OD600 of 2.0 (for the protease, chitinase, and elastase assays), as described above, with 8 or 2 μg/ml AZM, 0.04 μg/ml CPR, 0.25 μg/ml CFT, or no antibiotic (control). The P. aeruginosa PAO1 QS mutant ΔlasI-ΔrhlI was also grown along with the treated and untreated P. aeruginosa PAO1 cultures. Cells were harvested by centrifugation, and the supernatants were filtered sterilized (TPP syringe filter; pore size, 0.22 μm). The supernatants were either used immediately or stored at −20°C.
Hemolysis assay.
The filter-sterilized supernatants were autoclaved to destroy enzymatic activity and mixed with venous blood from a healthy individual at a volumetric ratio of 30:1 (supernatant to blood), observed for hemolysis after 20 min, and left at room temperature for 4 h to allow the sedimentation of intact erythrocytes. Hemolytic activity was observed as a clearing and a nonprecipitating red coloring of the blood solution, while a lack of hemolytic activity was characterized by the precipitation of intact erythrocytes. The experiment was performed four times with supernatants from four individual growth experiments.
Protease assay.
The filter-sterilized supernatants (300 μl each) were added to the wells of ABT agar plates (ABT medium supplemented with 2% agar) containing 5% skim milk, and the plates were incubated overnight at 37°C. The zones of clearance (radii) were measured on photographs of the plates by the use of a ruler. The experiment was performed three times as four replicates with supernatants from three individual growth experiments. The values from the four replicates were averaged and used as one value to represent each of the three experiments.
Elastase assay.
The filter-sterilized supernatants were mixed 2:1 with phosphate buffer (0.1 M, pH 6.3), and 2 mg/ml elastin Congo red (Sigma) was added. The mixture was incubated at 37°C with shaking (200 rpm) for 1 week. After centrifugation, the absorbance of the supernatant at 495 nm was measured with a spectrophotometer zeroed on an elastin Congo red sample incubated with medium alone. The procedure was modified from that described previously (63). The experiment was performed three times in triplicate with supernatants from three individual growth experiments. The values from the triplicate experiments were averaged and used as one value to represent each of the three experiments.
Chitinase assay.
Chitinase was measured by a modified chitin azure assay (30, 36). The filter-sterilized supernatants were mixed 2:1 with sodium citrate buffer (0.1 M, pH 4.8), and 0.5 mg/ml chitin azure (Sigma) was added. The supernatant-chitin azure mixtures were incubated at 37°C with shaking (200 rpm) for 1 week. The samples were then centrifuged at 15,000 × g for 10 min, and the absorbance at 570 nm was determined. The samples were compared to blanks incubated with medium only. The experiment was performed three times in triplicates with supernatants from three individual growth experiments. The values from the triplicate experiments were averaged and used as one value to represent each of the three experiments.
Quantification of rhamnolipid.
A standard curve for rhamnolipid B (concentration versus total ionization current) was calculated from liquid chromatography-electrospray ionization-mass spectrometry (MS) data. To minimize potential differences in the ionization levels of rhamnolipid between the samples, the rhamnolipid standards used to calculate the concentration curve were analyzed immediately prior to as well as after the samples were analyzed. The total ionization current was determined on the [M + NH4]+ ion at 668.4 over the 7 s over which rhamnolipid B was eluted. High-pressure liquid chromatography (LC)-MS analysis was performed with an Agilent 1100 series high-pressure liquid chromatograph connected to a Micromass LCT time-of-flight mass spectrometer. The concentration of rhamnolipid in the untreated culture was set equal to an index value of 100.
Docking of AZM, CFT, and CPR against the LBD of the LasR protein.
The X-ray crystallographic structure of the ligand binding domain (LBD) of the LasR protein (Protein Data Bank accession no. 2UV0) was used for the docking of AZM, CFT, and CPR to the LasR receptor site by using the program Molegro Virtual Docker (86) and the template docking model with default settings. OdDHL was set as the template ligand in the LBD of the LasR protein. The ligands were ranked by using the Molegro Virtual Docker rerank scoring function.
Data analysis and statistics.
One-way analysis of variance followed by Dunnett's test was used to evaluate the effects of AZM, CFT, and CPR treatment and the effect of the lasI-rhlI mutation on the expression of elastases, chitinase, and protease compared to their expression in untreated wild-type strain P. aeruginosa PAO1. Tests were performed with the InStat (version 3) program from GraphPad Software (San Diego, CA). Differences were considered significant if the P value was <0.05 or less.
Microarray data accession number.
The DNA microarray data (CHP, CEL, and EXP files) have been submitted to the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) and may be found under experiment series number GSE8953.
RESULTS
Screening for QSI activity.
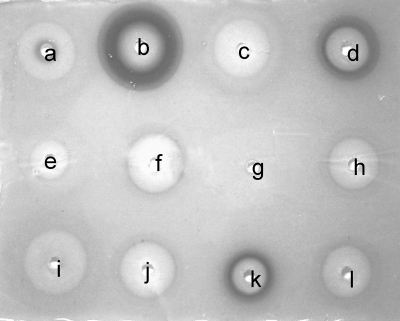
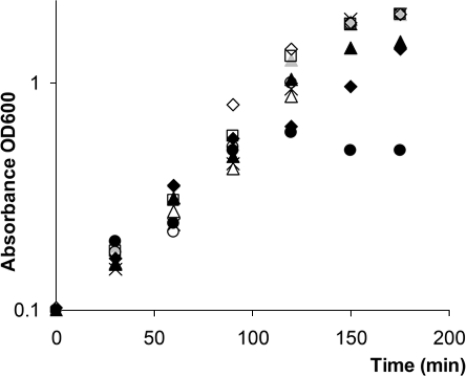
AZM has been shown to inhibit the expression of a subfraction of QS-controlled genes (60, 84). Along with AZM, we have investigated 11 other antibiotics (including one antifungal compound, griseofulvin) for their abilities to interfere with bacterial QS using a simple plate diffusion screen based on QSIS1 (73). By employing our standard conditions (73), we found that chloramphenicol, gentamicin, griseofulvin, kanamycin, piperacillin, spectinamycin, tetracycline, tobramycin, and streptomycin showed low levels of or no QSI activity, whereas AZM, in accordance with previous investigations, was very active. The fluoroquinolone CPR and the cephalosporin CFT also showed strong QSI activities in the assay with QSIS1 (Fig. 1). CPR and CFT are employed in the clinic as antipseudomonal treatments, whereas AZM exhibits a low level of, if any, bactericidal or bacteriostatic effect against P. aeruginosa. CPR targets DNA gyrase (topoisomerase II) and thereby inhibits bacterial DNA synthesis. CFT is a β-lactam and functions by disrupting the synthesis of the peptidoglycan layer of bacterial cell walls. The mode of action of AZM is to bind to the 50S subunit of the bacterial ribosome, thereby inhibiting the translation of mRNA. These three antibiotics therefore exhibit diverse mechanisms of antimicrobial action, and they are structurally very different. We chose to further investigate the effects on P. aeruginosa QS-regulated gene expression and virulence factor production with AZM, CPR, and CFT as representatives of these widely diverse groups of antibiotics. The growth curves for P. aeruginosa PAO1 grown in the presence or absence of AZM, CPR, and CFT at different concentrations (including concentrations with slightly growth-inhibiting effects) and for the ΔlasI ΔrhlI mutant are presented in Fig. 2. We found that addition of 8 μg/ml AZM, 0.25 μg/ml CFT, or 0.04 μg/ml CPR did not affect the growth of P. aeruginosa cultures when drug was added when the OD600 was 0.1. The MICs of the three antibiotics were found to be 800 μg/ml (AZM), 8 μg/ml (CFT), and 12.5 μg/ml (CPR) under the specified growth conditions. Thus, for the following series of experiments, the concentrations applied were 30- to 300-fold lower than the MICs.
FIG. 1.
Screening of antibiotics by the QSIS1 assay. (a) Piperacillin; (b) azithromycin; (c) chloramphenicol; (d) ciprofloxacin; (e) gentamicin; (f) spectinamycin; (g) griseofulvin; (h) kanamycin; (i) tetracycline; (j) tobramycin; (k) ceftazidime; (l) streptomycin.
FIG. 2.
Growth curves for the ΔlasI-ΔrhlI mutant (□) and strain PAO1 grown without antibiotics (×) or PAO1 grown in the presence of 2 μg/ml AZM (Δ), 8 μg/ml AZM ( ), 16 μg/ml AZM (▴), 0.04 μg/ml CPR (○), 0.08 μg/ml CPR (•), 0.25 μg/ml CFT (⋄), or 0.5 μg/ml CFT (⧫).
Heat-stable hemolysin.
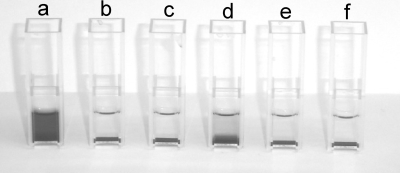
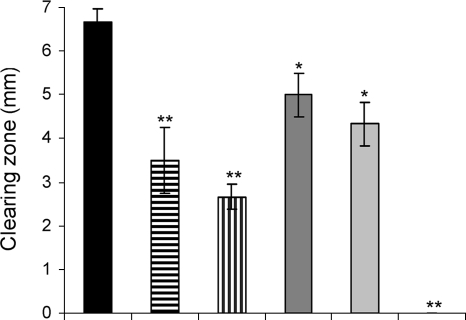
We investigated the effects of AZM, CPR, and CFT on the production of heat-stable hemolysin. P. aeruginosa produces at least two hemolysins, the heat-labile phospholipase C (phlC, PA0844) and the heat-stable rhamnolipid (rhlA, rhlB, rhlC, and rhlG; PA3479, PA3478, PA1130, and PA3387, respectively) (9, 46, 83). Rhamnolipid synthesis is turned on in early stationary phase of P. aeruginosa PAO1 cultures (62, 89). After 14 to 20 h of growth (under the conditions outlined in Materials and Methods), the concentration of rhamnolipid in the supernatant exceeds 50 μg/ml, which is sufficient to lyse erythrocytes within 20 min (unpublished observations). The lysis of erythrocytes by rhamnolipid can be used as a simple assay for the detection of rhamnolipid by mixing autoclaved supernatant with blood and incubating the mixture at room temperature for 20 min. The autoclaving eliminates the activity of the heat-labile enzyme phospholipase. A clearing and a nonprecipitating red coloring of the blood-supernatant solution indicates that rhamnolipid is present at a concentration well above 50 μg/ml, while a lack of hemolytic activity (characterized by the precipitation of intact blood cells) indicates that little (less than 50 μg/ml) or no rhamnolipid is present in the supernatant. Investigating autoclaved supernatants from P. aeruginosa grown in the presence or the absence of AZM, CPR, and CFT and supernatants from the QS mutant ΔlasI-ΔrhlI, we found that only the untreated wild type produced enough rhamnolipid to cause the rapid hemolysis of red blood cells. However, treatment with CFT did not fully abolish the hemolytic activity (Fig. 3).
FIG. 3.
Hemolysis of autoclaved supernatants from P. aeruginosa PAO1 cultures grown for 20 h. PAO1 was grown without antibiotics (a) and in the presence of 2 μg/ml AZM (b), 8 μg/ml AZM (c), 0.25 μg/ml CFT (d), or 0.04 μg/ml CPR (e). The results for the ΔlasI-ΔrhlI mutant of PAO1 are also shown (f).
