Abstract
We determined the entire nucleotide sequence of φSa2958-carrying Panton-Valentine leukocidin (PVL) gene, which was lysogenized in a sequence type 5 staphylococcal cassette chromosome mec (SCCmec) type II strain of methicillin-resistant Staphylococcus aureus (MRSA). Based on the nucleotide sequences of PVL phages, we developed PCRs to discriminate among five PVL phages, with a preliminary classification into two morphological groups (elongated-head type and icosahedral-head type) with four PCRs, including two PCRs for identifying the gene lineage between lukS-PV and the tail gene. The phages were then classified into five types by four PCRs identifying each phage-specific structure. With these PCRs, we examined the PVL phage types of 67 MRSA strains isolated in Japan from 1979 through 1985 and since 2000 and found that two morphologically distinct phages were predominant in Japan. The icosahedral-head-type phage, represented by the φ108PVL type, was identified for 39 of 53 strains isolated from 1979 through 1985. Of 26 other Japanese isolates, 25 belonged either definitively or presumably to elongated-head types as follows: 3 belonged to the φSa2958 type; 8 were determined to belong to an elongated-head type, but a determination of greater specificity was not made; and 14 belonged to a φSa2958-like phage of unknown type. We induced prophages by treatment with mitomycin C from six strains of the φSa2958 type or of φSa2958-like unknown-type phages; five of six strains carried intact PVL-carrying phages, which can infect other S. aureus strains and might generate novel PVL-positive strains of S. aureus. That various SCCmec elements were carried by different strains of the same phage type suggests that S. aureus strains might independently acquire PVL phages before they acquire various SCCmec elements.
Since the early 1990s, the number of strains of methicillin-resistant Staphylococcus aureus (MRSA) isolated from community-acquired infections (CA-MRSA) has increased (5, 11, 31). Characteristics of CA-MRSA strains have been investigated and compared with those of health care-associated MRSA strains. The genotypes and staphylococcal cassette chromosome mec (SCCmec) types of CA-MRSA strains differed greatly from those of health care-associated MRSA strains. In addition, most of CA-MRSA strains are reported to carry the Panton-Valentine leukocidin (PVL) gene at a high level of incidence (11).
PVL was first reported in 1932 (35). It is a two-component toxin composed of LukF-PV, with a molecular mass of 34 kDa, and LukS-PV, with a molecular mass of 32 kDa (36). These two proteins, LukF-PV and LukS-PV, act synergistically and cause damage on cell membranes by forming pores, resulting in the lysis of polymorphonuclear leukocytes and macrophages (6, 13).
The genes for these two proteins, lukS-PV and lukF-PV, respectively, are carried by prophages that are integrated into the S. aureus chromosome. To our knowledge, five PVL phages, φPVL, φSLT, φSa2mw, φ108PVL, and φSa2usa, have been reported to date (1, 9, 20, 21, 28, 32). These phages carry the lukS-PV and lukF-PV genes, which show more than 99% nucleotide identity and are integrated at the same position in the S. aureus chromosome. However, the morphologies of the phage particles are not identical. The phage morphologies can be classified into two types, namely, the icosahedral-head type and the elongated-head type (4). We have examined PVL-positive MRSA strains isolated from 1979 through 1985 and have determined the nucleotide sequence of φ108PVL, carried by SCCmec type IV.3 (SCCmec types are designated hereafter by a capital roman numeral [e.g., type IV] or by a capital roman numeral, a decimal point, and an Arabic numeral [e.g., type IV.3]) sequence type 30 (ST30) MRSA strain 81/108 (28). When we examined the carriage of φ108PVL phage in representative MRSA strains isolated from 1979 through 1985, phage φ108PVL was most often identified in ST30 MRSA strains, whereas the SCCmec type II.1 ST5 MRSA strain JCSC2958 was negative by five of six PCRs using different sets of primers to identify φ108PVL, indicating that the strain might carry another new PVL phage. Both PVL-positive methicillin-sensitive S. aureus (MSSA) strains and MRSA strains have been isolated worldwide, including in the United States (2, 3, 10, 37), France (39), Australia (7), England (15), Canada (30), Singapore (16), Belgium (8), and Uruguay (27). We wondered how these PVL-positive strains evolved by acquiring each PVL phage and how each PVL phage evolved by acquiring the lukS-PV and lukF-PV genes. In an attempt to answer these questions, we determined the nucleotide sequence of a new PVL phage carried by JCSC2958 and developed a PCR system to identify the lysogenized PVL phages. By applying the newly developed PCRs, we have found that Japanese PVL-positive S. aureus strains carry two morphologically distinct PVL phages, namely, the icosahedral-head type, represented by φ108PVL phage, and the elongated-head type, represented by φSa2958, and that these types differ from those for strains disseminated in the United States and France.
MATERIALS AND METHODS
MRSA and MSSA strains.
Sixty-five PVL-positive MRSA strains isolated in Japan were tested. Fifty-three strains were isolated from 1979 through 1985: 12 were isolated at Tokyo University Hospital, 23 at Gunma University Hospital, 10 at Tokyo Geriatric Hospital, and 9 at The Jikei University Hospital. Twelve PVL-positive MRSA strains were isolated over the past 8 years as follows: 1 strain was isolated from pus from the skin of an outpatient at Juntendo University Hospital in 2002; 1 strain was isolated from an inpatient with pneumonia at Moji Rosai Hospital (identified by the surveillance of PVL-positive strains conducted by the SRL Laboratory and kindly provided by Hiroshi Kuramoto); 1 strain was isolated in 2007 by Masato Higashiide from a 7-year-old boy with an abdominal wound; 2 strains were kindly provided by the LVFX Surveillance Group, headed by Keizo Yamaguchi, and consisted of 1 isolated from the pus of a 27-year-old man in 2002 (strain EB00449) and 1 isolated from the centesis of a 61-year-old man in 2000 (strain DB00319) (40); and 7 strains were isolated by Atsuo Katai at Kinan General Hospital between 2005 and 2007. Strains MW2 and 81/108 were used as representative PVL-positive MRSA strains, strain ATCC 49775 was used as a representative PVL-positive MSSA strain, and strain RN4220/φSLT was also used as a control for the induction experiment for PVL-positive phages.
To identify phages induced from lysogenized bacteria, two MSSA strains, RN4220 and 1039, were used as indicator strains. These mutant strains, which lack prophages and restriction systems, were obtained from the NCTC8325 and Terashima strains, respectively (41).
SCCmec typing and identification of virulence genes.
SCCmec typing and identification of virulence genes were performed with PCRs as described previously (22, 27, 34).
Determination of the entire nucleotide sequence of φSa2958.
The fosmid library from the genomic DNA of JCSC2958 was constructed by using the CopyControl fosmid library production kit (Epicentre Biotechnologies, Madison, WI) according to the protocols recommended by manufacturer. Briefly, genomic DNA was prepared from cells of JCSC2958 cells with Isoplant (Nippon Gene Co., Tokyo, Japan). Approximately 400 μg of the genomic DNAs was sheared into small fragments by aspiration and expulsion through an injection needle. Subsequent size selection of 40-kb DNA fragments was performed by running samples in agarose gel (SeaKem gold agarose; Cambrex Bio Science Rockland, Inc., Rockland, ME). To recover the size-fractionated DNA after electrophoresis, agar slices containing the corresponding sizes of DNA were cut and then melted in GElase solution. The recovered DNA was purified and treated with the end repair enzyme mix that came with the kit. The purified insert DNA was ligated into CopyControl pCC1FOS cloning-ready vector with DNA ligase and then packaged according to the manufacturer's instructions. Each 10 μl of the fosmid library was mixed with 100 μl of EPI300-T1R cells and incubated for 20 min to promote phage infection. Each reaction mixture was spread on an l-agar plate containing 12.5 μg/ml of chloramphenicol and incubated at 37°C overnight. Five hundred colonies were screened with two primer pairs for the PVL gene and the integrase gene (Table 1), which are conserved in nearly all extant PVL phages. Plasmids were extracted from positive clones and used as templates for nucleotide sequencing. In some cases, DNA fragments amplified by long-range PCR were used as the templates. Primers used for long-range PCR are listed in Table 1. Nucleotide sequences were subsequently determined with the primer-walking method.
TABLE 1.
