Abstract
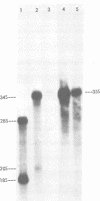
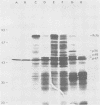
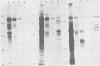
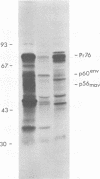
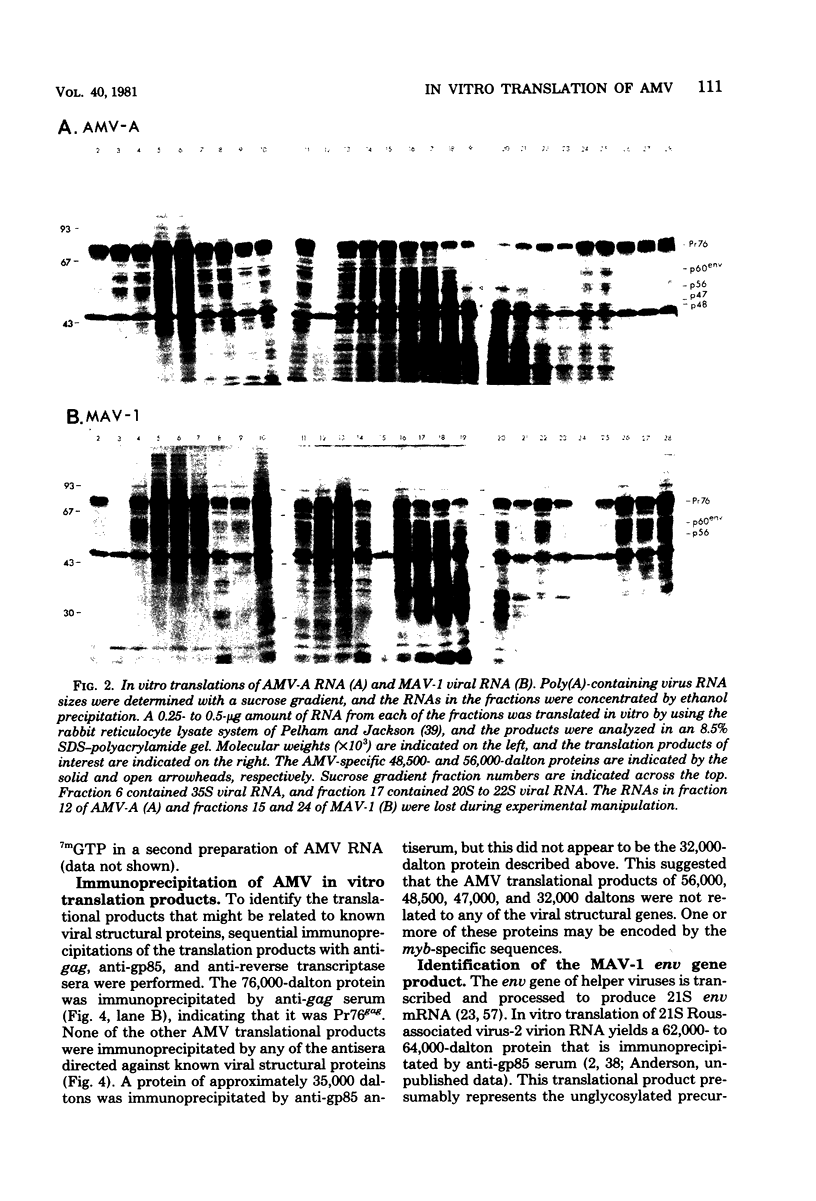
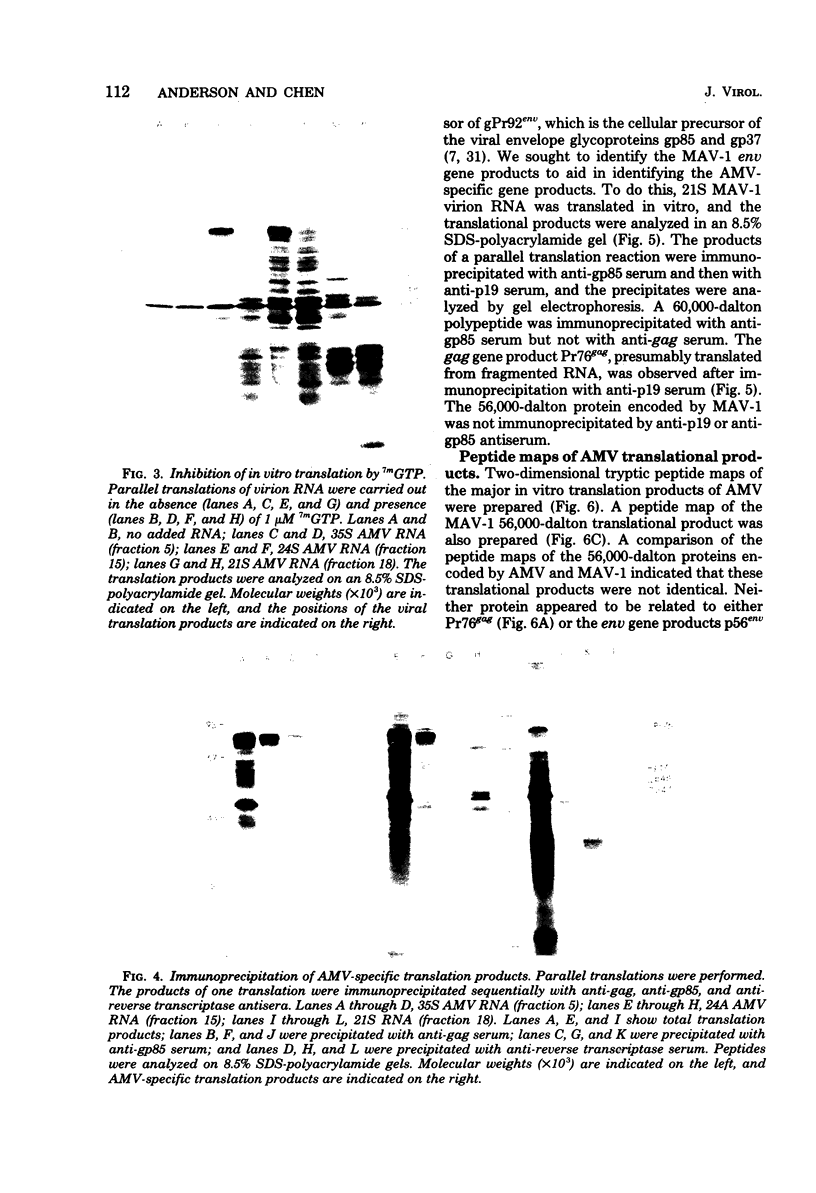
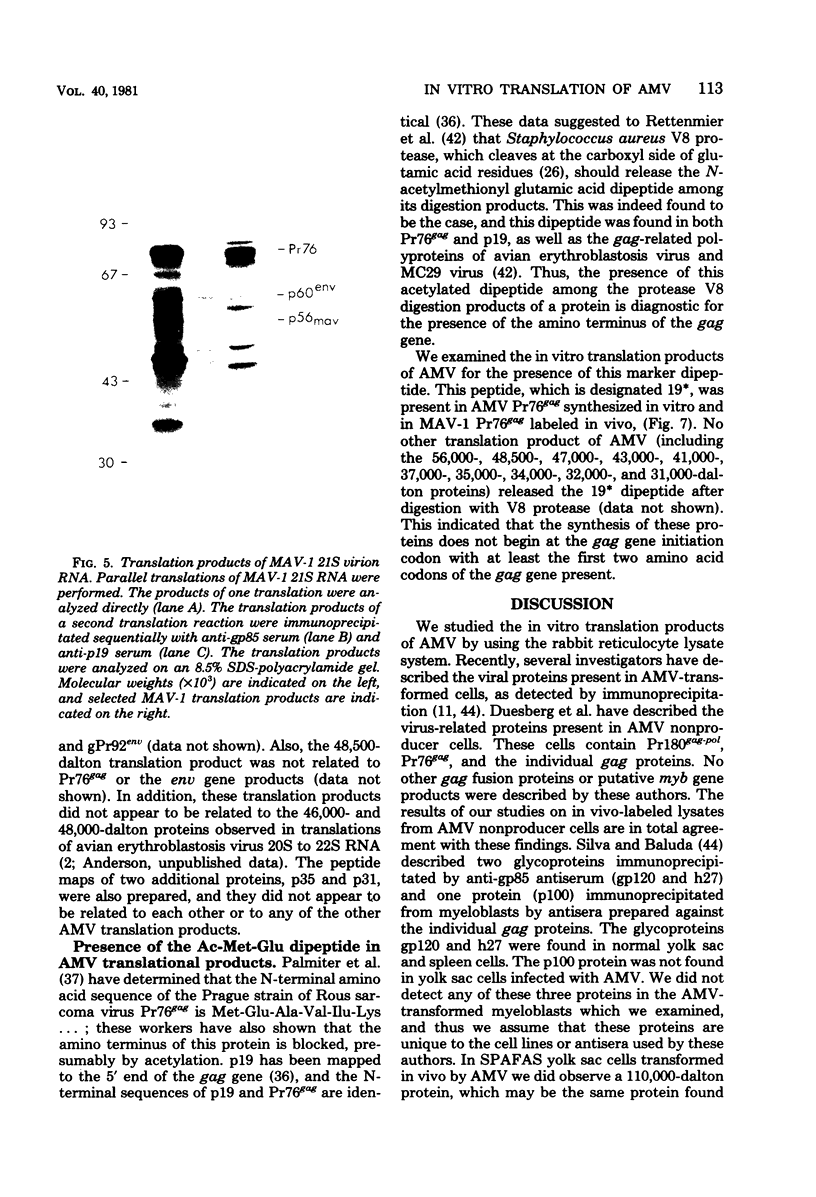
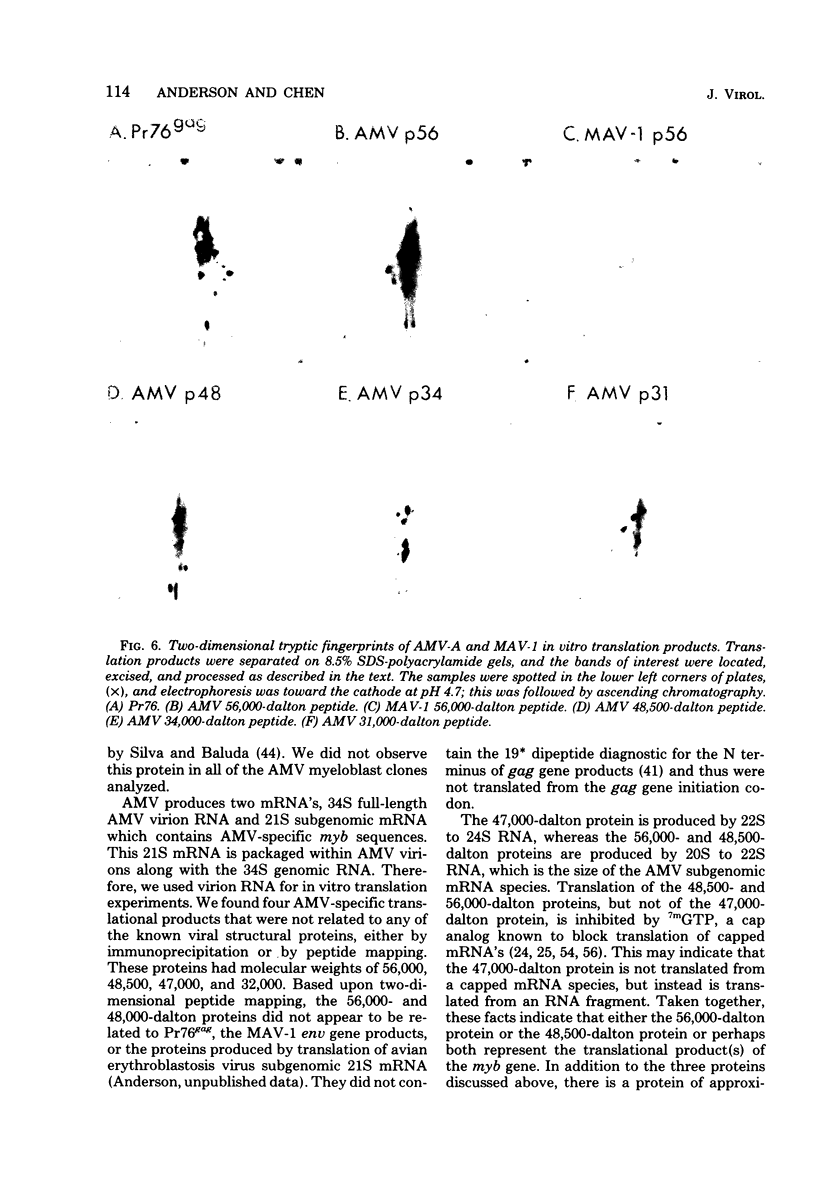
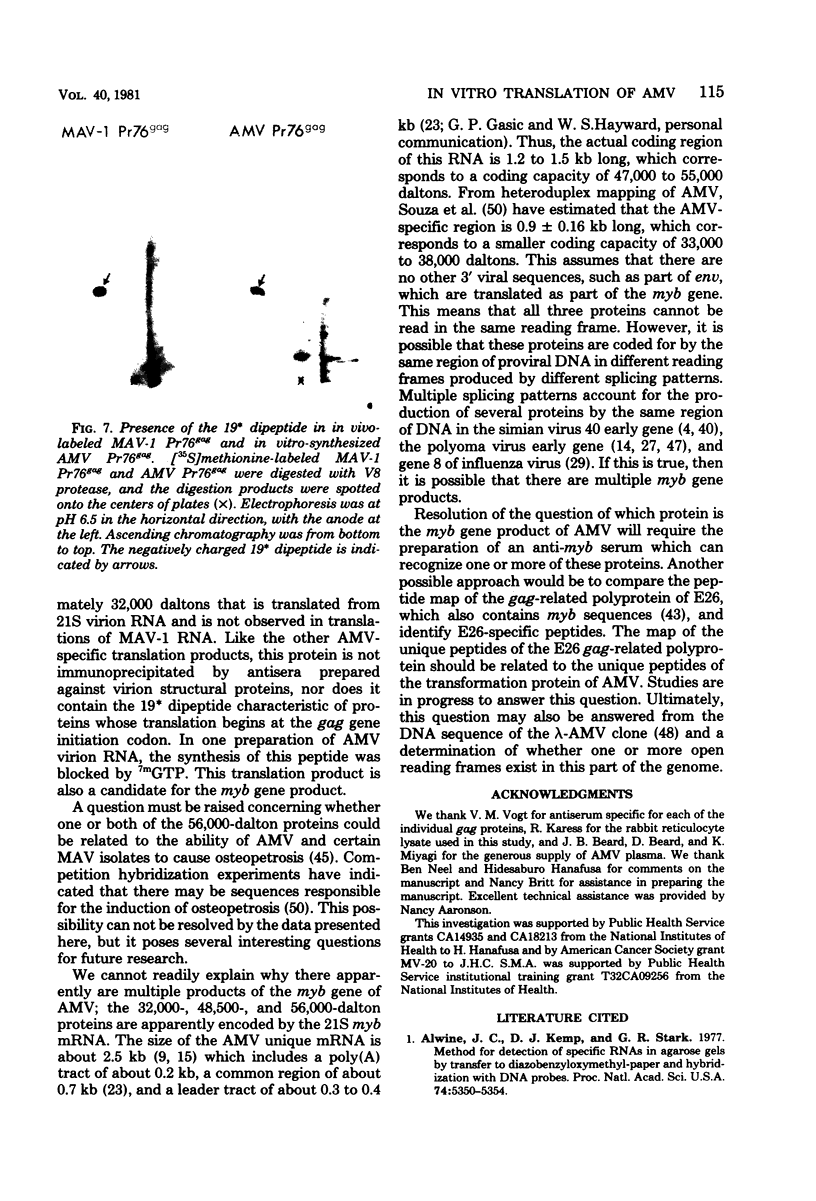
Avian myeloblastosis virus (AMV)-infected cells contain two viral mRNA's, a genome-sized 34S (7.5-kilobase) mRNA and a 21S (2.5-kilobase) subgenomic mRNA, which contains the AMV-specific sequences (myb sequences). We found that AMV virions packaged both the 7.5-kilobase full-length genomic RNA and the 2.5-kilobase subgenomic RNA. In vitro translation of AMV virion RNA sized by sucrose density gradient centrifugation yielded 76,000-, 56,000-, 48,500-, 47,000-, and 32,000-dalton products. The 76,000-dalton protein was coded for by RNA throughout the gradient, but the peak of activity was at 34S to 35S. The 56,000-, and 48,500-, and 32,000-dalton proteins were encoded in a 21S RNA, and 47,000-dalton protein was encoded in an RNA of approximately 24S. The 76,000-dalton protein was identified as Pr76gag, based upon immunoprecipitation with specific antiserum and the presence of the 19* dipeptide. 7-Methylguanosine triphosphate inhibited the syntheses of Pr76gag and the 56,000-, 48,500-, and 32,000-dalton proteins, but not the synthesis of the 47,000-dalton protein. The 56,000-, 48,500-, 47,000-, and 32,000-dalton proteins were not immunoprecipitated by anti-gag, anti-reverse transcriptase, or anti-gp85 antiserum. Two-dimensional peptide maps of the 56,000- and 48,500-dalton proteins indicated that they were unique. In vitro