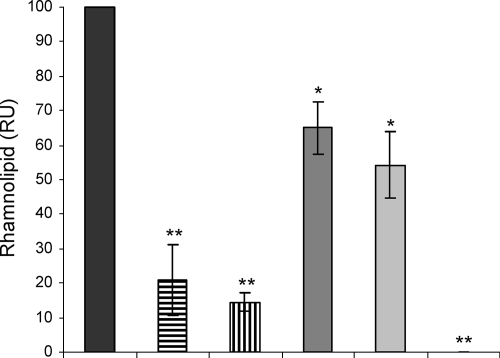
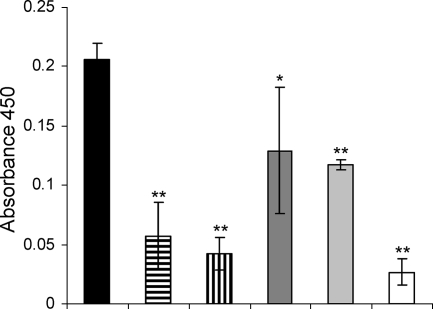
AZM at sub-MICs has previously been reported to affect an rhlAB::lacZ gene fusion (84). We decided to directly quantify the effect of AZM as well as the effects of CFT and CPR on rhamnolipid production. We thus determined the concentration of rhamnolipid in the supernatants of treated and untreated P. aeruginosa cells by LC-MS. When the cells were grown in the presence of 8 μg/ml AZM, the concentration of rhamnolipid was reduced by 85% compared to that in the untreated cultures, and 2 μg/ml AZM reduced the rhamnolipid content of the supernatants by 80% compared to that in the untreated cultures, whereas CPR (0.04 μg/ml) and CFT (0.25 μg/ml) reduced the concentration of rhamnolipid to 55 to 65% of that in the untreated cultures. The QS mutant ΔlasI-ΔrhlI did not produce any measurable amount of rhamnolipid (Fig. 4).
FIG. 4.
Rhamnolipid contents, in relative units (RU), in supernatants from P. aeruginosa cultures grown either without antibiotics (black bar) or in the presence of 2 μg/ml AZM (bar with horizontal lines), 8 μg/ml AZM (bar with vertical lines), 0.25 μg/ml CFT (dark gray bar), or 0.04 μg/ml CPR (light gray bar). The ΔlasI-ΔrhlI mutant of PAO1 was also included in the experiment but did not produce a measurable concentration of rhamnolipid (last slot [no column visible]). The concentration of rhamnolipid in the untreated culture was set equal to an index value of 100. The graph is based on the average of the indexes of three independent experiments. *, P < 0.05; **, P < 0.01 (Dunnett's post test).
Protease.
The production of extracellular protease in P. aeruginosa PAO1 cultures grown in the absence or the presence of AZM, CPR, or CFT and in ΔlasI-ΔrhlI mutant cultures was assayed by adding filter-sterilized supernatants from overnight cultures to wells made in agar plates containing 5% skim milk. The protease activity was lowered by AZM, CPR, and CFT (Fig. 5).
FIG. 5.
Protease activities of supernatants from P. aeruginosa cultures grown either without antibiotics (black bar) or in the presence of 2 μg/ml AZM (bar with horizontal lines), 8 μg/ml AZM (bar with vertical lines), 0.25 μg/ml CFT (dark gray bar), or 0.04 μg/ml CPR (light gray bar). The ΔlasI-ΔrhlI mutant of PAO1 was also included in the experiment but did not produce a measurable concentration of protease (last slot [no column visible]). The graph is based on the average results of three independent experiments. *, P < 0.05; **, P < 0.01 (Dunnett's test).
Elastase.
The extracellular elastase activities in the supernatants of P. aeruginosa PAO1 cultures (untreated or grown with AZM, CPR, or CFT) were quantified by use of a previously described procedure (63). The QS mutant ΔlasI-ΔrhlI was assayed by the same procedure. AZM treatment reduced the elastase activity of PAO1 almost to the level of that for the ΔlasI-ΔrhlI mutant. CPR and CFT also reduced the elastase activity (Fig. 6).
FIG. 6.
Elastolytic activities of supernatants from PAO1 cultures grown to an OD600 of 2.0 either without antibiotics (black bar) or in the presence of: 2 μg/ml AZM (bar with horizontal lines), 8 μg/ml AZM (bar with vertical lines), 0.25 μg/ml CFT (dark gray bar), or 0.04 μg/ml CPR (light gray bar). The elastolytic activity of the ΔlasI-ΔrhlI mutant of PAO1 is also shown (white bar). The graph is based on the average results of three independent experiments. *, P < 0.05; **, P < 0.01 (Dunnett's post test).
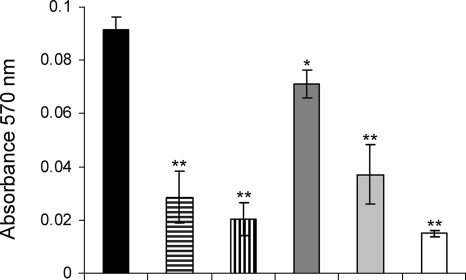
Chitinase.
The chitinase activities in the supernatants of P. aeruginosa PAO1 cultures (untreated or grown with AZM, CPR, or CFT) and QS mutant ΔlasI-ΔrhlI cultures were quantified by measuring the degradation of chitin azure. AZM and CPR reduced the chitinolytic activity of PAO1 almost to the level of that for the ΔlasI-ΔrhlI mutant. CFT did not decrease chitinase activity as much as AZM and CPR did, although it still had an effect (Fig. 7).
FIG. 7.
Chitinase activities of supernatants from PAO1 cultures grown to an OD600 of 2.0 either without antibiotics (black bar) or in the presence of: 2 μg/ml AZM (bar with horizontal lines), 8 μg/ml AZM (bar with vertical lines), 0.25 μg/ml CFT (dark gray bar), or 0.04 μg/ml CPR (light gray bar). The chitinase activity of the ΔlasI-ΔrhlI mutant of PAO1 is also shown (white bar). The graph is based on the average results of three independent experiments. *, P < 0.05; **, P < 0.01 (Dunnett's post test).
DNA microarray analysis of gene expression in the presence of AZM, CFT, and CPR.
In order to obtain knowledge of the mechanism by which virulence factor production was altered by the presence of the three antibiotics, we performed DNA microarray analyses with P. aeruginosa cultures treated with AZM, CPR, and CFT at concentrations that did not affect growth. The medium with exponentially growing P. aeruginosa cultures (OD600, 0.1) was supplemented with either AZM (2 and 8 μg/ml), CFT (0.25 μg/ml), or CPR (0.04 μg/ml) or the medium was left untreated as a control. By applying the growth conditions described in Materials and Methods, an OD600 of 2.0 corresponds to the transition from exponential phase into stationary phase, and at this point a substantial fraction of QS-regulated genes are maximally regulated (induced or repressed) (80). An OD600 of 2.0 was therefore chosen as the sample point for the transcriptomic analysis. Total RNA was extracted from the samples and subjected to cDNA synthesis. The absolute expression values from cultures grown in the presence of antibiotics were compared with those from untreated cultures, and the changes in expression are reported. As described previously (38, 89), changes in expression of less than fivefold were disregarded. Only probe sets that were present, as validated with Affymetrix GeneChip operating system (version 1.4) software, were included in the analyses. According to these criteria, non-growth-inhibitory concentrations of AZM altered the expression of 477 (8 μg/ml) and 323 (2 μg/ml) genes, with most genes (419 and 277, respectively) being downregulated. Non-growth-inhibitory concentrations of CFT (0.25 μg/ml) and CPR (0.04 μg/ml) affected the expression of 122 (114 repressed) and 271 genes (215 repressed), respectively (Table 2).
TABLE 2.
Effect on gene expression
| Compound (concn) | No. of genes downregulated more than fivefold | No. of genes upregulated more than fivefold | No. (%) of QS genesa affected more than fivefold | Specificity (%)b |
|---|---|---|---|---|
| AZM (8 μg/ml, 11 μM) | 427 | 61 | 121 (70) | 28 |
| AZM (2 μg/ml, 2.7 μM) | 227 | 49 | 81 (47) | 35 |
| CFT (0.25 μg/ml, 0.4 μM) | 136 | 10 | 49 (28) | 36 |
| CPR (0.04 μg/ml, 0.1 μM) | 223 | 58 | 89 (51) | 40 |
| C30c (2.5 μg/ml, 10 mM) | 260 | 0 | 125 (72) | 48 |
AZM, CFT, and CPR affect expression of QS-regulated genes.
The QS regulon has been defined by (among others) Rasmussen et al. (74). That particular study (74) considered genes to be regulated by QS if their levels of expression in both ΔlasI-ΔrhlI and ΔlasR-ΔrhlR mutants were consistently altered more than fivefold compared to the levels of expression in their wild-type counterpart. The majority of all QS-regulated genes defined in this way are inducible with exogenous AHLs in a ΔlasI-ΔrhlI background. Only one gene (PA1556, a probable cytochrome c oxidase subunit) is consistently upregulated in both the ΔlasI-ΔrhlI and the ΔlasR-ΔrhlR mutants and is thus QS repressible. Using this QS regulon, we analyzed the fraction of QS-regulated genes among the genes repressed by the three antibiotics. These analyses showed that AZM (8 μg/ml) repressed 71% of the 174 QS-regulated genes in P. aeruginosa PAO1 and that 29% of all genes being repressed by AZM were members of the QS regulon. Lowering of the concentration of AZM to 2 μg/ml caused a relief in the inhibition of QS-regulated genes (47% of all QS genes repressed), and the total number of genes downregulated fell to 277, while the specificity toward the QS regulon increased slightly to 30%. CPR had the highest specificity for the QS regulon (48%) and targeted 49% of all QS genes in P. aeruginosa, whereas the specificity for CFT was 39% and CFT repressed only 25% of all QS genes. For comparison, the specificity of C30 was 48%, and it downregulated 72% of all QS genes in P. aeruginosa (raw data for C30 are from a previous report [38] and have been subjected to the same data treatment and criteria as the raw data for AZM, CFT, and CPR). The effects of AZM, CFT, and CPR on the expression of genes belonging to the QS regulon are shown in Table 3.
TABLE 3.