List of primers used in this study
| Objective of primer construction | Primer name | Nucleotide sequence (5′-3′) | Size (bp) of PCR product | Gene or structure on which primer was designed | PVL phage(s) | Location(s) of primer(s) |
|---|---|---|---|---|---|---|
| For screening of fosmid library | int-R2 | CATTTTAATTGCCAGCATCTTA | 558 | int | φSa2958 | 1547-1525 |
| int-F2 | ATGTTTTCGAGTTTTTGAGTTAG | int | 989-1011 | |||
| PVL-F | ATGTCTGGACATGATCCAA | 970 | lukS-PV | 45386-45404 | ||
| PVL-R | AACTATCTCTGCCATATGGT | lukF-PV | 46356-46337 | |||
| For long-range PCR to amplify entire structure of φSa2958 | ||||||
| From chromosome to integrase | phiMW2-DN1 | GCAGAAAAAGATGCGATTGAA | (2.5 kb) | Chromosome of JCSC2958 | φSa2958 | Upstream of φ2958PVL |
| intR | AGGATATCGAAAAAGATGAATC | int | 1655-1634 | |||
| From integrase to DNA polymerase | intc | TTTGTAGTGTCTTTGTATCCG | 9,214 | int | 1376-1396 | |
| MW1425-R | TTGTTGCCATTTTTCAAGATC | pol | 10589-10569 | |||
| From DNA polymerase to ORF JP030 | MW1425-F | CTAAAGTAGATAATGAGCCTT | 8,060 | pol | 10237-10257 | |
| 2958-1405R1 | TCCCTTTTCTTGCTTCATTTC | JP030 | 18296-18276 | |||
| From virulence-associated protein E to portal protein | CF35 | ACGAAGACGATTTTATCAAGG | 6,097 | por | 17509-17529 | |
| 2958-portalR1 | CACTATATCTTCAGAGACATA | virE | 23605-23585 | |||
| From terminase large subunit to tail length tape measure protein | CF47 | AAAGTTATCTAATTCGATGGC | 10,480 | terL | 22990-23010 | |
| CF67 | GGGCTCTTGAATACATATCT | mtp | 33469-33450 | |||
| From tail length tape measure protein to ORF JP052 | 2958-1392-F10 | ATACTGAAAAGTGGTGGAATG | 8,741 | mtp | 32807-32827 | |
| phi-M-TR1 | GACTTCCTAAGTCGAAATAG | JP052 | 41547-41528 | |||
| From ORF JP052 to lukF-PV gene | phi-M-T1 | TGGATTAACTAAATCTAGTCG | 4,877 | JP052 | 41480-41500 | |
| PVL-R | AACTATCTCTGCCATATGGT | lukF-PV | 46356-46337 | |||
| From ORF JP058 to chromosome | LukS-RR | TGGTCAACTATATCGTGGTTTT | (2 kb) | JP058 | 44574-44595 | |
| phiMW-UP | TCGCCACGTTTAGCAATTTTAT | Chromosome of JCSC2958 | Downstream of φSa2958 | |||
| For classifying phages | ||||||
| PCR-1 (for identifying φ108PVL and φPVL) | portal-1F | ACACGTGATAAAACAGGAGAA | 569 | por | φ108PVL | 21069-21089 |
| portal-1R | TCTAAATTAGCATCCGTGATAC | por | 21637-21616 | |||
| tail-1R | ATAATTGGGATAGCAACGCAA | 489 | mtp | 31237-31257 | ||
| tail-1F | CTTGATTAGACTCAACCAAACT | mtp | 31725-31704 | |||
| PCR-2 (for identifying φ2958PVL, φSLT, and φSa2mw) | portal-2F | GATGGCTAGTTTGCCCTTGA | 656 | por | φSa2958 | 23005-23024 |
| portal-2R | CTGAGGGCAATTGAAAAACG | por | 23660-23641 | |||
| tail-2F | CATAGCGCTAATGTCGCAAA | 468 | mtp | 30040-30059 | ||
| tail-2R | AGCCTCCATTGTTTGTTTGG | mtp | 30507-30488 | |||
| PCR-3 (for identifying the gene lineage between genes of φ108PVL and φPVL, and lukS-PV) | lukSR1 | ACGAAGTAGCAATAGGAGTGA | 10,497 | lukS-PV | φ108PVL | 42326-42306 |
| teil-ico-F | AGATTTAGAAGAGGAGGCACGA | mtp | 31830-31851 | |||
| PCR-4 (for identifying the gene lineage between genes of φSa2958, φSa2mw, and φSLT, and lukS-PV) | lukSR1 | ACGAAGTAGCAATAGGAGTGA | 9,483 | lukS-PV | φSa2958 | 44861-44841 |
| For identifying each PVL phage | ||||||
| PCR-5 (for identifying φPVL and φ108PVL) | intF2 | ATGTTTTCGAGTTTTTGAGTTAG | 4,340 | int | φ108PVL, φPVL | 393-415, 24310-24332 |
| 108-aR | TCAAATCCGTAATCACTCATTCT | ant | φ108PVL | 4732-4710 | ||
| PVL-aR | TTCACTAACTAAACCTATCATTGT | 1,411 | JP030 | φPVL | 25720-25697 | |
| PCR-6 (for identifying φSa2958) | int-F2 | ATGTTTTCGAGTTTTTGAGTTAG | 2,238 | int | φSa2958 | 989-1011 |
| 2958-aR | TGGTAATCAACCATTCACTTATGA | JP004 | 3226-3203 | |||
| PCR-7 (for identifying φSa2mw) | int-F2 | ATGTTTTCGAGTTTTTGAGTTAG | 4,065 | int | φSa2mw | 1574920-1574898 |
| MW2-aR | TAAGTTCCTGGTGTCATTCCTAAT | cro | 1570856-1570879 | |||
| PCR-8 (for identifying φSLT) | int-F2 | ATGTTTTCGAGTTTTTGAGTTAG | 8,770 | int | φSLT | 123-145 |
| SLT-aR | TCTTACCAAATGCAACACAACGAAT | ssb | 8892-8868 |
MLST and coagulase typing.
The genotypes of representative strains were determined by means of multilocus sequence typing (MLST) by the method of Enright et al. (12). The coagulase types of all tested strains were determined with the serological method established by Ushioda et al. (38).
Induction of prophages from S. aureus cells.
A 0.3-ml portion of overnight culture was inoculated to 3 ml of BHI broth. After the culture had been shaken for 2 h at 37°C, mitomycin C (Kyowa Hakko Kogyo Co., Ltd., Tokyo, Japan) was added to a final concentration of 1 μg/ml, and the culture was cultivated at 37°C with shaking for 50 min. Cells were then precipitated by centrifugation and resuspended with 300 μl of L broth. A 100-μl portion was added to 3 ml of L broth and incubated further at 37°C until the cell suspension became transparent. The supernatant was sterilized by means of a membrane filter with 0.45-μm-diameter pores (Whatman Corp., Clifton, NJ).
Identification of PVL-carrying phages by plaque hybridization.
Phage solutions were diluted 10-fold serially, and a 0.1-ml portion of each solution was mixed with a 0.3-ml overnight culture of the RN4220 or 1039 strain and kept at room temperature for 15 min. Three milliliters of L broth containing 0.6% agar was added to each mixture, which was then poured onto heart infusion agar plates. The plates were incubated overnight at 30°C to form plaques on the lawns. Plates with an appropriate numbers of plaques were selected, and the plaques were transferred onto a piece of Biodyne membrane (Pall Biodyne A, pore size, 1.2 μm; Pall Life Sciences, Ann Arbor, MI). The filters were denatured by submersion for 5 min in 0.5 M NaOH and 1.5 M NaCl and then neutralized by submersion twice for 5 min in a solution of 0.5 M Tris-HCl (pH 7.3), 1.5 M NaCl, and 1 mM EDTA.
The DNAs were cross-linked to the nylon membrane by use of the Stratalinker UV cross-linker (Stratagene Japan K. K., Tokyo, Japan). Probes to identify PVL-carrying phages were prepared by labeling DNA fragments with digoxigenin by use of the digoxigenin DNA labeling and detection kit (Roche Applied Science, Penzberg, Germany). The DNA fragments used for plaque hybridization were amplified with PCR and genomic DNA of the JCSC2958 strain as a template with a pair of primers to identify the lukS-PV and lukF-PV genes (Table 1). The subsequent experiment was performed as described by the manufacturer.
PCRs to classify PVL phages.
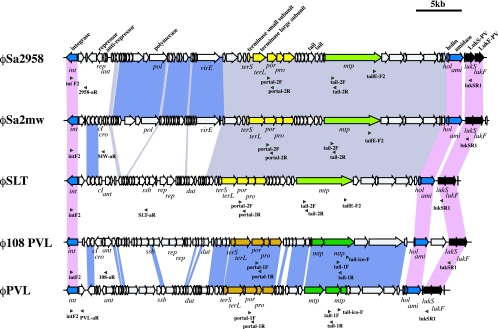
We developed eight PCRs to classify the PVL-encoding prophages by use of chromosomal DNA preparations from PVL-positive S. aureus strains as templates. Primers used for identifying PVL-encoding phages are listed in Table 1. These primers were designed on the bases of nucleotide sequences of five PVL phages, namely, φ108PVL, φSa2mw, φPVL, φSLT, and φSa2958, which are deposited in DDBJ/EMBL/GenBank databases under accession nos. AB009866, BA000033, AB009866, NC_002661, and AP009363, respectively. The locations of the primers are shown below (see Fig. 3).
FIG. 3.
Structural comparison of 5 PVL phages. Structures of φ108PVL, φPVL, φSLT, φSa2mw, and φSa2958 are illustrated on the bases of the nucleotide sequences as follows: φ108PVL (DDBJ/EMBL/GenBank databases under accession no. AB243556), φPVL (DDBJ/EMBL/GenBank accession no. AB009866), φSLT (DDBJ/EMBL/GenBank accession no. NC_002661), φSa2mw (DDBJ/EMBL/GenBank accession no. BA000033), and φSa2958 (DDBJ/EMBL/GenBank accession no. AP009363). Genes having nucleotide identities of more than 90% are linked by blocks filled with colors as follows: pink, genes conserved among five PVL phages; light purple, genes conserved among φSa2958, φSa2mw, and φSLT; blue, genes conserved between φPVL and φ108PVL. The positions of primers for M-PCR 1 to 7 are indicated by green arrowheads. Blue arrows indicate ORFs well conserved in all PVL phages other than lukS and lukF genes; black arrows indicate lukS and lukF genes; light green and dark green arrows indicate genes encoding major tail proteins of the elongated-head type and the icosahedral-head type, respectively; and yellow arrows and ocher arrows indicate genes encoding terminase subunits (large and small), portal proteins, prohead proteases, and capsid proteins of the elongated-head type and the icosahedral-head type, respectively.