translational products of myeloblastosis-associated virus 1 were also analyzed to aid in the identification of the AMV myb gene product(s); the translational products analyzed included Pr76gag, p60env, and a 56,000-dalton polypeptide which apparently was not identical to the 56,000-dalton AMV translational product, as determined by two-dimensional peptide mapping. Our data indicated that one of these proteins (56,000, 48,500, or 32,000 daltons) may represent the product of the AMV myb gene and, therefore, the putative transforming protein(s) of AMV.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. M., Hayward W. S., Neel B. G., Hanafusa H. Avian erythroblastosis virus produces two mRNA's. J Virol. 1980 Dec;36(3):676–683. doi: 10.1128/jvi.36.3.676-683.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann D. G., Souza L. M., Baluda M. A. Characterization of avian myeloblastosis-associated virus DNA intermediates. J Virol. 1980 May;34(2):366–372. doi: 10.1128/jvi.34.2.366-372.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Structure and specific sequences of avian erythroblastosis virus RNA: evidence for multiple classes of transforming genes among avian tumor viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5023–5027. doi: 10.1073/pnas.76.10.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhagen D. L., Hanafusa H. Intracellular precursors to the major glycoprotein of avian oncoviruses in chicken embryo fibroblasts. J Virol. 1978 Mar;25(3):845–851. doi: 10.1128/jvi.25.3.845-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H. Expression of endogenous avian myeloblastosis virus information in different chicken cells. J Virol. 1980 Oct;36(1):162–170. doi: 10.1128/jvi.36.1.162-170.