Effects of sub-MICs of AZM, CFT, and CPR on P. aeruginosa genes belonging to the QS regulona
| Gene in P. aeruginosa | Gene | pprBb | Regulation by AZMc | Reg.d | Relative expression |
Description | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AZM (2 μg/ml) | AZM (8 μg/ml) | CFT | CPR | lasl-rhlI | lasR-rhlR | lasR | rhlR | ||||||
| PA0059 | osmC | C | −19.8 | −63.4 | −8.6 | −16.8 | −22.0 | −31.8 | −44.0 | −25.8 | Osmotically inducible protein OsmC | ||
| PA0105 | coxB | C | −20.0 | −25.3 | −8.3 | −10.5 | −11.5 | −13.5 | −18.7 | −7.9 | Cytochrome c oxidase, subunit II | ||
| PA0107 | C | −91.9 | −29.0 | −11.0 | −17.0 | −17.4 | −14.1 | −18.6 | −11.4 | Conserved hypothetical protein | |||
| PA0108 | colII | C | −14.2 | −33.8 | −13.2 | −12.2 | −27.8 | −8.5 | −15.8 | −6.3 | Cytochrome oxidase, subunit III | ||
| PA0122 | − | C | −3.0 | −14.2 | −1.5 | −2.9 | −44.8 | −26.1 | −7.1 | −6.0 | Conserved hypothetical protein | ||
| PA0188 | C | −2.9 | −32.5 | −4.8 | −4.6 | −8.1 | −21.3 | −28.2 | −8.8 | Hypothetical protein | |||
| PA0355 | pfpl | C | −7.5 | −12.5 | −3.6 | −6.6 | −11.8 | −21.5 | −17.7 | −11.6 | Protease Pfpl | ||
| PA0567 | C | −9.2 | −22.0 | −3.5 | −9.1 | −249.4 | −25.2 | −22.1 | −9.5 | Conserved hypothetical protein | |||
| PA0572 | A | −3.5 | −6.0 | −2.3 | −3.4 | −10.4 | −13.7 | −8.2 | −1.1 | Hypothetical protein | |||
| PA0737 | C | −3.3 | −3.4 | −4.1 | −4.3 | −9.4 | −7.7 | −94.4 | −5.8 | Hypothetical protein | |||
| PA0843 | plcR | A | −4.7 | −7.8 | −2.7 | −3.3 | −7.1 | −6.2 | −25.0 | −4.6 | Phospholipase accessory protein PlcR precursor | ||
| PA0852 | cbpD | − | C | −7.0 | −35.0 | −5.0 | −10.1 | −63.7 | −74.4 | −33.2 | −8.3 | Chitin-binding protein CbpD precursor | |
| PA0990 | A | −7.0 | −9.3 | −3.3 | −5.9 | −5.7 | −19.9 | −6.6 | −4.4 | Conserved hypothetical protein | |||
| PA0996 | pqsA | − | + | A | 2.0 | 1.7 | 1.3 | −1.3 | −7.0 | −20.8 | −34.5 | 3.3 | Probable coenzyme A ligase |
| PA0997 | pqsB | − | A | 1.2 | −1.2 | 1.1 | −1.0 | −12.6 | −15.4 | −59.2 | 3.1 | Homologous to beta-keto-acyl-acyl carrier protein synthase | |
| PA0998 | pqsC | − | A | 1.2 | −1.3 | 1.2 | −1.1 | −8.2 | −13.9 | −29.9 | 2.8 | Homologous to beta-keto-acyl-acyl carrier protein synthase | |
| PA0999 | pqsD | − | A | 1.6 | −1.2 | −1.0 | −1.3 | −8.2 | −9.8 | −20.2 | 2.6 | 3-Oxoacyl-[acyl carrier protein] synthase III | |
| PA1000 | pqsE | A | 2.1 | 1.1 | 1.4 | 1.3 | −7.9 | −12.0 | −28.9 | 3.6 | Quinolone signal response protein | ||
| PA1001 | phnA | − | A | 1.9 | 1.0 | −1.0 | −1.6 | −6.6 | −9.7 | −20.9 | 3.2 | Anthranilate synthase component I | |
| PA1002 | phnB | A | 1.5 | −1.1 | 1.2 | −1.3 | −7.3 | −5.2 | −6.3 | 5.5 | Anthranilate synthase component II | ||
| PA1131 | B | −1.9 | −5.2 | −1.4 | −2.4 | −5.9 | −11.1 | −2.9 | −6.6 | Probable major facilitator superfamily transporter | |||
| PA1168 | A | −6.5 | −8.1 | −3.8 | −3.3 | −21.5 | −19.1 | −26.6 | −3.9 | Hypothetical protein | |||
| PA1190 | C | −19.2 | −26.3 | −9.8 | −8.9 | −14.6 | −12.4 | −12.5 | −10.4 | Conserved hypothetical protein | |||
| PA1242 | C | −5.7 | −10.1 | −5.5 | −18.7 | −19.6 | −21.3 | −6.7 | −9.2 | Hypothetical protein | |||
| PA1249 | aprA | D | −23.5 | −54.2 | −3.5 | −5.3 | −7.3 | −8.4 | −4.1 | −2.7 | Alkaline metalloproteinase precursor | ||
| PA1250 | aprl | D | −1.2 | −1.5 | 1.1 | −1.5 | −9.7 | −5.5 | −3.0 | −1.0 | Alkaline proteinase inhibitor Aprl | ||
| PA1323 | C | −11.2 | −35.6 | −4.3 | −9.6 | −25.3 | −39.3 | −56.5 | −15.5 | Hypothetical protein | |||
| PA1324 | C | −10.5 | −23.4 | −4.8 | −8.1 | −22.1 | −26.7 | −39.2 | −17.0 | Hypothetical protein | |||
| PA1412 | C | −5.5 | −11.2 | −22.3 | −7.0 | −5.7 | −5.6 | −8.8 | −6.6 | Hypothetical protein | |||
| PA1431 | rsaL | − | A | −2.6 | −2.2 | −1.3 | −2.2 | −3,619.1 | −3,441.9 | −3,038.0 | −1.9 | Regulatory protein RsaL | |
| PA1432 | lasl | − | A | −1.9 | 1.2 | 1.8 | 1.7 | −97.0 | −133.1 | −39.0 | −1.5 | Autoinducer synthesis protein LasI | |
| PA1471 | C | −4.5 | −5.8 | −2.0 | −2.9 | −7.8 | −5.6 | −10.3 | −9.7 | Hypothetical protein | |||
| PA1556 | C | 15.6 | 17.5 | 9.9 | 9.2 | 7.9 | 6.0 | 7.8 | 5.3 | Probable cytochrome c oxidase subunit | |||
| PA1625 | C | −3.7 | −5.1 | −3.7 | −7.4 | −6.6 | −9.0 | −6.5 | −5.5 | Conserved hypothetical protein | |||
| PA1657 | C | 1.6 | −1.5 | −1.5 | 3.5 | −12.7 | −15.4 | −12.7 | −5.2 | Conserved hypothetical protein | |||
| PA1662 | D | −1.5 | −3.5 | −2.3 | −1.4 | −5.7 | −9.0 | −4.3 | −4.4 | Probable ClpA/B-type protease | |||
| PA1664 | − | C | 1.6 | −2.4 | −1.3 | 1.1 | −5.2 | −11.0 | −15.5 | −8.7 | Hypothetical protein | ||
| PA1665 | A | 1.1 | −2.7 | −2.5 | 1.1 | −5.6 | −46.9 | −15.1 | −2.8 | Hypothetical protein | |||
| PA1667 | A | −1.5 | −4.1 | −1.1 | −1.1 | −5.5 | −5.1 | −5.0 | −3.1 | Hypothetical protein | |||
| PA1669 | B | −6.3 | −2.8 | −2.0 | −1.4 | −28.6 | −12.7 | −3.8 | −10.3 | Hypothetical protein | |||
| PA1784 | A | −5.5 | −14.6 | −2.4 | −4.4 | −9.4 | −11.2 | −9.9 | −1.4 | Hypothetical protein | |||
| PA1869 | − | C | 1.2 | −3.2 | −1.0 | −2.7 | −137.1 | −287.9 | −23.6 | −25.1 | Probable acyl carrier protein | ||
| PA1870 | − | C | −4.3 | −11.8 | −6.9 | −4.8 | −22.0 | −12.7 | −8.8 | −13.8 | Hypothetical protein | ||
| PA1871 | lasA | − | − | C | −10.3 | −32.9 | −2.9 | −9.0 | −105.4 | −147.8 | −31.3 | −7.1 | LasA protease precursor |
| PA1874 | A | −7.8 | −9.3 | −6.0 | −6.6 | −8.0 | −6.4 | −7.9 | 1.9 | Hypothetical protein | |||
| PA1875 | A | −7.5 | −16.7 | −12.3 | −20.5 | −34.2 | −9.0 | −6.7 | 1.1 | Probable outer membrane protein precursor | |||
| PA1901 | phzC2 | − | C | −1.4 | −27.6 | −3.1 | −4.1 | −21.8 | −25.3 | −19.1 | −51.2 | Phenazine biosynthesis protein PhzC | |
| PA1902 | phzD2 | − | C | −1.3 | −42.9 | −3.5 | −4.5 | −133.5 | −132.0 | −97.7 | −91.0 | Phenazine biosynthesis protein PhzD | |
| PA1903 | phzE2 | − | C | −1.3 | −20.0 | −2.7 | −4.5 | −17.2 | −24.4 | −18.6 | −19.7 | Phenazine biosynthesis protein PhzE | |
| PA1904 | phzF2 | − | C | 1.1 | −20.8 | −3.5 | −3.8 | −134.0 | −61.6 | −77.4 | −44.7 | Probable phenazine biosynthesis protein | |
| PA1905 | phzG2 | − | C | 1.2 | −15.9 | −2.6 | −4.4 | −14.1 | −32.7 | −20.8 | −30.0 | Probable pyridoxamine 5′-phosphate oxidase | |
| PA1914 | A | −24.0 | −31.9 | −5.5 | −6.8 | −92.8 | −15.1 | −100.0 | −1.4 | Conserved hypothetical protein | |||
| PA2021 | C | −12.2 | −14.1 | −5.0 | −11.5 | −8.6 | −12.1 | −12.2 | −13.2 | Hypothetical protein | |||
| PA2046 | C | −5.9 | −25.2 | −5.9 | −8.6 | −32.6 | −22.1 | −38.7 | −45.5 | Hypothetical protein | |||
| PA2067 | A | −3.5 | −13.4 | −2.1 | −5.9 | −6.0 | −5.7 | −5.3 | −4.2 | Probable hydrolase | |||
| PA2068 | − | C | −3.2 | −13.4 | −1.8 | −5.5 | −8.5 | −19.4 | −18.5 | −25.0 | Probable major facilitator superfamily transporter | ||
| PA2069 | − | C | −4.2 | −70.4 | −2.3 | −6.7 | −29.3 | −72.9 | −26.1 | −26.8 | Probable carbamoyl transferase | ||
| PA2137 | C | −29.3 | −107.3 | −50.3 | −20.8 | −11.4 | −15.9 | −46.4 | −71.3 | Hypothetical protein | |||
| PA2139 | C | −28.3 | −20.2 | −14.1 | −20.7 | −9.5 | −10.0 | −7.7 | −24.3 | Hypothetical protein | |||
| PA2141 | C | −31.2 | −23.4 | −8.2 | −33.2 | −5.8 | −45.3 | −80.1 | −69.0 | Hypothetical protein | |||
| PA2142 | C | −27.1 | −18.9 | −12.3 | −19.5 | −13.4 | −16.4 | −18.2 | −26.8 | Probable short-chain dehydrogenase | |||
| PA2143 | C | −82.1 | −43.2 | −23.0 | −42.5 | −67.1 | −60.1 | −191.3 | −83.7 | Hypothetical protein | |||
| PA2144 | glgp | A | −11.0 | −22.1 | −6.9 | −7.4 | −7.3 | −20.8 | −11.4 | −4.8 | Glycogen phosphorylase | ||
| PA2146 | − | C | −8.5 | −11.4 | −8.0 | −7.4 | −6.9 | −10.8 | −15.5 | −9.7 | Conserved hypothetical protein | ||
| PA2148 | A | −5.3 | −8.0 | −4.5 | −5.5 | −8.1 | −8.1 | −6.4 | −4.0 | Conserved hypothetical protein | |||
| PA2149 | C | −6.7 | −10.6 | −4.5 | −12.3 | −6.3 | −7.0 | −39.5 | −11.2 | Hypothetical protein | |||
| PA2151 | C | −47.9 | −71.1 | −11.2 | −13.5 | −39.3 | −124.4 | −41.2 | −30.6 | Conserved hypothetical protein | |||
| PA2153 | glgB | C | −15.0 | −30.0 | −4.2 | −6.4 | −6.9 | −11.3 | −19.1 | −9.2 | 1,4-Alpha-glucan branching enzyme | ||
| PA2158 | C | −23.8 | −68.3 | −12.3 | −43.9 | −65.0 | −10.3 | −35.9 | −52.4 | Probable alcohol dehydrogenase (Zn dependent) | |||
| PA2163 | C | −9.0 | −9.6 | −3.9 | −7.9 | −8.2 | −8.2 | −6.9 | −5.3 | Hypothetical protein | |||