Two PCRs were designed to identify the carriage of two morphologically distinct phages. PCR-1 was designed to identify the carriage of phages with isometric hexagonal heads by amplifying the portal gene and the head gene, which are conserved in φPVL and φ108PVL. PCR-2 was designed to identify the carriage of phages with elongated heads by amplifying the portal gene and the head gene, which are conserved in φSa2mw, φSa2958, and φSLT. If two DNA fragments could be amplified with PCR-1, we then proceeded to PCR-3, which was designed to identify the gene lineage between the tail gene and the lukS gene and to verify that the tail gene identified with PCR-1 belonged to a PVL-carrying phage with a hexagonal head.
If two DNA fragments were amplified with PCR-2, we proceed to PCR-4, which was designed to identify the gene lineage between the tail gene and the lukS gene to verify that the tail gene of the elongated shape is located in relation to the lukS gene with a primer pair commonly conserved among φSa2958, φSa2mw, and φSLT.
PCR-5 to -8 were designed to identify five PVL phages by amplifying the gene lineage between the integrase gene, which is commonly carried by all reported PVL-carrying phages, and genes located on the region related to the lysogeny or recombination of each PVL phage.
PCR-5 was designed to identify φPVL and φ108PVL by integrase and two open reading frames (ORFs), the repressor for φPVL and the antirepressor for φ108PVL. PCR-6, -7, and -8 were designed to identify φSa2958, φSa2mw, and φSLT by detecting the gene lineage between the integrase gene and the genes located downstream of the gene: those for a hypothetical protein (JCP004) for φSa2958, cro repressor protein for φSa2mw, and a single-stranded binding protein for φSLT.
Chromosomal DNAs were prepared with the small-scale phenol extraction method and used as PCR templates. The PCRs were performed using a Gene Amp 9600 thermal cycler (Perkin-Elmer Cetus Instruments, Emeryville, CA). The reaction mixtures for PCR-1, -2, and -5 to -8 contained 50 ng of template DNA, each oligonucleotide primer (0.2 mM), 400 mM of each deoxynucleoside triphosphate, 1× Ex Taq buffer with magnesium, and 4 U of Ex Taq polymerase (Takara Shuzo Co. Ltd., Kyoto, Japan) in final volumes of 50 μl. PCRs for five PCRs (PCR-1, -2, -5, -6, and -7) consisted of 30 cycles of denaturation (95°C, 60 s), annealing (50°C, 60 s), and extension (72°C, 2 min).
PCR-3, -4, and -8 were carried out with long-range PCR using an Expand high-fidelity PCR kit adhering to the protocols recommended by manufacturer. The 6-μl PCR mixture was subjected to agarose gel electrophoresis to detect amplified DNA fragments.
Nucleotide sequence accession number.
The entire nucleotide sequence of φSa2958 has been deposited in the DDBJ/EMBL/GenBank database under accession no. AP009363.
RESULTS
The structure of a novel PVL phage, φSa2958.
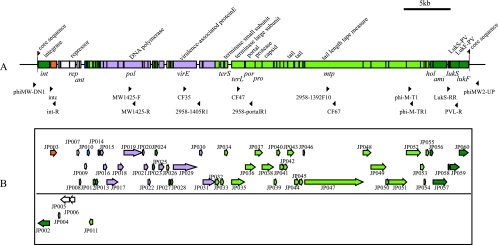
The φSa2958 phage is 46,046 bp in length, which is a size comparable to those of other PVL phages identified to date. A total of 59 predicted ORFs of larger than 99 bp were identified in φSa2958 (Fig. 1 and Table 2). The whole G+C content of the novel PVL phage was 33.1%, which is comparable to those of S. aureus genomes. The gene coding potential for φSa2958 was 90.6%, with approximately 1.28 genes/kbp of nucleotide sequence.
FIG. 1.
(A) The structure of φSa2958. Black arrowheads indicate the locations of primers used to amplify the entire φSa2958 genome. The two red arrowheads flanking the core sequence indicate the att sites on the phage element. (B) The ORFs in φSa2958. The ORFs are shown as squares in six possible reading frames. The direction of the arrow indicates the transcriptional direction for each ORF. Color codes are as follows: dark green, ORFs (or the parts of ORFs) that are well conserved among four other PVL-carrying phages, namely, φSa2mw, φSLT, φPVL, and φ108PVL; light green, ORFs that are highly homologous to φSa2mw and φSLT; light purple, ORFs that are highly homologous to φSa2mw; orange, an ORF that is homologous to φSLT and φPVL; yellow, an ORF that is homologous to φSLT and φ108PVL; white, ORFs that are unique to φSa2958; light blue, ORFs that are homologous to φSa2mw; dark blue, ORFs that are homologous to φSLT.
TABLE 2.
ORFs in and around φ2958PVL and their similarities to φSa2mw and φ108PVLa
| ORF and special structure | Start (bp) | End (bp) | Size (bp) | aaa | Gene | Function | φSa2mw
|
φ108PVL
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Identity to φSa2mw genome | Corresponding ORF (size in bp) | % aa identity | % Identity to φ108PVL genome | Corresponding ORF (size in bp) | % aa identity | |||||||
| (JP001)b | 295 | 936 | 642 | 214 | Hypothetical protein | |||||||
| attB1 | 842 | 866 | 25 | Attachment site on chromosome | ||||||||
| Core | 867 | 895 | 29 | Core sequence | ||||||||
| attP-left | 896 | 920 | 25 | Attachment site on phage | ||||||||
| JP002 | 2184 | 979 | 1,206 | 401 | int | Phage integrate | 98.7 | Integrase (1,206) | 99.8 | 98.6 | Integrase (1,206) | 99.8 |
| JP003 | 2310 | 2924 | 615 | 204 | Na/K ATPase | 47.4 | Putative membrane protein MW1441 (624) | 26.7 | 52.1 | |||
| JP004 | 3285 | 3103 | 183 | 60 | Hypothetical protein | 52.2 | 52.2 | |||||
| JP005 | 4301 | 3381 | 921 | 306 | Hypothetical protein | 45.9 | 45 | |||||
| JP006 | 4931 | 4317 | 615 | 204 | rep | Putative transcriptional repressor | 45.2 | Repressor (324) | 34.3 | 49.1 | cI-like repressor (717) | 24 |
| JP007 | 5103 | 5330 | 228 | 75 | Hypothetical protein | 54.4 | 54.7 | Putative cro-like repressor (243) | 23.1 | |||
| JP008 | 5356 | 5520 | 165 | 54 | ant | Putative antirepressor | 52.1 | 50.3 | Putative antirepressor (753) | 31.4 | ||
| JP009 | 5927 | 6145 | 219 | 72 | Hypothetical protein | 51.8 | 52 | |||||
| JP010 | 6195 | 6440 | 246 | 81 | Hypothetical protein | 100 | MW1434 (246) | 100 | 47.9 | |||
| JP011 | 6774 | 6409 | 366 | 121 | Hypothetical protein | 100 | MW1433 (366) | 100 | 46.1 | |||
| JP012 | 6829 | 7044 | 216 | 71 | Hypothetical protein | 77.3 | MW1432 (216) | 66.2 | 55.6 | |||
| JP013 | 7069 | 7332 | 264 | 87 | Hypothetical protein | 82.6 | MW1431 (264) | 78.8 | 99.6 | Putative DNA-binding protein (264) | 98.9 | |
| JP014 | 7347 | 7505 | 159 | 52 | Hypothetical protein | 93.7 | MW1430 (162) | 96.2 | 98.7 | |||
| JP015 | 7584 | 7907 | 324 | 107 | Hypothetical protein | 92.9 | MW1429 (324) | 91.6 | 51 | |||
| JP016 | 7922 | 8284 | 363 | 120 | Hypothetical protein | 97.8 | MW1428 (363) | 97.5 | 48.9 | |||
| JP017 | 8281 | 9447 | 1,167 | 388 | Hypothetical protein | 97.4 | MW1427 (1,167) | 97.9 | 46.5 | |||
| JP018 | 9473 | 10030 | 558 | 185 | Hypothetical protein | 98.7 | MW1426 (558) | 99.5 | 49.7 | |||
| JP019 | 10098 | 12050 | 1,953 | 650 | pol | DNA polymerase | 99.8 | DNA polymerase (1,962) | 99.7 | 47.5 | ||
| JP020 | 12063 | 12248 | 186 | 61 | Hypothetical protein | 100 | MW1424 (186) | 100 | 69.9 | |||
| JP021 | 12245 | 12649 | 405 | 134 | Putative DNA-binding protein | 100 | MW1423 (402) | 100 | 57 | |||
| JP022 | 12649 | 12906 | 258 | 85 | Hypothetical protein | 97.7 | MW1422 (258) | 97.6 | 52.8 | |||
| JP023 | 13118 | 13366 | 249 | 82 | Hypothetical protein | 92.4 | MW1420 (243) | 93.8 | 91.6 | P028 (249) | 89 | |
| JP024 | 13407 | 13661 | 255 | 84 | Hypothetical protein | 89.8 | MW1419 (255) | 86.6 | 96.8 | P030 (372) | 98.8 | |
| JP025 | 13811 | 14347 | 537 | 178 | Hypothetical protein | 95.2 | MW1415 (534) | 96.6 | 49.1 | |||
| JP026 | 14645 | 14881 | 237 | 78 | Hypothetical protein | 54 | 100 | P032 (237) | 100 | |||
| JP027 | 14865 | 15026 | 162 | 53 | Hypothetical protein | 91.7 | MW1411 (156) | 86.3 | 92.1 | |||
| JP028 | 15094 | 15294 | 201 | 66 | Hypothetical protein | 98.5 | MW1410 (201) | 97 | 61.6 | |||
| JP029 | 15347 | 17794 | 2,448 | 815 | virE | Virulencc-associated protein E | 98.9 | Virulence-associated protein E (2,448) | 99.6 | 46.7 | ||