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Hayward W. S., Moscovici C. Size and genetic content of virus-specific RNA in myeloblasts transformed by avian myeloblastosis virus (AMV). Virology. 1981 Apr 15;110(1):128–136. doi: 10.1016/0042-6822(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Moscovici M. G., Moscovici C. Isolation of complementary DNA unique to the genome of avian myeloblastosis virus (AMV). Virology. 1980 May;103(1):112–122. doi: 10.1016/0042-6822(80)90130-0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Moscovici C. Genetic structure of avian myeloblastosis virus, released from transformed myeloblasts as a defective virus particle. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5120–5124. doi: 10.1073/pnas.77.9.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Avian acute leukemia viruses MC29 and MH2 share specific RNA sequences: evidence for a second class of transforming genes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1633–1637. doi: 10.1073/pnas.76.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Mason W. S., Linial M. Synthesis and processing of polymerase proteins of wild-type and mutant avian retroviruses. J Virol. 1980 Oct;36(1):62–78. doi: 10.1128/jvi.36.1.62-78.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Esty A., LaPorte P., Deininger P. The nucleotide sequence and genome organization of the polyoma early region: extensive nucleotide and amino acid homology with SV40. Cell. 1979 Jul;17(3):715–724. doi: 10.1016/0092-8674(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Fanshier L., Bishop J. M., Moscovici C., Moscovici M. G. The genome and the intracellular RNAs of avian myeloblastosis virus. Cell. 1981 Jan;23(1):279–290. doi: 