| PA2165 | C | −16.5 | −19.7 | −7.5 | −14.4 | −8.7 | −5.4 | −6.2 | −7.5 | Probable glycogen synthase | |||
| PA2166 | C | −15.8 | −19.0 | −6.7 | −14.8 | −15.4 | −38.5 | −24.4 | −19.2 | Hypothetical protein | |||
| PA2167 | C | −11.0 | −86.2 | −7.4 | −18.1 | −8.7 | −8.1 | −7.1 | −15.4 | Hypothetical protein | |||
| PA2170 | C | −4.1 | −5.1 | −3.1 | −8.6 | −8.2 | −14.1 | −5.4 | −6.5 | Hypothetical protein | |||
| PA2171 | − | C | −10.1 | −9.9 | −7.0 | −21.7 | −27.6 | −109.9 | −58.6 | −22.5 | Hypothetical protein | ||
| PA2176 | C | −50.5 | −112.3 | −10.0 | −14.8 | −19.2 | −25.2 | −30.0 | −12.0 | Hypothetical protein | |||
| PA2178 | D | −7.0 | −3.6 | −3.6 | −21.9 | −11.2 | −6.3 | −4.3 | −4.9 | Hypothetical protein | |||
| PA2184 | C | −5.0 | −6.7 | −4.7 | −7.3 | −15.5 | −13.9 | −17.5 | −20.8 | Conserved hypothetical protein | |||
| PA2190 | − | C | −13.3 | −60.5 | −6.3 | −11.3 | −17.0 | −14.9 | −9.9 | −6.9 | Conserved hypothetical protein | ||
| PA2193 | hcnA | − | + | B | 3.5 | −1.5 | 1.7 | 1.4 | −262.2 | −256.6 | −3.0 | −9.1 | Hydrogen cyanide synthase HcnA |
| PA2194 | hcnB | − | B | 2.3 | −3.9 | −1.1 | −1.3 | −123.1 | −43.1 | −2.3 | −8.3 | Hydrogen cyanide synthase HcnB | |
| PA2195 | hcnC | − | B | 1.7 | −4.3 | 1.1 | −1.5 | −20.5 | −38.9 | −2.7 | −6.9 | Hydrogen cyanide synthase HcnC | |
| PA2300 | chiC | − | C | −20.2 | −45.4 | −8.1 | −20.7 | −33.8 | −66.7 | −82.8 | −34.0 | Chitinase | |
| PA2302 | − | A | −3.3 | −9.6 | −1.8 | −3.3 | −13.2 | −17.5 | −10.1 | −1.2 | Probable nonribosomal peptide synthetase | ||
| PA2303 | − | A | −3.1 | −10.5 | −1.5 | −2.1 | −87.9 | −212.8 | −59.2 | −1.3 | Hypothetical protein | ||
| PA2304 | − | A | −2.8 | −6.9 | −1.5 | −2.3 | −18.1 | −23.2 | −9.2 | 1.1 | Hypothetical protein | ||
| PA2305 | A | −1.7 | −2.8 | 1.1 | −1.5 | −6.5 | −16.7 | −8.8 | 1.1 | Probable nonribosomal peptide synthetase | |||
| PA2414 | C | −12.2 | −51.7 | −4.6 | −11.3 | −32.2 | −17.6 | −16.4 | −7.7 | l-Sorbosone dehydrogenase | |||
| PA2415 | C | −5.1 | −8.6 | −2.9 | −6.4 | −13.8 | −9.3 | −9.2 | −5.2 | Hypothetical protein | |||
| PA2423 | + | A | −1.2 | −1.2 | −1.1 | −1.6 | −5.8 | −5.9 | −7.6 | 1.6 | Hypothetical protein | ||
| PA2433 | C | −25.6 | −70.7 | −6.4 | −24.8 | −25.9 | −67.3 | −70.0 | −32.8 | Hypothetical protein | |||
| PA2485 | + | C | −7.9 | −8.1 | −2.3 | −7.0 | −12.0 | −18.0 | −15.5 | −12.3 | Hypothetical protein | ||
| PA2486 | C | −9.3 | −18.4 | −2.3 | −4.5 | −8.9 | −10.6 | −8.5 | −5.7 | Hypothetical protein | |||
| PA2566 | A | −4.2 | −6.8 | 1.1 | −3.3 | −9.6 | −7.1 | −6.3 | −1.7 | Conserved hypothetical protein | |||
| PA2570 | lecA | C | −25.9 | −76.4 | −4.5 | −14.9 | −45.4 | −49.2 | −27.2 | −30.3 | LecA | ||
| PA2587 | pqsH | − | A | −1.6 | −2.1 | −1.6 | −2.3 | −46.1 | −36.1 | −20.4 | 1.3 | Probable FAD-dependent mono-oxygenase | |
| PA2588 | A | −3.3 | −4.5 | −2.4 | −4.3 | −6.7 | −34.3 | −87.8 | −2.4 | Probable transcriptional regulator | |||
| PA2591 | D | −1.1 | −1.2 | 1.1 | −1.3 | −7.1 | −14.8 | −2.2 | 1.1 | Probable transcriptional regulator | |||
| PA2592 | D | 1.3 | −1.2 | 1.4 | −1.1 | −6.7 | −17.9 | −3.8 | −1.6 | Probable periplasmic spermidine/putrescine-binding protein | |||
| PA2708 | C | −4.1 | −4.7 | −4.2 | −6.1 | −5.8 | −7.8 | −8.7 | −12.5 | Hypothetical protein | |||
| PA2747 | + | C | −3.8 | −7.2 | −2.6 | −7.7 | −32.6 | −37.3 | −37.5 | −10.3 | Hypothetical protein | ||
| PA2751 | C | −3.4 | −4.4 | −2.4 | −4.3 | −8.5 | −7.0 | −10.3 | −6.0 | Conserved hypothetical protein | |||
| PA2754 | C | −5.0 | −9.2 | −2.4 | −3.5 | −11.5 | −7.3 | −9.1 | −5.4 | Conserved hypothetical protein | |||
| PA2777 | C | −4.4 | −26.5 | −4.6 | −10.1 | −8.4 | −6.2 | −10.5 | −8.9 | Conserved hypothetical protein | |||
| PA2873 | D | −1.2 | −7.8 | −1.3 | −3.1 | −13.2 | −5.8 | −1.6 | −1.3 | Hypothetical protein | |||
| PA2937 | C | −23.3 | −45.2 | −5.2 | −13.2 | −6.9 | −8.6 | −9.1 | −5.4 | Hypothetical protein | |||
| PA2939 | − | A | −10.6 | −38.6 | −3.5 | −6.5 | −39.8 | −9.1 | −12.7 | 1.8 | Probable aminopeptidase | ||
| PA3231 | C | −17.7 | −21.9 | −6.4 | −41.9 | −33.7 | −13.2 | −27.7 | −10.0 | Hypothetical protein | |||
| PA3273 | C | −10.2 | −20.7 | −4.0 | −19.7 | −13.3 | −13.2 | −13.2 | −27.1 | Hypothetical protein | |||
| PA3274 | C | −11.5 | −49.1 | −7.0 | −27.4 | −45.1 | −37.5 | −112.5 | −17.5 | Hypothetical protein | |||
| PA3326 | D | −1.5 | −4.2 | 1.3 | −1.0 | −12.3 | −11.0 | −2.4 | −4.1 | Probable Clp family ATP-dependent protease | |||
| PA3327 | − | C | −1.4 | −5.0 | −1.0 | 1.3 | −5.4 | −12.7 | −5.0 | −9.8 | Probable nonribosomal peptide synthetase | ||
| PA3328 | − | B | −1.3 | −4.5 | −1.1 | −1.2 | −6.5 | −26.0 | −3.2 | −12.4 | Probable FAD-dependent mono-oxygenase | ||
| PA3329 | − | C | 1.3 | −3.8 | 1.9 | 1.3 | −54.9 | −173.5 | −6.1 | −69.3 | Hypothetical protein | ||
| PA3330 | − | B | −4.3 | −1.1 | 1.7 | 1.3 | −62.4 | −179.6 | −4.4 | −19.5 | Probable short-chain dehydrogenase | ||
| PA3331 | − | B | 1.1 | −4.1 | 1.8 | 1.6 | −19.8 | −62.0 | −3.8 | −23.8 | Cytochrome P450 | ||
| PA3332 | − | B | 1.3 | −4.3 | 1.3 | 1.2 | −10.2 | −18.7 | −5.0 | −17.7 | Conserved hypothetical protein | ||
| PA3333 | fabH2 | − | B | −1.3 | −7.4 | 1.0 | −1.2 | −17.0 | −33.4 | −4.1 | −10.4 | 3-Oxoacyl-[acyl carrier protein] synthase III | |
| PA3334 | − | C | 1.2 | −3.8 | 1.8 | 1.7 | −42.8 | −17.4 | −5.1 | −11.2 | Probable acyl carrier protein | ||
| PA3335 | − | D | 1.1 | −3.9 | 1.8 | 1.2 | −18.4 | −9.4 | −2.3 | −3.9 | Hypothetical protein | ||
| PA3336 | B | −9.3 | −2.4 | 1.5 | −1.3 | −8.2 | −9.3 | −4.3 | −22.8 | Probable major facilitator superfamily transporter | |||
| PA3361 | lecB | C | −1.4 | −12.3 | −1.6 | −2.5 | −22.4 | −28.9 | −13.2 | −15.3 | Fucose-binding lectin PA-IIL | ||
| PA3369 | + | C | −7.1 | −11.9 | −4.2 | −12.7 | −19.9 | −42.2 | −36.5 | −14.2 | Hypothetical protein | ||
| PA3370 | + | C | −12.9 | −26.1 | −8.7 | −37.1 | −39.0 | −66.4 | −87.1 | −31.2 | Hypothetical protein | ||
| PA3371 | + | C | −6.7 | −11.7 | −7.4 | −18.8 | −23.8 | −51.7 | −69.2 | −25.7 | Hypothetical protein | ||
| PA3460 | A | −12.9 | −28.0 | −2.0 | −3.2 | −5.0 | −6.0 | −7.0 | −4.7 | Probable acetyltransferase | |||
| PA3476 | rhll | − | D | −1.6 | −2.0 | −1.2 | −1.6 | −19.0 | −41.4 | −2.4 | −1.8 | Autoinducer synthesis protein Rhll | |
| PA3478 | rhlB | − | C | −7.3 | −31.7 | −2.4 | −6.3 | −120.2 | −122.7 | −19.5 | −53.0 | Rhamnosyltransferase chain B | |
| PA3479 | rhlA | − | C | −5.0 | −28.3 | −1.8 | −5.3 | −320.2 | −196.0 | −14.5 | −55.0 | Rhamnosyltransferase chain A | |
| PA3520 | C | −3.7 | −8.9 | −2.7 | −4.4 | −28.3 | −18.3 | −13.9 | −7.4 | Hypothetical protein | |||
| PA3581 | glpF | C | −163.4 | −43.8 | −14.0 | −99.3 | −61.0 | −88.9 | −19.1 | −35.1 | Glycerol uptake facilitator protein | ||
| PA3584 | glpD | C | −9.2 | −12.2 | −8.1 | −6.3 | −5.7 | −20.4 | −12.0 | −8.4 | Glycerol-3-phosphate dehydrogenase | ||
| PA3691 | C | −5.1 | −13.3 | −2.0 | −3.1 | −11.6 | −19.2 | −28.8 | −12.3 | Hypothetical protein | |||
| PA3692 | C | −7.9 | −26.7 | −3.1 | −4.4 | −30.7 | −16.8 | −37.5 | −12.3 | Probable outer membrane protein precursor | |||
| PA3724 | lasB | − | C | −3.5 | −22.8 | −2.5 | −6.1 | −167.2 | −224.3 | −15.1 | −5.2 | Elastase LasB | |
| PA3734 | C | −5.0 | −9.3 | −3.5 | −4.9 | −7.2 | −21.3 | −6.6 | −8.5 | Hypothetical protein | |||
| PA3788 | A | −4.7 | −6.5 | −2.7 | −4.8 | −9.2 | −5.8 | −6.1 | −4.0 | Hypothetical protein | |||
| PA3819 | C | −3.5 | −5.0 | −3.7 | −3.6 | −6.3 | −7.0 | −9.7 | −7.1 | Conserved hypothetical protein | |||
| PA3888 | A | −2.5 | −3.2 | −2.1 | −3.5 | −5.8 | −10.2 | −10.5 | −4.6 | Probable permease of ABC transporter | |||
| PA3890 | C | −3.8 | −5.1 | −2.6 | −6.7 | −17.8 | −87.2 | −64.7 | −31.8 | Probable permease of ABC transporter | |||
| PA3904 | − | A | 1.9 | 1.5 | 1.5 | 1.6 | −68.3 | −40.7 | −172.1 | −1.0 | Hypothetical protein | ||
| PA3906 | − | A | 1.1 | −1.2 | −1.2 | −1.1 | −7.5 | −176.5 | −9.5 | −1.4 | Hypothetical protein | ||
| PA3907 | A | 1.4 | −1.1 | 1.2 | 2.0 | −5.8 | −13.4 | −5.2 | 1.0 | Hypothetical protein | |||
| PA3908 | − | D | −1.0 | −2.6 | −1.5 | 1.2 | −6.2 | −49.8 | −4.5 | 1.1 | Hypothetical protein | ||
| PA4078 | * | A | −6.2 | −15.8 | −4.3 | −6.4 | −5.6 | −11.0 | −7.4 | −4.8 | Probable nonribosomal peptide synthetase | ||
| PA4130 | − | D | 1.4 | −1.9 | −1.3 | 1.1 | −9.6 | −6.2 | −3.3 | 1.5 | Probable sulfite or nitrite reductase | ||