| JP030 | 18135 | 18425 | 291 | 96 | Hypothetical protein | 95.2 | MW1406 (198) | 98.5 | 50 | |||
| JP031 | 18502 | 19773 | 1,272 | 423 | Phage helicase | 99.4 | Phage helicase (1,359) | 100 | 47.8 | |||
| JP032 | 19786 | 20223 | 438 | 145 | Phage regulatory protein | 89.7 | MW1404 (438) | 93.8 | 51.4 | |||
| JP033 | 20380 | 20694 | 315 | 105 | Hypothetical protein | 97.5 | MW1403 (315) | 95.2 | 51.5 | |||
| JP034 | 20805 | 21128 | 324 | 107 | terS | Terminase small subunit | 97.5 | Terminase small subunit (306) | 100 | 49.4 | Terminase small subunit (468) | 28.6 |
| JP035 | 21448 | 22809 | 1,362 | 453 | terL | Terminase large subunit | 91.6 | Terminase large subunit (1,692) | 96.5 | 46.1 | Terminase large subunit (1,695) | 24.8 |
| JP036 | 23006 | 24052 | 1,047 | 348 | por | Portal protein | 98.9 | Portal protein (1,239) | 98.9 | 48.7 | Portal protein (1,326) | 21.9 |
| JP037 | 24036 | 24809 | 774 | 257 | pro | Prohead protease | 99.7 | Protease (774) | 100 | 46.7 | Prohead protease (585) | 24.6 |
| JP038 | 24776 | 25984 | 1,209 | 402 | Putative capsid protein | 99.9 | Capsid (1,164) | 100 | 49.3 | Capsid (1,248) | 19.2 | |
| JP039 | 26053 | 26331 | 279 | 92 | Hypothetical protein | 100 | MW1397 (279) | 100 | 49.4 | |||
| JP040 | 26343 | 26675 | 333 | 110 | Hypothetical protein | 96.4 | MW1396 (333) | 97.3 | 49.5 | |||
| JP041 | 26672 | 27073 | 402 | 133 | Hypothetical protein | 99.5 | MW1395 (402) | 100 | 48.5 | |||
| JP042 | 27074 | 27469 | 396 | 131 | Hypothetical protein | 100 | MW1394 (396) | 100 | 50.4 | |||
| JP043 | 27504 | 28145 | 642 | 213 | Major tail protein | 99.8 | Major tail protein (642) | 100 | 47.8 | |||
| JP044 | 28237 | 28692 | 456 | 151 | Major tail protein | 99.8 | Major tail protein (456) | 99.3 | 49.3 | |||
| JP045 | 28750 | 29100 | 351 | 116 | Hypothetical protein | 99.7 | MW1391 (342) | 100 | 50.4 | |||
| JP046 | 29142 | 29300 | 159 | 52 | Hypothetical protein | 100 | No corresponding gene | 56.8 | ||||
| JP047 | 29314 | 35514 | 6,201 | 2066 | mtp | Tail length tape measure protein | 98.1 | Tail length tape measure protein (6,201) | 98.9 | 46.4 | Tail length tape measure protein (3,333) | 25.3 |
| JP048 | 35514 | 36338 | 825 | 274 | Hypothetical protein | 100 | MW1389 (825) | 100 | 48.5 | |||
| JP049 | 36347 | 37930 | 1,584 | 527 | Hypothetical protein | 100 | MW1388 (1,584) | 100 | 45.7 | |||
| JP050 | 37930 | 38220 | 291 | 96 | Hypothetical protein | 100 | MW1387 (291) | 100 | 49.5 | |||
| JP051 | 38236 | 40146 | 1,911 | 636 | Hypothetical protein | 99.6 | MW1386 (1,911) | 99.8 | 45.4 | |||
| JP052 | 40113 | 41612 | 1,500 | 499 | Hypothetical protein | 99.7 | MW1385 (1,467) | 99.8 | 47.5 | |||
| JP053 | 41612 | 42001 | 390 | 129 | Hypothetical protein | 100 | MW1384 (390) | 100 | 48.1 | |||
| JP054 | 41994 | 42158 | 165 | 54 | Hypothetical protein | 100 | MW1383 (165) | 100 | 54 | |||
| JP055 | 42204 | 42503 | 300 | 99 | Hypothetical protein | 100 | MW1382 (300) | 100 | 51.1 | |||
| JP056 | 42639 | 42941 | 303 | 100 | hol | Holin | 100 | Holin (303) | 100 | 100 | Holin (303) | 100 |
| JP057 | 42952 | 44406 | 1,455 | 484 | aml | Amidase (peptidoglycan hydrolase) | 98.3 | Amidase (1,455) | 99.2 | 98.3 | Amidase (1,455) | 99.2 |
| JP058 | 44556 | 44687 | 132 | 43 | Hypothetical protein | 98.5 | No corresponding gene | 98.5 | ||||
| JP059 | 44795 | 45733 | 939 | 315 | lukS-PV | LukS-PV | 99.8 | LukS-PV (939) | 99.7 | 100 | LukS-PV (939) | 100 |
| JP060 | 45735 | 46712 | 978 | 325 | lukF-PV | LukF-PV | 99.9 | LukF-PV (978) | 100 | 99.9 | LukF-PV (978) | 100 |
| attP-right | 46917 | 46941 | 25 | Attachment site on phage | ||||||||
| Core | 46942 | 46970 | 29 | Core sequence | ||||||||
| attB2 | 46971 | 46995 | 25 | Attachment site on chromosome | ||||||||
aa, amino acid.
ORF in parentheses was located outside of the φ2958PVL. Two sets of core sequences and attachment site sequences were also identified flanking the φ2958PVL.
The 29-bp core sequence in φSa2958 exactly matched the corresponding core sequence conserved in φSa2mw but differed from those of φSLT, φPVL, and φ108PVL by 1 bp. The 25-bp sequences of the attB site located on the chromosome side were identified flanking both ends of φSa2958. The rightmost (attB-R) sequence was identical with that of φSa2mw, whereas the leftmost (attB-L) sequence differed by 2 bp from that of φSa2mw and differed significantly from those of φPVL and φ108PVL. The 25-bp attachment sites (attP) at both ends of the phage were well conserved among all five PVL phages.
The organization of the φSa2958 genome was similar to those of other extant PVL phages, indicating that the following order of modules on the phage genome is well conserved: regions related to lysogeny, DNA replication/transcriptional regulation regions, the packaging/head, the tail, and the lysis module as well as lukS-PV and lukF-PV. Fifty-nine ORFs were roughly classified into five groups on the basis of similarities with extant PVL phages.
Group 1 contained 10 genes (JP013, JP014, JP023, JP024, JP027, JP056, JP057, JP058, JP059, and JP060) conserved among five extant PVL phages, with nucleotide identities of more than 90% (Fig. 1). The integrase gene (int) was located at the leftmost side of φSa2958. It showed high similarity to int genes carried by extant PVL phages, with more than 98% identity in amino acids. ORFs JP056, JP057, JP059, and JP060 encoded holin, amidase, and LukS-PV and LukF-PV proteins, respectively. Both the lukF-PV and lukS-PV genes showed nucleotide identities of more than 99% with those of other PVL phages. In particular, the lukF-PV gene in φSa2958 exhibited 100% homology with that of φPVL. ORF JP058 encodes 48 amino acids with no assigned function identified by means of a BLASTP search. This ORF was also identified in φPVL, although it was disrupted and its function could not be assigned because of the insertion of a “T” nucleotide.
Group 2 contained 27 ORFs (JP011, JP012, JP028, and JP032 to -055) conserved among φSa2958, φSa2mw, and φSLT (Fig. 1). Because eight ORFs that might be associated with phage DNA packaging and head and tail formation are involved (JP034 [terminase small subunit], JP035 [terminase large subunit], JP036 [portal protein], JP037 [prohead protease], JP038 [capsid protein], JP043 and JP044 [major tail protein], and JP047 [phage tail tape measure protein]), φSa2958 could be determined on the basis of taxonomy criteria as belonging to the same subgroup as φSa2mw and φSLT.
Group 3 contained 12 ORFs (JP015 to JP022, JP025, JP029, JP030, and JP031) that showed high similarity only with φSa2mw (Fig. 1). Three genes were associated with phage replication during the phage lyric process. Representative genes in group 3 encode DNA polymerase (JP019), DNA-binding protein (JP021), helices (JP031), and virulence-associated protein E (JP029).
Group 4 contained three ORFs that were homologous only to one or two other phages. JP026, JP003, and JP010 (Fig. 1) were homologous only to φ108PVL and φSLT, only to φPVL and φSLT, and only to φ108PVL, respectively.
Group 5 contained six ORFs (JP004 to -009) that were uniquely conserved in φSa2958 (Fig. 1). Interestingly, JP006, containing the transcription repressor gene, was not homologous to the corresponding genes in other extant PVL phages. A search with the BLASTP program revealed that JP006 showed 100% identity with the corresponding gene conserved in lytic phage 47 and showed 65% identity with that in lytic phage 29, whose entire genomic sequences have been determined by Kwan et al. (23).
The JP006 protein contains a helix-turn-helix DNA-binding motif that is present in a large family of transcriptional regulators. Another unique ORF, JP008, which is conserved in φSa2958 and was identified as an antirepressor gene, was shown to have 93% similarity with the corresponding gene in phage 47. The remaining four unique ORFs in φSa2958 could not be assigned any functions through searches with the BLASTP program.