10.1016/0092-8674(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T. In vitro transformation of chicken bone marrow cells with avian erythroblastosis virus. Z Naturforsch C. 1975 Nov-Dec;30(6):847–849. doi: 10.1515/znc-1975-11-1232. [DOI] [PubMed] [Google Scholar]
- Graf T., Oker-Blom N., Todorov T. G., Beug H. Transforming capacities and defectiveness of avian leukemia viruses OK10 and E 26. Virology. 1979 Dec;99(2):431–436. doi: 10.1016/0042-6822(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Royer-Pokora B., Schubert G. E., Beug H. Evidence for the multiple oncogenic potential of cloned leukemia virus: in vitro and in vitro studies with avian erythroblastosis virus. Virology. 1976 Jun;71(2):423–433. doi: 10.1016/0042-6822(76)90370-6. [DOI] [PubMed] [Google Scholar]
- Graf T. Two types of target cells for transformation with avian myelocytomatosis virus. Virology. 1973 Aug;54(2):398–413. doi: 10.1016/0042-6822(73)90152-9. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C. Inhibition of initiation of protein synthesis by 7-methylguanosine-5'-monophosphate. Proc Natl Acad Sci U S A. 1976 Jan;73(1):19–23. doi: 10.1073/pnas.73.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C., Kim C. H., Sarma R. H. A relation between inhibition of protein synthesis and conformation of 5'-phosphorylated 7-methylguanosine derivatives. J Mol Biol. 1977 Jan 15;109(2):173–183. doi: 10.1016/s0022-2836(77)80027-2. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen R., Favaloro J., Parker J., Treisman R., Lania L., Fried M., Mellor A. Comparison of polyoma virus transcription in productively infected mouse cells and transformed rodent cell lines. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):63–75. doi: 10.1101/sqb.1980.044.01.009. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell. 1980 Sep;21(2):475–485. doi: 10.1016/0092-8674(80)90484-5. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Hayami M. Analysis of precursors to the envelope glycoproteins of avian RNA tumor viruses in chicken and quail cells. J Virol. 1977 Jun;22(3):598–607. doi: 10.1128/jvi.22.3.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Gazzolo L., Moscovici M. G. Focus assay and defectiveness of avian myeloblastosis virus. Virology. 1975 Nov;68(1):173–181. doi: 10.1016/0042-6822(75)90159-2. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G. Tissue culture of avian hematopoietic cells. Methods Cell Biol. 1973;7:313–328. doi: 10.1016/s0091-679x(08)61784-7. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Zanetti M. Studies on single foci of hematopoietic cells transformed by avian myeloblastosis virus. Virology. 1970 Sep;42(1):61–67. doi: 10.1016/0042-6822(70)90238-2. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Vogt V. M., Ripley S., Eisenman R. N. The NH2-terminal sequence of the avian oncovirus gag precursor polyprotein (Pr76gag). Virology. 1978 Dec;91(2):423–433. doi: 10.1016/0042-6822(78)90388-4. [DOI] [PubMed] [Google Scholar]
- Pawson T., Mellon P., Duesberg P. H., Martin G. S. env Gene of Rous sarcoma virus: identification of the gene product by cell-free translation. J Virol. 1980 Mar;33(3):993–1003. doi: 10.1128/jvi.33.3.993-1003.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Piatak M., Weissman S. M. Simian virus 40 early mRNA's. I. Genomic localization of 3' and 5' termini and two major splices in mRNA from transformed and lytically infected cells. J Virol. 