| PA4133 | − | A | 2.6 | −1.8 | −2.1 | 1.1 | −57.4 | −13.0 | −6.1 | 1.6 | Cytochrome c oxidase subunit (cbb3 type) | ||
| PA4134 | − | A | 1.9 | −3.3 | −3.3 | −1.8 | −12.0 | −5.6 | −18.0 | −1.2 | Hypothetical protein | ||
| PA4139 | A | −9.2 | −11.6 | −4.3 | −3.2 | −6.1 | −10.2 | −7.0 | −4.2 | Hypothetical protein | |||
| PA4141 | − | C | −2.0 | −12.2 | −1.1 | −1.6 | −73.1 | −105.0 | −27.6 | −57.6 | Hypothetical protein | ||
| PA4142 | C | −2.1 | −11.7 | −2.1 | −4.2 | −27.2 | −71.7 | −25.6 | −26.4 | Probable secretion protein | |||
| PA4143 | C | −2.6 | −14.4 | −2.1 | −3.4 | −8.1 | −15.3 | −7.3 | −9.0 | Probable toxin transporter | |||
| PA4171 | C | −7.4 | −10.0 | −4.9 | −6.3 | −5.4 | −15.5 | −13.6 | −9.5 | Probable protease | |||
| PA4175 | prpL | A | −12.0 | −25.4 | −6.7 | −15.8 | −28.4 | −47.1 | −30.4 | −1.6 | Pvds-regulated endoprotease, lysyl class | ||
| PA4209 | phzM | C | 1.8 | −10.8 | −1.9 | −1.9 | −9.8 | −18.8 | −11.9 | −7.5 | Probable phenazine-specific methyltransferase | ||
| PA4210 | phzA1 | C | −1.0 | −11.8 | −2.3 | −5.6 | −65.1 | −123.9 | −79.7 | −20.2 | Probable phenazine biosynthesis protein | ||
| PA4211 | phzB1 | − | C | 1.4 | −15.5 | −2.2 | −2.3 | −51.0 | −61.2 | −59.7 | −46.1 | Probable phenazine biosynthesis protein | |
| PA4217 | phzS | − | C | 1.4 | −9.6 | −2.2 | −2.2 | −11.4 | −12.9 | −8.9 | −10.9 | Flavin-containing mono-oxygenase | |
| PA4290 | C | −23.4 | −19.0 | −26.8 | −25.3 | −7.2 | −6.0 | −14.2 | −15.9 | Probable chemotaxis transducer | |||
| PA4345 | C | −5.3 | −7.4 | −3.9 | −6.9 | −10.6 | −9.1 | −7.7 | −6.6 | Hypothetical protein | |||
| PA4377 | − | A | −13.9 | −12.2 | −3.5 | −6.2 | −10.1 | −7.0 | −7.8 | −3.2 | Hypothetical protein | ||
| PA4677 | A | −6.8 | −4.2 | −3.1 | −5.1 | −17.0 | −40.0 | −22.5 | −1.1 | Hypothetical protein | |||
| PA4738 | C | −6.8 | −22.5 | −5.2 | −11.5 | −39.6 | −54.4 | −58.1 | −16.6 | Conserved hypothetical protein | |||
| PA4739 | − | C | −4.8 | −12.9 | −3.4 | −5.7 | −34.3 | −34.3 | −43.8 | −16.6 | Conserved hypothetical protein | ||
| PA4876 | osmE | C | −5.3 | −10.4 | −3.4 | −4.8 | −18.2 | −9.1 | −10.8 | −6.7 | Osmotically inducible lipoprotein Osme | ||
| PA4880 | C | −6.8 | −17.3 | −4.5 | −4.4 | −6.0 | −29.6 | −12.9 | −12.9 | Probable bacterioferritin | |||
| PA5097 | D | −4.2 | −18.2 | −12.5 | −5.8 | −9.5 | −5.2 | −3.4 | −4.8 | Probable amino acid permease | |||
| PA5099 | C | −188.0 | −353.2 | −54.1 | −450.6 | −16.2 | −14.7 | −40.5 | −39.9 | Probable transporter | |||
| PA5212 | C | −3.1 | −5.6 | −1.7 | −2.5 | −8.8 | −8.7 | −12.1 | −5.7 | Hypothetical protein | |||
| PA5220 | B | −2.1 | −5.5 | −1.7 | −2.3 | −7.7 | −8.9 | −4.2 | −6.9 | Hypothetical protein | |||
| PA5235 | glpT | B | −4.3 | −2.7 | −2.6 | −5.0 | −15.2 | −32.4 | −2.8 | −13.9 | Glycerol-3-phosphate transporter | ||
| PA5297 | poxB | D | −3.1 | −9.4 | −2.0 | −3.5 | −17.8 | −6.0 | −3.8 | −2.8 | Pyruvate dehydrogenase (cytochrome) | ||
| PA5480 | B | −10.6 | −2.6 | 1.0 | −4.2 | −14.2 | −19.9 | −2.5 | −6.1 | Hypothetical protein | |||
| PA5481 | + | C | −9.6 | −30.8 | −7.3 | −15.0 | −45.6 | −50.7 | −96.6 | −22.6 | Hypothetical protein | ||
| PA5482 | + | C | −5.1 | −15.2 | −3.5 | −9.2 | −59.6 | −43.0 | −81.1 | −12.2 | Hypothetical protein | ||
The effects of sub-MICs of AZM, CFT and CPR on genes belonging to the QS regulon, which is defined elsewhere (74). This regulon consists of genes downregulated in both a ΔlasI-ΔrhlI mutant and a ΔlasR-ΔrhlR mutant of P. aeruginosa PAO1. Genes downregulated more than five times are in boldface, and genes upregulated more than five times are underlined.
pprB genes upregulated more than five times (+) or downregulated five times (−) in a ΔpprB mutant of PAO1, as described elsewhere (25).
Genes previously reported to be upregulated (+) or downregulated (−) by AZM, as described elsewhere (60).
Genes were assigned to one of the following four QS regulation (Reg.) groups: group A, Las-dependent genes, group B, Rhl-dependent genes; group C, genes requiring both a functional Las system and a functional Rhl system; group D, Las-Rhl-regulated genes. See the text for further explanations.
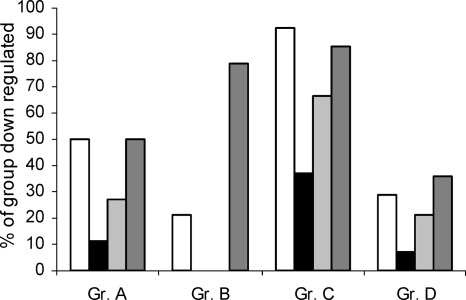
The DNA array data show that members of the QS regulon are overrepresented among the genes repressed by sub-MICs of AZM, CFT, and CPR. This suggests that these antibiotics exhibit some specificity for QS-regulated gene expression through an interaction with or an effect on QS regulatory components. Investigating the expression profiles of genes regulated by LasR, RhlR, or LasR-RhlR, we further aimed to elucidate the potential QS target(s) of the antibiotics. The QS-regulated genes can be divided into four groups. Group A contains 44 LasR-dependent genes, i.e., genes downregulated in both a ΔlasR mutant and a ΔlasR-ΔrhlR mutant. Group B consists of RhlR-dependent genes (14 genes), i.e., genes downregulated in both a ΔrhlR mutant and a ΔlasR-ΔrhlR mutant. Group C comprises the main fraction of QS-regulated genes (102 genes in total) and consists of genes which are downregulated in all three types of mutants: ΔlasR, ΔrhlR, and ΔlasR-ΔrhlR. Finally, group D consists of 14 LasR-RhlR-dependent genes, which are downregulated only if both LasR and RhlR are knocked out, as in the ΔlasR-ΔrhlR mutant. Interestingly it seems that AZM, CFT, and CPR had less of a preference for the group B genes (the RhlR-specific genes) (Fig. 8).
FIG. 8.
LasR, RhlR, and LasR-RhlR specificities of AZM (white bars), CFT (black bars), CPR (light gray bars), and C30 (dark gray bars). See the text for the definitions of groups (Gr.) A, B, C, and D.
Even though all three antibiotics exhibited a higher degree of specificity for the LasR-regulated genes than for the RhlR-dependent genes, none of these antibiotics downregulated the AHL synthetase genes. AZM, however, caused the downregulation of lasR sevenfold, which may also contribute to the QSI effect of AZM. This is in accordance with the observation that AZM (2 μg/ml) has been reported to decrease the level of expression of a lasR::lacZ gene fusion (84).
Among the genes affected by AZM, CFT, and CPR, many encode previously identified QS-regulated genes such as chitinase (chiC, PA2300) and chitin-binding protein (cbpD, PA0852) (30, 80, 89). Other known QS-induced genes, such as the genes encoding the osmotically inducible protein (osmC, PA0059) and cytochrome c oxidase (coxAB and coIII, PA0105 to PA0108), are downregulated by the presence of the three antibiotics (38, 80). The endoprotease gene piv (also known as prpL [PA4175]) was also downregulated by AZM, CFT, and CPR. This protease is capable of degrading elastin, decorin, and the transferrin family of proteins, including lactoferrin (92). PA5099 (a probable transporter), which belongs to the QS regulon, was downregulated more than 300 times by AZM and CPR and 50 times by CFT. The regulon contains only one gene, PA1556, which is repressed by QS. This gene, encoding cytochrome c oxidase, was upregulated by all three antibiotics, and so was the neighboring gene PA1557, a probable cytochrome oxidase subunit. Many genes from the region from PA2134 to PA2190 of the genome were downregulated by AZM, CFT, and CPR. This region has been found to be associated with stationary growth and to be under QS/rpoS control (79, 80, 89, 90). Of the 57 genes in this 60.69-kbp region, 46 were found to be present in all the arrays. Of the 46 genes, 35 genes were repressed more than fivefold by AZM and CPR. CPR and especially AZM seemed to exhibit higher target specificities for QS genes than CFT, which is in accordance with the findings of the virulence factor assays described earlier in this section. Many QS-regulated virulence factors, such as the alkaline metalloproteinase (aprA, PA1249), the PfpI protease (pfpI, PA0355), the LasA protease (lasA, PA1871), elastase B (lasB, PA3724), the cytotoxic galactophilic lectin (PA-IL; lecA, PA2570), and rhamnolipid (rhlA and rhlB; PA3478 and PA3479, respectively), were repressed more than fivefold by AZM and CPR but not by CFT. These results were confirmed by RT-PCR, which showed that AZM downregulated rhlA expression 45-fold, while CPR and CFT reduced the level of rhlA expression 10- and 3-fold, respectively.
AZM, CFT, and CPR alter the expression of a variety of genes.