Characterization of PVL-carrying prophages in Japanese MRSA strains.
We conducted PCR experiments to identify PVL phages carried by 53 MRSA strains isolated in from 1979 through 1985 and 12 MRSA strains isolated in the 2000s with PCRs as described in Materials and Methods. The representative results of these PCRs are shown in Fig. 2 and the results of PCR experimentation are summarized in Table 3. When PCR-1 was performed with the chromosomal DNAs of these same five strains carrying each PVL phage, DNA fragments of two expected sizes, 489 bp for the tail gene and 569 bp for the head gene, were amplified with chromosomal DNAs of 81/108 (φ108PVL), ATCC 49775 (φPVL), and MW2 (φSa2mw). When PCR-2 was performed with chromosomal DNAs of these same five strains, two DNA fragments of expected sizes, 466 bp for the tail gene and 655 bp for the head gene, were amplified with chromosomal DNAs of JCSC2958 (φSa2958), RN4220 (φSLT), MW2 (φSa2mw), and 81/108 (φ108PVL).
FIG. 2.
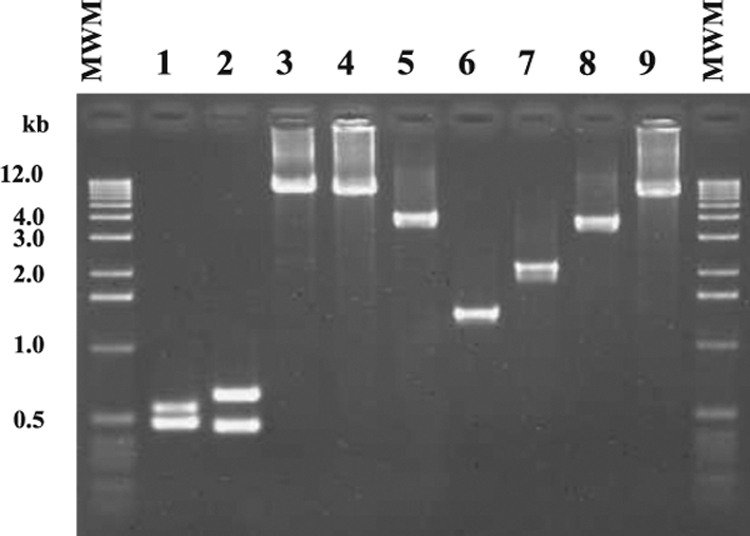
Representative results of seven multiplex PCRs (M-PCRs) for identifying PVL phages. A 1-kb molecular weight marker (MWM) was run on both sides of the gel. Lanes: 1, PCR-1 using chromosomal DNA of 81/108; 2, PCR-2 using chromosomal DNA of JCSC2958; 3, PCR-3 using chromosomal DNA of 81/108; 4, PCR-4 using chromosomal DNA of JCSC2958; 5, PCR-5 using chromosomal DNA of 81/108; 6, PCR-5 using chromosomal DNA of ATCC 49775; 7, PCR-6 using chromosomal DNA of JCSC2958; 8, PCR-7 using chromosomal DNA of MW2; and 9, PCR-8 using chromosomal DNA of RN4220 (φSLT).
TABLE 3.
Results of PCR experimentationa
| Yr of isolation (no. of strains) | Identification of (no. of strains):
|
PVL-carrying phage type (no. of strains) | ||||
|---|---|---|---|---|---|---|
| Two kinds of phages by:
|
Gene lineage of lukS-PV to tail gene by:
|
Phage-specific modules related to lysogeny and recombination by PCR-5, -6, and -7 | ||||
| PCR-1 (icosahedrally shaped head) | PCR-2 (elongated head) | PCR-3 (tail gene common to φ108PVL and φPVL) | PCR-4 (tail gene common to φSa2mw, φSLT, and φSa2958) | |||
| 1979-1985 (53) | + (39) | + (39) | + (35) | ND | φ108PVL (33) | φ108PVL type (33) |
| NT (2) | Icosahedral-head type (2) | |||||
| − (4) | ND | φ108PVL (4) | φ108PVL-like type (4) | |||
| − (14) | + (14) | ND | + (1) | φSa 2958 (1) | φSa2958 type (1) | |
| − (13) | φSa 2958 (13) | φSa2958-like phage of unknown type (13) | ||||
| 2000s (12) | − (12) | + (11) | ND | + (10) | φSa 2958 (2) | φSa2958 type (2) |
| NT (8) | Elongated-head type (8) | |||||
| − (1) | φSa 2958 (1) | φSa2958-like phage of unknown type (1) | ||||
| − (1) | ND | − (1) | φSa 2958 (1) | NT (1) | ||
ND, not determined; NT, nontypeable.
MW2 was positive by PCR-1 and PCR-2, because this S. aureus strain carries two additional different phages in its chromosomal DNA, and one of them, φSa3mw, has the same morphogenesis region as does φPVL (1, 19). Strain 81/108 was also positive with PCR-2 and seemed to carry other phages that did not carry lukS-PV and lukF-PV genes or have the structure to react with primers for PCR-2. Most of the PCR-1-positive strains we had tested up to this point in our study were also PCR-2 positive.
Therefore, to confirm whether the structures related to morphology belonged to PVL-carrying phages, we performed PCR-3 and PCR-4 to verify whether the PVL-carrying prophage belonged to either the icosahedral-head-type phages or the elongated-head-type phages by amplifying gene lineages between lukS and mtp that belong to either the icosahedral-head type or the elongated-head type.
DNA fragments of the expected sizes were amplified using PCR-3 and chromosomal DNAs of 81/108 (φ108PVL) and ATCC 49775 (φPVL) and using PCR-4 and chromosomal DNAs of MW2 (φSa2mw), JCSC2958 (φSa2958), and RN4220 (φSLT).
Up to that point, 38 of 39 PCR-1-positive strains were positive by PCR-2. However, PCR-3 was performed with chromosomal DNAs of 39 strains, and a DNA fragment of 10.5 kb was successfully amplified from 35 of 39 PCR-1-positive strains, indicating that these strains carried PVL phages of the icosahedral-head type. Sixty-six of 67 strains were positive by PCR-2, whereas DNA fragments of different sizes were amplified with chromosomal DNA of JCSC4274. When we performed PCR-4 with chromosomal DNAs with 26 strains for which no or only one DNA fragment was amplified by PCR-1, DNA fragments were amplified with chromosomal DNAs of 11 of these 26 strains.
Furthermore, we performed PCR-5 to -8 to classify these PVL phages into five different types. We conducted PCR-5 to determine whether these phages belonged to either the φPVL type or the φ108PVL type. A DNA fragment of 4,340 bp, indicating the carriage of a φ108PVL-specific gene (fragment PCR-1 A), was amplified with the DNAs of 33 of 35 strains, whereas a DNA fragment of 1,411 bp, indicating the carriage of φPVL (fragment PCR-2 A), was amplified in two cases, together with a DNA fragment of 4,340 bp. Because two PCR experiments to identify the other component of φPVL yielded negative results and four PCR experiments to identify the φ108PVL-specific regions yielded positive results (data not shown), we conclude that these 33 strains that were positive by PCR-1 amplified fragment PCR-1 A, which carried the phage belonging φ108PVL type. Four strains that were negative by PCR-4 were confirmed to belong to the φ108PVL type, with four PCR experiments to identify φ108PVL-specific regions and two long-range PCRs to amplify the DNA fragments covering the region from mtp to lukS. Therefore we regarded these four strains as being of the φ108PVL-like type. Two strains, with the gene lineage between lukS and mtp common to φ108PVL and φPVL, could not be classified into one of the five extant PVL phage types tested for up to that point.
In contrast, of the 26 strains for which no or only one DNA fragment was amplified by PCR-1, 25 were positive by PCR-2. In the case of strain JCSC7247, only one DNA fragment was amplified, suggesting the carriage of mtp in common with the elongated-head type.
PCR-4 was performed with DNAs of 26 strains to verify the gene lineage between the tail gene and the lukS gene by use of a primer pair designed for the tail gene that is commonly conserved in φSa2958, φSa2mw, and φSLT and for lukS. Of the 14 strains isolated from 1979 through 1985, only 1 was positive by PCR; in contrast, 10 of 20 strains isolated in the 2000s were positive by PCR. In addition, we performed PCR-6, -7, and -8 to classify elongated-head-type phages as of either the φSa2958 type, the φSa2mw type, or the φSLT type. Interestingly, neither the φSa2mw type nor the φSLT type, both of which are carried by S. aureus strains isolated in the United States or France, was identified, whereas three strains carrying φSa2958-type phage and eight strains carrying the elongated-head-type phage were identified. We determined the nucleotide sequence of a prophage lysogenized in MRSA strain JCSC2958, which was isolated in Japan in 1981. However, 13 of 14 strains isolated from 1979 through 1985 were definitively determined to be of the φSa2958 type, because we could not amplify the region between mtp and lukS with long-range PCR. Because these 13 strains as well as a strain isolated in the 2000s were positive by PCR-6, identifying a specific ORF in φSa2958, and by several types of long-range PCR, amplifying the gene lineage between mtp to some ORFs of φSa2958 located between the tail gene and lukS, e.g., JP052 or JP053, we consider them to be φSa2958-like phage of an unknown type. One strain carried a prophage that could not be classified into either one of the two types; this strain was exceptional, because it was negative by the seven PCRs other than PCR-6.
Characterization of PVL-positive MRSA clones.