1979 Apr;30(1):279–296. doi: 10.1128/jvi.30.1.279-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Anderson S. M., Riemen M. W., Hanafusa H. gag-Related polypeptides encoded by replication-defective avian oncoviruses. J Virol. 1979 Dec;32(3):749–761. doi: 10.1128/jvi.32.3.749-761.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Karess R. E., Anderson S. M., Hanafusa H. Tryptic peptide analysis of avian oncovirus gag and pol gene products. J Virol. 1979 Oct;32(1):102–113. doi: 10.1128/jvi.32.1.102-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Silva R. F., Baluda M. A. Avian myeloblastosis virus proteins in leukemic chicken myeloblasts. J Virol. 1980 Sep;35(3):766–774. doi: 10.1128/jvi.35.3.766-774.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Davids L. J., Neiman P. E. Comparison of an avian osteopetrosis virus with an avian lymphomatosis virus by RNA-DNA hybridization. J Virol. 1975 Jan;17(1):160–167. doi: 10.1128/jvi.17.1.160-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Moscovici C. The oncogenic effects of nontransforming viruses from avian myeloblastosis virus. Cancer Res. 1969 Jul;29(7):1356–1366. [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Griffin B. E. Sequence from early region of polyoma virus DNA containing viral replication origin and encoding small, middle and (part of) large T antigens. Cell. 1979 Jun;17(2):357–370. doi: 10.1016/0092-8674(79)90162-4. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Briskin M. J., Hillyard R. L., Baluda M. A. Identification of the avian myeloblastosis virus genome. II. Restriction endonuclease analysis of DNA from lambda proviral recombinants and leukemic myeoblast clones. J Virol. 