A few genes were consistently upregulated by AZM, CFT, and CPR. The QS repressible genes PA1556 (cytochrome c oxidase subunit) and PA1557 were induced more than five times. PA2260 (a hypothetical protein previously identified [89] to be a QS repressible gene) was induced by AZM and CFT but not by CPR.
Several genes were induced only by AZM. A high proportion of these genes have also previously been reported to be AZM inducible (60), although there were a number of differences in the experimental setup between the current study and the previous study (60). For example, the previous study (60) used the P. aeruginosa PAO1 strain DSM 1707 instead of the P. aeruginosa PAO1 strain from the Pseudomonas Genetic Stock Center used here. Moreover, the study by Nalca et al. (60) also applied the complex and rich brain heart infusion medium instead of minimal medium. Many of the genes induced by AZM encode ribosomal subunits, such as PA3742 (rplS) and PA4671 (probable ribosomal protein). The overexpression of ribosomal genes may be a mechanism that compensates for the effect of AZM, which targets the 50S ribosomal subunit. The initiation factor infA (PA2619) was also induced in the presence of AZM, in accordance with previous findings (60). In agreement with the findings of two recent studies (12, 53), we found that CPR induces the expression of the pyocin S genes (PA0985 for pyocin S5, PA1150 for pyocin S2, and PA3866 for the pyocin S3-like protein). The S-type pyocins are protease-sensitive bacteriocins and have colicin-like structures and modes of action.
The ribosome modulation factor (rmf, PA4296) was repressed by AZM but was not affected by treatment with CFT or CPR. Previous transcriptome analyses (60) also showed that rmf is repressed by AZM. The rmf of P. aeruginosa shows a high degree of homology to the rmf of E. coli (similarity, 66%). rmf mutants of E. coli have been shown to gradually lose their viability in stationary-phase cultures (61); however, a recent study concluded that rmf (in E. coli) may be required only under competitive growth conditions (13).
AZM induces expression of genes related to T3S.
In accordance with previous findings (60), we also found that AZM induced the expression of genes involved in type III secretion (T3S). P. aeruginosa uses its T3S system to deliver several cytotoxic products into the cytosol of eukaryotic cells. The expression of T3S increases the pathogenicity of P. aeruginosa and is associated with more severe infections and higher rates of mortality in animal models (1, 75, 78). QS has been shown to negatively modulate the expression of the T3S system in P. aeruginosa (10), which means that the T3S system is downregulated under high-cell-density conditions and thus presumably plays a role primarily in the early stages of the infection. Genes in the T3S system, such as PA1700 and PA1701 (conserved hypothetical proteins in T3S), pcrGV (PA1705 regulator in T3S and PA1706), exsB (PA1712, exoenzyme S synthesis protein B), exsD (PA1714, part of the pscB to pscL operon), and pscBCEO (PA1715, T3S export apparatus protein; PA1716, T3S outer membrane protein; PA1718, T3S export protein; PA1696, translocation protein in T3S), were upregulated by AZM. Neither CFT nor CPR, however, induced the genes in the T3S system gene cluster from PA1690 to PA1725.
AZM, CPR, CFT, and the two-component regulator system pprAB.
The QS-induced chemotaxis transducer PA4290 was downregulated by AZM, CFT, and CPR. AZM and CPR also repressed the main part of the genes in the region from PA4290 to PA4306. Many of these genes have been shown in previous studies to be regulated by QS. In those studies, QS genes were identified by mapping genes downregulated in ΔlasI-ΔrhlI mutants and restored to wild-type levels by addition of signal molecules (38, 80, 89). The two-component regulator system pprAB (PA4293, PA4296) was downregulated by AZM and CPR but not by CFT. pprA was downregulated 16 and 6 times by AZM and CPR, respectively, and pprB downregulated 10 and 5 times by AZM and CPR, respectively. These results obtained by DNA microarray analysis were confirmed by RT-PCR, which showed that AZM reduced the levels of pprA and pprB expression 19- and 15-fold, respectively, whereas CPR downregulated the level of pprA expression 11-fold and the level of pprB expression 18-fold. RT-PCR also confirmed that the effect of CFT on pprAB expression was insignificant.
Docking of AZM, CFT, and CPR against the LBD of the LasR protein.
In order to explore the possibility that AZM, CFT, and CPR directly interact with the LasR protein, we performed in silico docking of the three antibiotics with the recently published LBD of the LasR protein (11). None of the three antibiotics gave a high-affinity score in the docking experiment. In contrast, previously reported QSI compounds (e.g., C30, patulin, furanone, and 4-nitropyridine-N-oxide) exhibited high-affinity scores (Table 4). The in silico analysis indicates that AZM, CFT, and CPR do not fit inside the binding site cavity of LasR because they exhibit steric clashes with the protein target. This suggests that the effects of AZM, CFT, and CPR on QS may be due to a mechanism of action other than a direct interaction with the LasR protein.
TABLE 4.
In silico docking
| Name of ligand | Rerank score |
|---|---|
| OdDHL (template) | −107.3 |
| 3-Oxo-C12 | −94.5 |
| 2-Heptylthioacetyl homoserine lactone | −88.8 |
| BHL | −82.2 |
| Patulin | −69.8 |
| 4-Nitropyridine-N-oxide | −62.9 |
| C30 | −50.4 |
| CPR | 317.3 |
| CFT | 373.5 |
| AZM | 3,316.2 |
DISCUSSION
In the present paper, we present the results from the screening of 12 antibiotics for QSI activity obtained with QSIS1. Three of the 12 antibiotics, AZM, CFT, and CPR, showed strong positive results in the screening system and were further investigated for their effects on the production of virulence factors in P. aeruginosa. AZM, CFT, and CPR exhibit diverse mechanisms of antimicrobial action and are structurally very different. It is thus intriguing that all three antibiotics were active in QSIS1. Both CFT and piperacillin are β-lactams that target penicillin binding protein 3 (PBP 3); however, only CFT showed actual activity in the QSI screening system, indicating that the QSI activity is likely not PBP 3 dependent. We carefully selected the concentrations of the three antibiotics which had no effect on growth. Our experience from the screening of large numbers of compounds from compound libraries is that toxic or growth-inhibitory compounds may show QSI-like activities (unpublished observations). This is likely to be caused by stress-like effects rather than “true” QSI activity (such as an interaction with the QS receptor), since the effect on QS-controlled gene expression disappears when the concentration of the compound is lowered to a level that allows the bacteria to attain the optimum growth rate in a given medium. Furthermore, since the obstruction of QS (either by mutation or by antagonistic drugs) does not inhibit growth, we consider it essential to investigate the potential QSI activities of the three antibiotics under conditions that do not affect growth. When they were administered at such concentrations, AZM, CPR, and CFT inhibited the production of the important virulence factors chitinase, protease, and elastase and the production of the heat-stable hemolysin rhamnolipid. Our recent data suggest that rhamnolipid functions as a shield to protect biofilms against the predatory behavior of neutrophils, and rhamnolipid may thus be a pivotal virulence factor (45). Investigations have shown that biofilms of P. aeruginosa QS mutants have reduced tolerance to antibiotics compared with that of their wild-type parents, and inhibition of QS has been shown to promote the eradication of biofilms by antimicrobial treatments and make the biofilm more susceptible to phagocytosis by neutrophils (20, 38, 73). Rhamnolipid and the adhesive lectin LecA were downregulated more than fivefold by AZM and CPR. Both products are regulated by QS and are involved in biofilm maturation (19, 23, 52, 65). Repression of these genes may contribute to the development of the less structured and more antibiotic sensitive biofilm seen in QS mutants. Moreover, rhamnolipid and lectin LecA have been reported to have cytotoxic effects on mammalian cells, including neutrophils and macrophages, and are also important in the P. aeruginosa infectious process (5, 45, 57, 58, 81, 82, 95).
Another important virulence component of P. aeruginosa is the T3S system, which is negatively regulated by QS in P. aeruginosa. The DNA microarray data presented here suggest that AZM increases the level of expression of T3S genes, in accordance with the QS-inhibiting properties of AZM reported here and by others. Interestingly, a previous study (50) showed that treatment of P. aeruginosa with sub-MICs of macrolides (AZM, clarithromycin, erythromycin) prior to the intranasal challenge of mice significantly enhanced the mortality rate (from 0% to 80 to 100%). In the same study, however, macrolide antibiotics were also administered to mice after the inoculation of P. aeruginosa PAO1, and in that case, the rate of mortality among the mice did not increase (50). Thus, it seems that the activation of genes by macrolides causes enhanced virulence only if the macrolides are applied prophylactically and not as a part of the treatment. Nevertheless, the potential risk, along with the potential benefits, of inducing virulence and cytotoxicity through the T3S system should be considered when patients infected with P. aeruginosa are treated with AZM (and possibly other macrolides).
As mentioned above, the two-component regulator system pprAB (PA4293 and PA4296, respectively) was downregulated more than fivefold by AZM and CPR but not by CFT; these results were confirmed by RT-PCR. The expression of pprAB has been shown to be regulated by QS (80, 89), but the gene products have also been identified to have activities that modulate QS, representing yet another autofeedback system of QS in P. aeruginosa (25). The knockout of pprB leads to reduced influx of the P. aeruginosa QS signal OdDHL, thereby globally influencing the expression of QS-regulated genes (25); but PprB is also involved in the regulation of sensitivity to antibiotics. The overexpression of pprB results in increased sensitivity to antibiotics, especially aminoglycosides, probably due to a decrease in membrane permeability (91). In vivo and in vitro studies have shown that P. aeruginosa develops increasing tolerance to aminoglycosides following pretreatment with these antimicrobials (18, 47, 93). Interestingly, cells from P. aeruginosa biofilms exposed to sub- and suprainhibitory concentrations of CPR have also recently been demonstrated to display transient increased levels of resistance to CPR (64). We speculate that the underlying mechanism by which AZM and CPR (and perhaps also CFT) exert their action on QS is by means of an adaptive response which downregulates pprB and perhaps also other membrane regulators or transporters, such as PA5099. This causes the membrane permeability to decrease, thereby providing the cells with transient protection against the toxic effects of the antibiotics. However, at the same time, the lower membrane permeability decreases the level of OdDHL influx, which results in a reduction in the levels of expression of QS-regulated genes. Support for this hypothesis can be found in the observation that the three antibiotics have higher specificities for las-regulated genes than rhl-regulated genes (group B). The rhl signal molecule BHL is capable of freely diffusing over the membrane and is, unlike OdDHL, not dependent on active transport.
A previous study (25) identified 53 genes that were downregulated more than fivefold by a Tn5 insertion in pprB. In comparison, as described in the present report, we found that the levels of expression of 38 of those pprB-regulated genes that were downregulated more than fivefold in the previously mentioned study (25) were reduced to half that level or less in the presence of AZM. This included 21 genes which were downregulated more than fivefold. We found that CPR downregulated 23 of the 53 genes more than twofold, including 6 genes that were downregulated more than fivefold. CFT caused a downregulation of 15 of the 53 genes downregulated by the pprB mutation, but none of the genes were downregulated more than fourfold. Genes reported to be affected by PprB include virulence factor genes such as lasB, rhlAB, lecA, and prpL (25), which are also downregulated by AZM and CPR. The levels of expression of the QS transcriptional regulator RhlR and the AHL synthetase genes lasI and rhlI were also decreased by the prpB-knockout mutation (25). This is not surprising, as rhl is subordinate to the las system and the AHL synthetase genes are under QS control, which may provide a negative feedback that enhances the QS-repressing effect of the pprB mutation.
QSIs constructed with AHL scaffolds are expected to bind to the LuxR homologue without activating it, thereby blocking the binding and activation by AHLs. Thus, these QS antagonists have a high degree of structural similarity to AHLs. Other QSIs which share the 2(5H)-furanone moiety, such as the natural occurring halogenated furanones, and the mycotoxins patulin and penicillic acid seem to exert their effects by destabilizing LuxR and thereby inducing the turnover of QS regulator proteins (56, 74). Figure 9 shows the structures of BHL, OdDHL, and previously published QSI compounds.