We next characterized these strains by determining the coagulase isotypes and SCCmec types of all 65 strains and the MLST types of selected strains (Table 4). Fifty-two of the 53 MRSA strains isolated from 1979 through 1985 and 11 of the 12 MRSA strains isolated in the 2000s carried type 4 coagulase. Only JCSC2958, which we used for sequencing the φ2958PVL genome, carried type 2 coagulase, and only JCSC7247 carried type 7 coagulase. When we performed MLST of the chosen isolates, we found that all coagulase type 4 strains belonged to ST30, whereas a coagulase type 2 strain belonged to ST5 and a coagulase type 7 strain belonged to ST59. The data showed that the genotypes of MRSA strains carrying PVL phages isolated in Japan were extremely homogeneous and were represented by ST30 coagulase type 4 strains.
TABLE 4.
Characterization of PVL-positive MRSA strains isolated in 1979 to 1985, 2002, and 2004
| Yr of isolation (no. of strains) | Phage typea (no. of strains) | Coagulase isotype | SCCmec type (no. of strains) | Strain genotype by MLST
|
||
|---|---|---|---|---|---|---|
| No. of tested strains | ST | Allelic profile | ||||
| 1979-1985 (53) | φ108PVL (33) | 4 | I (1) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 |
| II (2) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 | |||
| IV.1 (8) | 3 | 30 | 2, 2, 2, 2, 6, 3, 2 | |||
| 2 | Nb | 2, 2, N, 2, 6, 3, 2 | ||||
| IV.3 (21) | 3 | 30 | 2, 2, 2, 2, 6, 3, 2 | |||
| IV.5 (1) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 | |||
| φ108PVL-like (4) | 4 | IV.1 (1) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 | |
| IV.3 (3) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 | |||
| Icosahedral-head type (2) | 4 | IV.3 (1) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 | |
| 4 | type II.n (1) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 | ||
| φSa2958 type (1) | 2 | II (1) | 1 | 5 | 1, 4, 1, 4, 12, 1, 10 | |
| Sa2958-like phage of unknown type (13) | 4 | I (10) | 2 | 30 | 2, 2, 2, 2, 6, 3, 2 | |
| II (1) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 | |||
| IV.3 (1) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 | |||
| IV.n (1) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 | |||
| 2000s (12) | φSa2958 (2) | 4 | IV.3 (2) | 2 | 30 | 2, 2, 2, 2, 6, 3, 2 |
| Elongated-head type (8) | IV.3 (1) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 | ||
| NTc (6) | 1 | 30 | 2, 2, 2, 2, 6, 3, 2 | |||
| φSa2958-like phage of unknown type (1) | 4 | I (1) | 1 | 765 | 2, 2, 2, 2, 6, 104, 2 | |
| Nontypeable (1) | 7 | V (1) | 1 | 59 | 19, 23, 15, 2, 19, 20, 15 | |
Phage type judged with M-PCRs.
N, the ST of this strain could not be assigned, since the glpF gene could not be amplified by PCR. The allele numbers other than glpF were the same as those for ST30.
NT, nontypeable.
However, the SCCmec elements carried by these strains were extremely diverse. Among the 33 MRSA strains identified as of the φ108PVL type, SCCmec type IV.3 strains (21 of 33) were the most frequent and were followed by SCCmec type IV.1 strains (8 of 33), SCCmec type II strains (2 of 33), and SCCmec type I strains (1 of 33). Three strains identified as being of the φSa2958 type carried type II SCCmec and type IV.1 SCCmec elements. Eight strains classified as the elongated-head type carried type IV.3 SCCmec and nontypeable SCCmec elements, which were similar to those of type IV.3 because they carried type 2 ccr and the J1 region of type IV.3 SCCmec and IS1272. Since we could not amplify the gene lineage between mecA and IS1272, we tentatively considered them as untypeable. Other strains also carried several SCCmec elements.
Interestingly, no strains with icosahedral-head-type phages were identified in isolates from the 2000s. The prophages of three strains were judged to be of the φSa2958 type. In contrast to JCSC2958, two other strains isolated in the 2000s belonged to the coagulase type 4 ST30 genotype and carried a type IV.3 SCCmec element. A φSa2958-like phage of unknown type was identified in the 2000s as belonging to the coagulase type 4 ST765 (CC30) group, carried type I SCCmec, and was similar to the isolates from 1979 through 1985. A strain carrying an untypeable PVL-carrying phage belonged to coagulase type 7 ST59 and carried the type V SCCmec element.
Induction of infective PVL phages.
To date, the φSLT is the only PVL phage that has been induced from cells and found capable of infecting other cells. Through a structural comparison of PVL phages, we found that both φSa2958 and φSa2mw carried possibly intact genes encoding head and tail proteins, and we inferred that these PVL phages might also be infective. To confirm this hypothesis, we induced prophages by treating these phages with mitomycin C and mixed them with cells of restriction-negative strains RN4220 and 1039. Because plaque formation does not indicate whether these plaques were generated by induced PVL phages, we conducted plaque hybridization experiments with a probe for the lukS-PV and lukF-PV genes to examine whether these plaques were of PVL-carrying phages.
The results are summarized in Table 5. We tested four MRSA strains (JCSC2973, JCSC4465, M11, and JCSC2958) isolated from 1979 through 1985, along with MRSA strains isolated in 2002 (JCSC4625) and 2004 (JCSC6605). Three strains carried φSa2958-type PVL phages, and three strains carried φSa2958-like PVL phages of unknown type. In addition, prophages φSa2mw and φ108PVL, whose genomes have been sequenced, were induced from strains MW2 and 81/108, as was lysogenized φSLT from strain RN4220 (φSLT). The cell suspensions of eight of nine tested strains became transparent after the addition of mitomycin C, indicating that phages might be generated upon the stress of DNA synthesis inhibition by mitomycin C. When these phage lysates were diluted and mixed with cells of indicator strains RN4220 and 1039, we found that most of the induced phages could propagate in 1039, whereas only some of them could propagate in RN4220.
TABLE 5.
Identification of PVL phages induced by mitomycin C treatment
| Strain | Coagulase isotype | MLST | SCCmec type | Phage typea | Indicator strain | No. of:
|
||
|---|---|---|---|---|---|---|---|---|
| Phages induced by mitomycin C (PFU/ml) | Tested plaques | PVL-positive plaques (%) | ||||||
| MW2 | 7 | 1 | IV.1 | φSa2mw (S) | RN4220 | 2.34 × 106 | 234 | 234 (100) |
| 1039 | 3.86 × 106 | 386 | 386 (100) | |||||
| JCSC2958 | 2 | 5 | II | φSa2958 (S) | RN4220 | 2.26 × 109 | 226 | 226 (100) |
| 1039 | 1.05 × 1010 | 316 | 316 (100) | |||||
| RN4220/φSLT | 3 | 8 | —b | φSLT (S) | RN4220 | 1.11 × 106 | 111 | 111 (100) |
| 1039 | 2.23 × 105 | 223 | 223 (100) | |||||
| 81/108 | 4 | 30 | IV.3 | φ108PVL (S) | RN4220 | 4.2 × 102 | 42 | 0 |
| 1039 | 1.82 × 105 | 182 | 0 | |||||
| JCSC2973 | 4 | 30 | I | φSa2958-like (P) | RN4220 | 2.32 × 105 | 232 | 0 |
| 1039 | 4.08 × 106 | 408 | 2 (0.49) | |||||
| M11 | 4 | 30 | IV.3 | φSa2958-like (P) | RN4220 | 2.05 × 108 | 205 | 205 (100) |
| 1039 | 2.33 × 108 | 233 | 233 (100) | |||||
| JCSC4465 | 4 | 30 | IV.n | φSa2958-like (P) | RN4220 | 1.34 × 108 | 134 | 0 |
| 1039 | 1.48 × 108 | 148 | 4 (2.7) | |||||
| JCSC4625 | 4 | 30 | IV.3 | φSa2958 type (P) | RN4220 | 2.61 × 105 | 261 | 261 (100) |
| 1039 | 3.97 × 106 | 397 | 397 (100) | |||||
| JCSC6055 | 4 | 30 | IV.3 | φSa2958 type (P) | RN4220 | No plaque | NTc | NT |
| 1039 | No plaque | NT | NT | |||||
Phage types assigned with PCR are indicated by (P), and those judged by sequences of phage genomes are indicated by (S).
—, none.
NT, nontypeable.
As expected, phage lysates from MW2 and JCSC2958 generated plaques on the 1039 and RN4220 lawns, which hybridized with the probe for the lukS-PV and lukF-PV genes, indicating that φSa2mw and φSa2958 can infect other S. aureus strains, as can the previously reported φSLT. The phage lysate from 81/108 generated plaques, but no PVL-positive plaque was observed, indicating that the φ108PVL phage did not induce or cannot infect the strains tested so far. This result was consistent with our speculation that φ108PVL might be defective, because the tail gene of φ108PVL was disrupted in a manner similar to that seen for φPVL (21).
Phage lysates of five of six MRSA strains carrying either the φSa2958-type PVL phage or the φSa2958-like PVL phage of unknown type generated PVL-positive plaques on indicator strains; however, the ratios of PVL-positive plaques ranged from 2% to 100%. In the case of JCSC6055, whose cell suspension did not become transparent after the addition of mitomycin C, no plaques were formed on the lawns of 1039 and RN4220 after the addition of the filtrate.
DISCUSSION
Characteristics of φSa2958.