1980 Nov;36(2):325–336. doi: 10.1128/jvi.36.2.325-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Komaromy M. C., Baluda M. A. Identification of a proviral genome associated with avian myeloblastic leukemia. Proc Natl Acad Sci U S A. 1980 May;77(5):3004–3008. doi: 10.1073/pnas.77.5.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Strommer J. N., Hillyard R. L., Komaromy M. C., Baluda M. A. Cellular sequences are present in the presumptive avian myeloblastosis virus genome. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5177–5181. doi: 10.1073/pnas.77.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G., Hanafusa H. Microinjection analysis of envelope-glycoprotein messenger activities of avian leukosis viral RNAs. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1614–1618. doi: 10.1073/pnas.74.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Guntaka R. V., Varmus H. E., Bishop J. M. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976 Mar 5;101(3):349–365. doi: 10.1016/0022-2836(76)90152-2. [DOI] [PubMed] [Google Scholar]
- Stéhelin D., Graf T. Avian myelocytomatosis and erythroblastosis viruses lack the transforming gene src of avian sarcoma viruses. Cell. 1978 Apr;13(4):745–750. doi: 10.1016/0092-8674(78)90224-6. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Effect of 7-methylguanosine-5'-phosphate on rabbit globin synthesis. FEBS Lett. 1976 Dec 31;72(2):309–313. doi: 10.1016/0014-5793(76)80993-3. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber L. A., Feman E. R., Hickey E. D., Williams M. C., Baglioni C. Inhibition of HeLa cell messenger RNA translation by 7-methylguanosine 5'-monophosphate. J Biol Chem. 1976 Sep 25;251(18):5657–5662. [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]