FIG. 9.
Inhibitors of and signal molecules for P. aeruginosa QS. (a) 4-Nitropyridine-N-oxide (73); (b) C30 (38); (c) 2-heptylthioacetyl homoserine lactone (69a); (d) 3-oxo-C12-2-aminophenol (83a); (e) BHL; (f) patulin (74); (g) OdDHL.
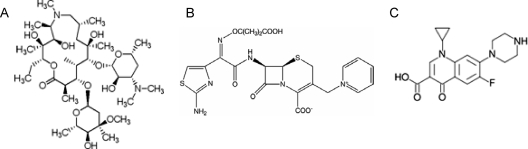
The three antibiotics AZM, CFT, and CPR are structurally very different from all QSIs described until now; moreover, like many other antibiotics, they are very bulky molecules (Fig. 10). In silico docking of AZM, CFT, and CPR to the LBD of the LasR protein showed that the antibiotics have a low affinity for the LasR receptor site, mainly due to spatial penalties. This supports the idea that these antibiotics may exert their QS regulatory effects through mechanisms different from those of the other QSIs, thereby opening new and interesting possibilities for combination therapy with QSIs with different modes of action. The usefulness of the combination of QSIs and traditional bactericidal compounds has been proved by several in vitro experiments (20, 38, 74). The understanding of some antibiotics’ dual course of action may have the potential to further add to therapies against pathogens in which the key element attenuates rather than kills the bacteria directly. This strategy seeks to allow the host defense system to eliminate the attenuated bacteria.
FIG. 10.
Structures of the three antibiotics investigated for potential quorum sensing inhibitory activities. (A) AZM; (B) CFT; (C) CPR.
Most antibiotics originate from natural sources, such as soil microorganisms, or are derivatives of compounds originating from nature. A general point of view has been that the ecological purpose of antimicrobials in the environment is to fight competitors. However, the concentrations of free antibiotics in soil are likely to be well below the MICs.
As described in this paper and by others (53, 94), sub-MICs of antibiotics may have various effects on bacteria which are totally different from the effects of high doses; for example, they may enhance biofilm formation, alter motility patterns, increase cytotoxicity, and cause changes in the expression of virulence factors (39, 53, 60). It has been suggested that antibiotics in nature may function as intermicrobial signals rather than as bullets aimed at killing enemies in order to defend niches or food (53). This hypothesis offers an evolutionary explanation of why some antibiotics may be capable of interfering with QS-controlled gene expression. In line with the findings presented in the present report, it has recently been shown that OdDHL (and other 3-oxo-AHLs) as well as its tetramic acid degradation product exhibits activities against gram-positive bacteria (48), suggesting that the signal molecules, like some antibiotics, possess dual activities. Furthermore, our data suggest that antibiotics which function to downregulate QS in P. aeruginosa may be administered at sub-MICs (where there is a reduced selection pressure for the development of resistance) to block QS and thereby attenuate the activity of the pathogen. Such interesting properties highlight the huge unexplored potential of using compounds derived from natural scaffolds in the search for new pharmaceuticals with a variety of targets (i.e., multifunctional antimicrobials). It seems that we have not fully explored and exploited the potential of the drugs that we know today.
The dual activities of some antibiotics (e.g., AZM, CFT, and CPR, investigated here) may help explain why CFT has been shown to be superior to comparative antipseudomonal antibiotic regimens for patients with CF in the Danish CF center (69). Caution must be taken, however. The subsequent shift to CFT as the predominant antipseudomonal antibiotic in the Danish CF center led to the epidemic spread of a multiresistant nonmucoid strain (68). A previous study (35) showed that sub-MICs of antibiotics may still modulate transcriptional patterns in members of the beneficial human flora, causing a variety of unwanted effects. Likewise, it has been shown that the long-term use of AZM by patients with CF and chronic P. aeruginosa infections may lead to macrolide resistance in Staphylococcus aureus in these patients (87). This underlines the requirement for the further development of QSIs without classical bactericidal or bacteriostatic activity.
Acknowledgments
This work was supported by grants from the German Mukoviszidose e.v., Deutsche Forschungsgemeinshaft, and the Danish Research Council (STF grant no. 2052-03-0013) to M.G.
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Ader, F., R. Le Berre, K. Faure, P. Gosset, O. Epaulard, B. Toussaint, B. Polack, E. Nowak, N. B. Viget, E. Kipnis, and B. P. Guery. 2005. Alveolar response to Pseudomonas aeruginosa: role of the type III secretion system. Infect. Immun. 73:4263-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, J. B., A. Heydorn, M. Hentzer, L. Eberl, O. Geisenberger, B. B. Christensen, S. Molin, and M. Givskov. 2001. gfp-based N-acyl-homoserine-lactone monitors for detection of bacterial communication. Appl. Environ. Microbiol. 67:575-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade, S. S., R. N. Jones, A. C. Gales, and H. S. Sader. 2003. Increasing prevalence of antimicrobial resistance among Pseudomonas aeruginosa isolates in Latin American medical centres: 5 year report of the SENTRY Antimicrobial Surveillance Program (1997-2001). J. Antimicrob. Chemother. 52:140-141. [DOI] [PubMed] [Google Scholar]
- 4.Anwar, H., and J. W. Costerton. 1990. Enhanced activity of combination of tobramycin and piperacillin for eradication of sessile biofilm cells of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 34:1666-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajolet-Laudinat, O., S. Girod-de Bentzmann, J. M. Tournier, C. Madoulet, M. C. Plotkowski, C. Chippaux, and E. Puchelle. 1994. Cytotoxicity of Pseudomonas aeruginosa internal lectin PA-I to respiratory epithelial cells in primary culture. Infect. Immun. 62:4481-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann, U., M. King, E. M. App, S. Tai, A. Konig, J. J. Fischer, T. Zimmermann, W. Sextro, and H. von der Hardt. 2004. Long term azithromycin therapy in cystic fibrosis patients: a study on drug levels and sputum properties. Can. Respir. J. 11:151-155. [DOI] [PubMed] [Google Scholar]
- 7.Beatson, S. A., C. B. Whitchurch, A. B. T. Semmler, and J. S. Mattick. 2002. Quorum sensing is not required for twitching motility in Pseudomonas aeruginosa. J. Bacteriol. 184:3598-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger, M., and M. Konstan. 1999. Immunopathogenesis of cystic fibrosis lung disease, p. 115-143. In J. R. Yankaskas and M. R. Knowles (ed.), Cystic fibrosis in adults. Lippincott-Raven, Philadelphia, PA.
- 9.Berka, R. M., and M. L. Vasil. 1982. Phospholipase C (heat-labile hemolysin) of Pseudomonas aeruginosa: purification and preliminary characterization. J. Bacteriol. 152:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleves, S., C. Soscia, P. Nogueira-Orlandi, A. Lazdunski, and A. Filloux. 2005. Quorum sensing negatively controls type III secretion regulon expression in Pseudomonas aeruginosa PAO1. J. Bacteriol. 187:3898-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottomley, M. J., E. Muraglia, R. Bazzo, and A. Carfi. 2007. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J. Biol. Chem. 282:13592-13600. [DOI] [PubMed] [Google Scholar]
- 12.Brazas, M. D., and R. E. Hancock. 2005. Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:3222-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bubunenko, M., T. Baker, and D. L. Court. 2007. Essentiality of ribosomal and transcription antitermination proteins analyzed by systematic gene replacement in Escherichia coli. J. Bacteriol. 189:2844-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciofu, O., V. Fussing, N. Bagge, C. Koch, and N. Hoiby. 2001. Characterization of paired mucoid/non-mucoid Pseudomonas aeruginosa isolates from Danish cystic fibrosis patients: antibiotic resistance, beta-lactamase activity and RiboPrinting. J. Antimicrob. Chemother. 48:391-396. [DOI] [PubMed] [Google Scholar]
- 16.Clark, D. J., and O. Maaloe. 1967. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 23:99-112. [Google Scholar]
- 17.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 18.Daikos, G. L., G. G. Jackson, V. T. Lolans, and D. M. Livermore. 1990. Adaptive resistance to aminoglycoside antibiotics from first-exposure down-regulation. J. Infect. Dis. 162:414-420. [DOI] [PubMed] [Google Scholar]
- 19.Davey, M. E., N. C. Caiazza, and G. A. O'Toole. 2003. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 185:1027-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 21.de Nys, R., M. Givskov, N. Kumar, S. Kjelleberg, and P. D. Steinberg. 2006. Furanones. Prog. Mol. Subcell. Biol. 42:55-86. [DOI] [PubMed] [Google Scholar]
- 22.DeVries, C. A., and D. E. Ohman. 1994. Mucoid-to-nonmucoid conversion in alginate-producing Pseudomonas aeruginosa often results from spontaneous mutations in algT, encoding a putative alternate sigma factor, and shows evidence for autoregulation. J. Bacteriol. 176:6677-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diggle, S. P., R. E. Stacey, C. Dodd, M. Camara, P. Williams, and K. Winzer. 2006. The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ. Microbiol. 8:1095-1104. [DOI] [PubMed] [Google Scholar]
- 24.DiMango, E., H. J. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Investig. 96:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong, Y. H., X. F. Zhang, H. M. Soo, E. P. Greenberg, and L. H. Zhang. 2005. The two-component response regulator PprB modulates quorum-sensing signal production and global gene expression in Pseudomonas aeruginosa. Mol. Microbiol. 56:1287-1301. [DOI] [PubMed] [Google Scholar]
- 26.Elborn, J. S., D. J. Shale, and J. R. Britton. 1991. Cystic fibrosis: current survival and population estimates to the year 2000. Thorax 46:881-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Equi, A., I. M. Balfour-Lynn, A. Bush, and M. Rosenthal. 2002. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet 360:978-984. [DOI] [PubMed] [Google Scholar]
- 28.Flamm, R. K., M. K. Weaver, C. Thornsberry, M. E. Jones, J. A. Karlowsky, and D. F. Sahm. 2004. Factors associated with relative rates of antibiotic resistance in Pseudomonas aeruginosa isolates tested in clinical laboratories in the United States from 1999 to 2002. Antimicrob. Agents Chemother. 48:2431-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fogarty, A., R. Hubbard, and J. Britton. 2000. International comparison of median age at death from cystic fibrosis. Chest 117:1656-1660. [DOI] [PubMed] [Google Scholar]
- 30.Folders, J., J. Algra, M. S. Roelofs, L. C. van Loon, J. Tommassen, and W. Bitter. 2001. Characterization of Pseudomonas aeruginosa chitinase, a gradually secreted protein. J. Bacteriol. 183:7044-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173:3000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Givskov, M., R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J. Bacteriol. 178:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Givskov, M., and S. Molin. 1993. Secretion of Serratia liquefaciens phospholipase from Escherichia coli. Mol. Microbiol. 8:229-242. [DOI] [PubMed] [Google Scholar]
- 34.Givskov, M., L. Olsen, and S. Molin. 1988. Cloning and expression in Escherichia coli of the gene for extracellular phospholipase A1 from Serratia liquefaciens. J. Bacteriol. 170:5855-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goh, E. B., G. Yim, W. Tsui, J. McClure, M. G. Surette, and J. Davies. 2002. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc. Natl. Acad. Sci. USA 99:17025-17030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hackman, R. H., and M. Goldberg. 1964. New substrates for use with chitinases. Anal. Biochem. 8:397-401. [DOI] [PubMed] [Google Scholar]
- 37.Hentzer, M., and M. Givskov. 2003. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Investig. 112:1300-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hentzer, M., H. Wu, J. B. Andersen, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, M. A. Schembri, Z. Song, P. Kristoffersen, M. Manefield, J. W. Costerton, S. Molin, L. Eberl, P. Steinberg, S. Kjelleberg, N. Hoiby, and M. Givskov. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman, L. R., D. A. D'Argenio, M. J. MacCoss, Z. Zhang, R. A. Jones, and S. I. Miller. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171-1175. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann, N., B. Lee, M. Hentzer, T. B. Rasmussen, Z. Song, H. K. Johansen, M. Givskov, and N. Hoiby. 2007. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr−/− mice. Antimicrob. Agents Chemother. 51:3677-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoiby, N. 1995. Microbiology of cystic fibrosis, p. 75-98. In M. E. Hodson and D. M. Geddes (ed.), Cystic fibrosis. Chapman & Hall Medical, London, United Kingdom.