Canchaya et al. have classified the Staphylococcal phages into five groups based on similarities in genes related to morphogenesis (4). All extant PVL phages are Sfi21-like cos-site phages of the Siphoviridae family, which can be further subdivided into two groups based on morphology: those with isomeric hexagonal heads and those with elongated heads. The PVL phages φSa2mw and φSa2usa, which are carried by MRSA strains isolated in the United States, have elongated heads. The φSLT phage was isolated from a French strain of S. aureus and has been shown by electron microscopy to have an elongated head measuring 100 by 50 nm and a flexible tail that is 400 nm long (32). Interestingly, the φSa2958 phage we described here belongs to the elongated-head group, whereas φ108PVL, identified in Japan, has an isometric hexagonal head, as does φPVL (20).
When we performed a detailed comparison of the genomic structure of φSa2958 with those of other extant PVL phages, represented by φSa2mw and φ108PVL (Fig. 3 and Table 2), we found that most of the region between φSa2958 and φSa2mw is conserved. Similarly, this region is also conserved among φSa2usa and φSLT. In contrast, the regions containing ORFs related to lysogeny (ORFs JP004 to -009) were unique to each phage. The average G+C content of the region from JP004 to JP009 was approximately 29.2%, which was lower than the average of the whole φSa2958 genome (33.1%). These data indicated that these genes might be acquired from organisms with lower G+C contents.
The theory of modular exchange in phages, which is known as the modular theory, has become the basis of a popular hypothesis for phage evolution (26, 33). According to this theory, φSa2958 and φSa2mw must share a common ancestor and must have evolved as close relatives, while the module exchange was likely a recent event. We note here that functionally important structures of the repressor and antirepressor genes are located in this module (25). We now know that in most known prophages, immunity is elicited by a repressor protein that prevents transcription initiation at promoters controlling the expression of lytic function (14, 24). Furthermore, the immunity of the phage is sometimes complicated by the presence of an antirepressor gene that can prevent the expression of the repressor activity. The nucleotide sequences of the repressor genes conserved in φSa2958 and φSa2mw exhibited differences from each other at the DNA level; however, when we investigated the protein structure encoded by these repressor genes, we found that the essential helix-turn-helix domain was well conserved, indicating that both repressor genes execute functional activities. This finding suggests that φSa2958 and φSa2mw might utilize different mechanisms for maintaining their immunity when they are integrated into bacterial genomes; to achieve this goal, these phages had to adopt the most beneficial module during the process when they interacted with host bacteria or with other phages.
Two lineages of PVL phage are predominant in MRSA and MSSA strains in Japan.
We designed several PCRs to identify PVL phages with the notion that the type of PVL-carrying phage should be determined on the bases of the gene lineage between lukS-PV and lukF-PV and other phage components. We first developed PCR-1 and -2 to identify portal or head genes, but we soon noticed that these PCRs might also be used to identify components of phages other than PVL-carrying phages. Therefore, we developed PCRs to identify the gene lineage between lukS and mtp, which is located rather far from the lukS-PV and lukF-PV genes. In addition, we developed PCRs to classify individual phages by identifying the genes in lysogeny-related regions or recombination-related regions in combination with the integrase gene, which is carried by all reported PVL-carrying phages. During this step, we noticed that some modules are commonly shared among phages. As shown in Fig. 3, φSa2958 was similar to φSa2mw, and φSa2958 was similar to φSLT (data not shown). The primer pairs we reported here are only those selected pairs that react only with one of five phages.
We have found that two PVL lineages, the icosahedral-head type and the elongated-head type, are present in Japan. Phages of the φ108PVL type were identified mostly in MRSA strains isolated from 1979 through 1985. Of the 53 MRSA strains examined, 33 isolates were identified as the carrier of PVL-carrying phage of the φ108PVL type, and 4 isolates were identified as carriers of the φ108PVL-like type; however, no phages of this type were identified in the isolates from the 2000s. Furthermore, no φPVL-type strain was identified among the MRSA strains tested. However, when 13 PVL-positive MSSA strains isolated in the 2000s were examined, 2 were found to belong to the icosahedral-head type and were likely to be of the φPVL type. Because the tail gene of φ108PVL was truncated, we assume that the phage had already lost its infectivity but carried an integrated truncated prophage, which might explain why φ108PVL-type strains have not been identified recently.
In contrast, phages with an elongated head, e.g., φSa2958 as well as φSa2mw and φSLT, carried intact tail and head genes. φSa2958 was identified from an ST5 SCCmec type II MRSA strain identified in 1981, whereas two φSa2958 type strains of ST30 SCCmec type IV.3 strains were identified in the 2000s, suggesting that the phage might infect other strains and become integrated into their chromosomes as a prophage. We considered the prophages carried by 14 strains (13 isolates from 1975 to 1985 and 1 isolate from the 2000s) as φSa2958-like phages of unknown type, because we could not clarify the gene lineage between mtp and lukS-PV. The strains carrying the prophage might be derived from the same clone because they belonged to ST30 and carry a type I SCCmec element and because the prophages of all three tested strains could be induced, proving their infectivity; these findings suggest that the recent isolate might be a descendant or that the phage might infect other strains. Interestingly, eight strains with elongated-head-type PVL-carrying prophages were identified. Because these strains were identified not only from seven isolates in Wakayama prefecture but also from an isolate from a 27-year-old man in Kanagawa prefecture, which is far from Wakayama, in 2002 (strain EB00449), we speculate that the prophage might have been induced from the cells of S. aureus and then infected other S. aureus cells to generate the novel PVL phage-carrying strain. A coagulase type 7 ST59 strain carried an as-yet-untypeable PVL phage. Vandenesch et al. reported that five major STs, namely, ST1, -8, -30, -59, and -80, carry PVL phages and that ST59 PVL-positive strains have been isolated in the United States (39). Because the ST59 strain is not common in Japan, the strains of other countries might have been introduced into Japan. Whether the strain carries a novel PVL phage is the next problem that should be clarified. It might carry a PVL phage that does not belong to the two extant groups.
When we characterized these PVL-positive strains by means of SCCmec typing and genotyping, we found that the most of them produced type 4 coagulase and belonged to ST30 or CC30 and carried SCCmec elements of various types, including type I, type II, type IV.1, and type IV.3 (17, 18, 29). We speculate that MSSA strains of coagulase type 4 ST30 independently acquired two phages, φ108PVL and φSa2958, or other elongated-head phages before they acquired SCCmec elements. The characterization of PVL phages carried by MSSA strains isolated in the 1960s has shown that MSSA strains carrying either of the PVL phages were present in the Japanese community. Therefore, we presume that the MSSA strains evolved into PVL-positive MRSA strains by acquiring SCCmec elements of various types. When we examined the types of SCCmec elements carried by PVL-positive MRSA strains, we found that 20 of 33 MRSA strains of the φ108PVL type carried type IV.3 SCCmec and that 8 of 33 carried type IV.1 SCCmec. It was noteworthy that most of the PVL-positive SCCmec type IV.1 strains had been isolated at the Tokyo Geriatric Hospital, whereas, and most of the PVL-positive SCCmec type IV.3 strains had been isolated at Gunma University Hospital. The data indicated that MRSA clones disseminating in different Japanese hospitals were not identical and that the spread of MRSA strains is regional.
φSa2958 and φSa2958-like phages of unknown type are intact prophages.
When we studied the structure of φSa2958, identified in this study, we realized that the amino acid sequence of the tail-length tape measure protein designated JP047 showed 98% similarity in with that of φSLT, which has been shown to be the first PVL phage that could infect S. aureus strains experimentally (32). Because the tail-length tape measure protein of φSa2mw carried by MW2, a CA-MRSA strain isolated in North Dakota, was similar to that of φSLT, we presumed that the newly identified φSa2958 as well as φSa2mw might also be able to infect to S. aureus strains.
To determine whether these two phages as well as phages of the φSa2958 type were intact and could infect S. aureus strains, we tested MW2 and six MRSA strains carrying φSa2958-type phage or φSa2958-like PVL phage of unknown type. The PVL phages carried by five of six MRSA strains could infect indicator cells, although the ratios of PVL phages in the phage lysates were not similar. In addition, the sizes of plaques generated on the lawn were larger with 1039 than with RN4220. With three strains, namely, M11, JCSC2958, and JCSC4625, all phages induced by treatment with mitomycin C were PVL positive. In contrast, with two strains, JCSC2973 and JCSC4625, the ratios of PVL-positive phages were very low. In the case of JCSC6055, which did not generate any plaques, the apparent reason that phages were not induced was that the cell suspension did not turn transparent after mitomycin C was added. These discrepancies may be due to many host cell factors, e.g., the presence of other prophages and differences in gene expression that confer the SOS response. Although we did not test a large number of strains, we chose φSa2958-type MRSA strains of a representative genotype identified in this study. Except for JCSC6055, the five strains (derived from four different clones as indicated), namely, JCSC2973 (ST30 SCCmec type I), M11 and JCSC4625 (ST30 SCCmec type IV.3), JCSC4465 (ST30 SCCmec type IV.n), and JCSC2958 (ST5 SCCmec type II), generated intact PVL phages. This result indicates that φSa2958-type and φSa2958-like phages of unknown type can be frequently induced from many S. aureus strains with diverse genetic backgrounds. These PVL phages might be induced with or without any stress from S. aureus and infect other appropriate recipient S. aureus strains, resulting in the phage conversion of nonvirulent bacteria to virulent bacteria. The facts that two recently isolated strains carried φSa2958 and that one of them generated infective PVL phages suggest that the φSa2958 type might be the cause of the recent emergence of PVL-positive MRSA strains in Japan. As shown in Table 5, φSa2mw carried by MW2 was infectious for two indicator strains, RN4220 and 1039. The USA300 strain has recently been the predominant CA-MRSA clone in the United States. φSa2usa carries PVL and belongs to the same family as φSa2mw and φSa2958, which have elongated heads. Although we could not identify any plaques of φSa2usa, we have tested FPR3757, whose genome has been sequenced; these φSa2mw-type or φSa2usa-type PVL phages might be the cause of the recent appearance of PVL-positive MRSA strains in the United States. Our data also indicate that ST30 MRSA strains carry two different PVL phages. Further study will clarify whether ST30 PVL-positive MRSA clones can be grouped with Western Samoan clones.