- 42.Hooi, D. S., B. W. Bycroft, S. R. Chhabra, P. Williams, and D. I. Pritchard. 2004. Differential immune modulatory activity of Pseudomonas aeruginosa quorum-sensing signal molecules. Infect. Immun. 72:6463-6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howe, R. A., and R. C. Spencer. 1997. Macrolides for the treatment of Pseudomonas aeruginosa infections? J. Antimicrob. Chemother. 40:153-155. [DOI] [PubMed] [Google Scholar]
- 44.Ichimiya, T., K. Takeoka, K. Hiramatsu, K. Hirai, T. Yamasaki, and M. Nasu. 1996. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 42:186-191. [DOI] [PubMed] [Google Scholar]
- 45.Jensen, P. O., T. Bjarnsholt, R. Phipps, T. B. Rasmussen, H. Calum, L. Christoffersen, C. Moser, P. Williams, T. Pressler, M. Givskov, and N. Hoiby. 2007. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 153:1329-1338. [DOI] [PubMed] [Google Scholar]
- 46.Johnson, M. K., and D. Boese-Marrazzo. 1980. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect. Immun. 29:1028-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlowsky, J. A., M. H. Saunders, G. A. Harding, D. J. Hoban, and G. G. Zhanel. 1996. In vitro characterization of aminoglycoside adaptive resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 40:1387-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaufmann, G. F., R. Sartorio, S. H. Lee, C. J. Rogers, M. M. Meijler, J. A. Moss, B. Clapham, A. P. Brogan, T. J. Dickerson, and K. D. Janda. 2005. Revisiting quorum sensing: discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl. Acad. Sci. USA 102:309-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi, H. 1995. Biofilm disease: its clinical manifestation and therapeutic possibilities of macrolides. Am. J. Med. 99:26S-30S. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi, T., K. Tateda, T. Matsumoto, S. Miyazaki, A. Watanabe, T. Nukiwa, and K. Yamaguchi. 2002. Macrolide-treated Pseudomonas aeruginosa induces paradoxical host responses in the lungs of mice and a high mortality rate. J. Antimicrob. Chemother. 50:59-66. [DOI] [PubMed] [Google Scholar]
- 51.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. Stewart, A. Lazdunski, and P. Williams. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 52.Lequette, Y., and E. P. Greenberg. 2005. Timing and localization of rhamnolipid synthesis gene expression in Pseudomonas aeruginosa biofilms. J. Bacteriol. 187:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linares, J. F., I. Gustafsson, F. Baquero, and J. L. Martinez. 2006. Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Natl. Acad. Sci. USA 103:19484-19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Livermore, D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634-640. [DOI] [PubMed] [Google Scholar]
- 55.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manefield, M., T. B. Rasmussen, M. Henzter, J. B. Andersen, P. Steinberg, S. Kjelleberg, and M. Givskov. 2002. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148:1119-1127. [DOI] [PubMed] [Google Scholar]
- 57.McClure, C. D., and N. L. Schiller. 1992. Effects of Pseudomonas aeruginosa rhamnolipids on human monocyte-derived macrophages. J. Leukoc. Biol. 51:97-102. [DOI] [PubMed] [Google Scholar]
- 58.McClure, C. D., and N. L. Schiller. 1996. Inhibition of macrophage phagocytosis by Pseudomonas aeruginosa rhamnolipids in vitro and in vivo. Curr. Microbiol. 33:109-117. [DOI] [PubMed] [Google Scholar]
- 59.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 60.Nalca, Y., L. Jansch, F. Bredenbruch, R. Geffers, J. Buer, and S. Haussler. 2006. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob. Agents Chemother. 50:1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niga, T., H. Ito, Y. Oyamada, J. Yamagishi, M. Kadono, T. Nishino, N. Gotoh, and M. Inoue. 2005. Cooperation between alteration of DNA gyrase genes and over-expression of MexB and MexX confers high-level fluoroquinolone resistance in Pseudomonas aeruginosa strains isolated from a patient who received a liver transplant followed by treatment with fluoroquinolones. Microbiol. Immunol. 49:443-446. [DOI] [PubMed] [Google Scholar]
- 62.Ochsner, U. A., and J. Reiser. 1995. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:6424-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohman, D. E., S. J. J. Cryz, and B. H. Iglewski. 1980. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J. Bacteriol. 142:836-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oldak, E., and E. A. Trafny. 2005. Secretion of proteases by Pseudomonas aeruginosa biofilms exposed to ciprofloxacin. Antimicrob. Agents Chemother. 49:3281-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pamp, S. J., and T. Tolker-Nielsen. 2007. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 189:2531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Passador, L., and B. H. Iglewski. 1995. Quorum sensing and virulence gene regulation in Pseudomonas aeruginosa, p. 65-78. In J. A. Roth (ed.), Virulence mechanisms of bacterial pathogens, 2nd ed. American Society for Microbiology, Washington, DC.
- 67.Pearson, J. P., C. Van Delden, and B. H. Iglewski. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pedersen, S. S., C. Koch, N. Hoiby, and K. Rosendal. 1986. An epidemic spread of multiresistant Pseudomonas aeruginosa in a cystic fibrosis centre. J. Antimicrob. Chemother. 17:505-516. [DOI] [PubMed] [Google Scholar]
- 69.Permin, H., C. Koch, N. Hoiby, H. O. Christensen, A. F. Moller, and S. Moller. 1983. Ceftazidime treatment of chronic Pseudomonas aeruginosa respiratory tract infection in cystic fibrosis. J. Antimicrob. Chemother. 12(Suppl A):313-323. [DOI] [PubMed] [Google Scholar]
- 69a.Persson, T., T. H. Hansen, T. B. Rasmussen, M. E. Skindersoe, M. Givskov, and J. Nielsen. 2005. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem. 21:253-262. [DOI] [PubMed] [Google Scholar]
- 70.Pesci, E. C., J. B. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramsey, B. W. 1996. Management of pulmonary disease in patients with cystic fibrosis. N. Engl. J. Med. 335:179-188. [DOI] [PubMed] [Google Scholar]
- 73.Rasmussen, T. B., T. Bjarnsholt, M. E. Skindersoe, M. Hentzer, P. Kristoffersen, M. Kote, J. Nielsen, L. Eberl, and M. Givskov. 2005. Screening for quorum-sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 187:1799-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rasmussen, T. B., M. E. Skindersoe, T. Bjarnsholt, R. K. Phipps, K. B. Christensen, P. O. Jensen, J. B. Andersen, B. Koch, T. O. Larsen, M. Hentzer, L. Eberl, N. Hoiby, and M. Givskov. 2005. Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology 151:1325-1340. [DOI] [PubMed] [Google Scholar]
- 75.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 76.Saiman, L., B. C. Marshall, N. Mayer-Hamblett, J. L. Burns, A. L. Quittner, D. A. Cibene, S. Coquillette, A. Y. Fieberg, F. J. Accurso, and P. W. Campbell III. 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749-1756. [DOI] [PubMed] [Google Scholar]
- 77.Savli, H., A. Karadenizli, F. Kolayli, S. Gundes, U. Ozbek, and H. Vahaboglu. 2003. Expression stability of six housekeeping genes: a proposal for resistance gene quantification studies of Pseudomonas aeruginosa by real-time quantitative RT-PCR. J. Med. Microbiol. 52:403-408. [DOI] [PubMed] [Google Scholar]
- 78.Sawa, T., M. Ohara, K. Kurahashi, S. S. Twining, D. W. Frank, D. B. Doroques, T. Long, M. A. Gropper, and J. P. Wiener-Kronish. 1998. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect. Immun. 66:3242-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schuster, M., A. C. Hawkins, C. S. Harwood, and E. P. Greenberg. 2004. The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol. Microbiol. 51:973-985. [DOI] [PubMed] [Google Scholar]
- 80.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharabi, Y., and N. Gilboa-Garber. 1979. Mitogenic stimulation of human lymphocytes by Pseudomonas aeruginosa galactosephilic lectins. FEMS Microbiol. Lett. 5:273-276. [Google Scholar]
- 82.Shryock, T. R., S. A. Silver, M. W. Banschbach, and J. C. Kramer. 1984. Effect of Pseudomonas aeruginosa rhamnolipid on human neutrophil migration. Curr. Microbiol. 10:323-328. [Google Scholar]
- 83.Sierra, G. 1960. Hemolytic effect of a glycolipid produced by Pseudomonas aeruginosa. Antonie van Leeuwenhoek 26:189-192. [DOI] [PubMed] [Google Scholar]
- 83a.Smith, K. M., Y. Bu, and H. Suga. 2003. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol. 10:563-571. [DOI] [PubMed] [Google Scholar]
- 84.Tateda, K., R. Comte, J. C. Pechere, T. Kohler, K. Yamaguchi, and C. Van Delden. 2001. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45:1930-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Telford, G., D. Wheeler, P. Williams, P. T. Tomkins, P. Appleby, H. Sewell, G. S. Stewart, B. W. Bycroft, and D. I. Pritchard. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 66:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomsen, R., and M. H. Christensen. 2006. MolDock: a new technique for high-accuracy molecular docking. J. Med. Chem. 49:3315-3321. [DOI] [PubMed] [Google Scholar]
- 87.Tramper-Stranders, G. A., T. F. Wolfs, A. Fleer, J. L. Kimpen, and C. K. van der Ent. 2007. Maintenance azithromycin treatment in pediatric patients with cystic fibrosis: long-term outcomes related to macrolide resistance and pulmonary function. Pediatr. Infect. Dis. J. 26:8-12. [DOI] [PubMed] [Google Scholar]
- 88.Tsai, W. C., M. L. Rodriguez, K. S. Young, J. C. Deng, V. J. Thannickal, K. Tateda, M. B. Hershenson, and T. J. Standiford. 2004. Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection. Am. J. Respir. Crit. Care Med. 170:1331-1339. [DOI] [PubMed] [Google Scholar]
- 89.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waite, R. D., A. Paccanaro, A. Papakonstantinopoulou, J. M. Hurst, M. Saqi, E. Littler, and M. A. Curtis. 2006. Clustering of Pseudomonas aeruginosa transcriptomes from planktonic cultures, developing and mature biofilms reveals distinct expression profiles. BMC Genomics 7:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang, Y., U. Ha, L. Zeng, and S. Jin. 2003. Regulation of membrane permeability by a two-component regulatory system in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 47:95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilderman, P. J., A. I. Vasil, Z. Johnson, M. J. Wilson, H. E. Cunliffe, I. L. Lamont, and M. L. Vasil. 2001. Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect. Immun. 69:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiong, Y. Q., J. Caillon, M. F. Kergueris, H. Drugeon, D. Baron, G. Potel, and A. S. Bayer. 1997. Adaptive resistance of Pseudomonas aeruginosa induced by aminoglycosides and killing kinetics in a rabbit endocarditis model. Antimicrob. Agents Chemother. 41:823-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yim, G., H. H. Wang, and J. Davies. 2006. The truth about antibiotics. Int. J. Med. Microbiol. 296:163-170. [DOI] [PubMed] [Google Scholar]
- 95.Zulianello, L., C. Canard, T. Kohler, D. Caille, J. S. Lacroix, and P. Meda. 2006. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect. Immun. 74:3134-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]