In conclusion, we have developed a method of combining several PCRs to identify PVL phage carried by an organism. Our results indicate that two lineages of PVL phages have existed in Japan for many years and that intact phages with elongated heads, such as φSa2958 and φSa2958-like phage of unknown type or other phages known simply as elongated-head types, might disseminate among MRSA strains in Japan and confer greater virulence.
Acknowledgments
We thank Yukio Utsui (Daiichi-Sankyo Pharmaceutical Co. Ltd.), Toyoji Okubo (Gunma University), and Takashi Inamatsu (Tokyo Geriatric Hospital) for providing MRSA strains isolated from 1979 through 1985. We thank Toyoko Oguri (Juntendo University Hospital) for providing MRSA strain JCSC4625 and Takashi Kuramoto (Moji Rosai Hospital) for providing MRSA strain JCSC6055. We thank the staff of SLR Laboratory for conducting PCR experiments to identify PVL-positive S. aureus strains. We thank Keizo Yamaguchi (Toho University) for providing MRSA strains collected by the surveillance study for the susceptibility testing for quinolone antibiotics.
This work was supported by a grant-in-aid for 21st-century COE research, a grant-in-aid for scientific research on priority areas, and a grant-in-aid for scientific research (C19590456) from the Ministry of Education, Science, Sports, Culture and Technology of Japan.
Footnotes
Published ahead of print on 6 August 2008.
REFERENCES
- 1.Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 3591819-1827. [DOI] [PubMed] [Google Scholar]
- 2.Baggett, H. C., T. W. Hennessy, K. Rudolph, D. Bruden, A. Reasonover, A. Parkinson, R. Sparks, R. M. Donlan, P. Martinez, K. Mongkolrattanothai, and J. C. Butler. 2004. Community-onset methicillin-resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a furunculosis outbreak in rural Alaska. J. Infect. Dis. 1891565-1573. [DOI] [PubMed] [Google Scholar]
- 3.Begier, E. M., K. Frenette, N. L. Barrett, P. Mshar, S. Petit, D. J. Boxrud, K. Watkins-Colwell, S. Wheeler, E. A. Cebelinski, A. Glennen, D. Nguyen, and J. L. Hadler. 2004. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin. Infect. Dis. 391446-1453. [DOI] [PubMed] [Google Scholar]
- 4.Canchaya, C., C. Proux, G. Fournous, A. Bruttin, and H. Brussow. 2003. Prophage genomics. Microbiol. Mol. Biol. Rev. 67238-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers, H. F. 2001. The changing epidemiology of Staphylococcus aureus. Emerg. Infect. Dis. 7178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colin, D. A., I. Mazurier, S. Sire, and V. Finck-Barbancon. 1994. Interaction of the two components of leukocidin from Staphylococcus aureus with human polymorphonuclear leukocyte membranes: sequential binding and subsequent activation. Infect. Immun. 623184-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coombs, G. W., J. C. Pearson, F. G. O'Brien, R. J. Murray, W. B. Grubb, and K. J. Christiansen. 2006. Methicillin-resistant Staphylococcus aureus clones, Western Australia. Emerg. Infect. Dis. 12241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denis, O., A. Deplano, H. De Beenhouwer, M. Hallin, G. Huysmans, M. G. Garrino, Y. Glupczynski, X. Malaviolle, A. Vergison, and M. J. Struelens. 2005. Polyclonal emergence and importation of community-acquired methicillin-resistant Staphylococcus aureus strains harbouring Panton-Valentine leucocidin genes in Belgium. J. Antimicrob. Chemother. 561103-1106. [DOI] [PubMed] [Google Scholar]
- 9.Diep, B. A., S. R. Gill, R. F. Chang, T. H. Phan, J. H. Chen, M. G. Davidson, F. Lin, J. Lin, H. A. Carleton, E. F. Mongodin, G. F. Sensabaugh, and F. Perdreau-Remington. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367731-739. [DOI] [PubMed] [Google Scholar]
- 10.Diep, B. A., G. F. Sensabaugh, N. S. Somboona, H. A. Carleton, and F. Perdreau-Remington. 2004. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J. Clin. Microbiol. 422080-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eady, E. A., and J. H. Cove. 2003. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus-an emerging problem for the management of skin and soft tissue infections. Curr. Opin. Infect. Dis. 16103-124. [DOI] [PubMed] [Google Scholar]
- 12.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 381008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finck-Barbancon, V., G. Duportail, O. Meunier, and D. A. Colin. 1993. Pore formation by a two-component leukocidin from Staphylococcus aureus within the membrane of human polymorphonuclear leukocytes. Biochim. Biophys. Acta 1182275-282. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich, J., M. Velleman, and H. Schuster. 1995. The tripartite immunity system of phages P1 and P7. FEMS Microbiol. Rev. 17121-126. [DOI] [PubMed] [Google Scholar]
- 15.Holmes, A., M. Ganner, S. McGuane, T. L. Pitt, B. D. Cookson, and A. M. Kearns. 2005. Staphylococcus aureus isolates carrying Panton-Valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J. Clin. Microbiol. 432384-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu, L. Y., T. H. Koh, T. Y. Tan, T. Ito, X. X. Ma, R. T. Lin, and B. H. Tan. 2006. Emergence of community-associated methicillin-resistant Staphylococcus aureus in Singapore: a further six cases. Singapore Med. J. 4720-26. [PubMed] [Google Scholar]
- 17.Ito, T., K. Okuma, X. X. Ma, H. Yuzawa, and K. Hiramatsu. 2003. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resist. Update 641-52. [DOI] [PubMed] [Google Scholar]
- 18.Ito, T., Y. Katayama, K. Asada, N. Mori, K. Tsutsumimoto, C. Tiensasitorn, and K. Hiramatsu. 2001. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 451323-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneko, J., and Y. Kamio. 2004. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 68981-1003. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko, J., T. Kimura, Y. Kawakami, T. Tomita, and Y. Kamio. 1997. Panton-Valentine leukocidin genes in a phage-like particle isolated from mitomycin C-treated Staphylococcus aureus V8 (ATCC 49775). Biosci. Biotechnol. Biochem. 611960-1962. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko, J., T. Kimura, S. Narita, T. Tomita, and Y. Kamio. 1998. Complete nucleotide sequence and molecular characterization of the temperate staphylococcal bacteriophage phiPVL carrying Panton-Valentine leukocidin genes. Gene 21557-67. [DOI] [PubMed] [Google Scholar]
- 22.Kondo, Y., T. Ito, X. X. Ma, S. Watanabe, B. N. Kreiswirth, J. Etienne, and K. Hiramatsu. 2007. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 51264-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwan, T., J. Liu, M. DuBow, P. Gros, and J. Pelletier. 2005. The complete genomes and proteomes of 27 Staphylococcus aureus bacteriophages. Proc. Natl. Acad. Sci. USA 1025174-5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindqvist, B. H., G. Deho, and R. Calendar. 1993. Mechanisms of genome propagation and helper exploitation by satellite phage P4. Microbiol. Rev. 57683-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ljungquist, E., K. Kockum, and L. E. Bertani. 1984. DNA sequences of the repressor gene and operator region of bacteriophage P2. Proc. Natl. Acad. Sci. USA 813988-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucchini, S., F. Desiere, and H. Brussow. 1999. Comparative genomics of Streptococcus thermophilus phage species supports a modular evolution theory. J. Virol. 738647-8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma, X. X., A. Galiana, W. Pedreira, M. Mowszowicz, I. Christophersen, S. Machiavello, L. Lope, S. Benaderet, F. Buela, W. Vincentino, M. Albini, O. Bertaux, I. Constenla, H. Bagnulo, L. Llosa, T. Ito, and K. Hiramatsu. 2005. Community-acquired methicillin-resistant Staphylococcus aureus, Uruguay. Emerg. Infect. Dis. 11973-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma, X. X., T. Ito, P. Chongtrakool, and K. Hiramatsu. 2006. Predominance of clones carrying Panton-Valentine leukocidin genes among methicillin-resistant Staphylococcus aureus strains isolated in Japanese hospitals from 1979 to 1985. J. Clin. Microbiol. 444515-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma, X. X., T. Ito, C. Tiensasitorn, M. Jamklang, P. Chongtrakool, S. Boyle-Vavra, R. S. Daum, and K. Hiramatsu. 2002. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 461147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulvey, M. R., L. MacDougall, B. Cholin, G. Horsman, M. Fidyk, and S. Woods. 2005. Community-associated methicillin-resistant Staphylococcus aureus, Canada. Emerg. Infect. Dis. 11844-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud, J. Etienne, S. K. Johnson, F. Vandenesch, S. Fridkin, C. O'Boyle, R. N. Danila, and R. Lynfield. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2902976-2984. [DOI] [PubMed] [Google Scholar]
- 32.Narita, S., J. Kaneko, J. Chiba, Y. Piemont, S. Jarraud, J. Etienne, and Y. Kamio. 2001. Phage conversion of Panton-Valentine leukocidin in Staphylococcus aureus: molecular analysis of a PVL-converting phage, phiSLT. Gene 268195-206. [DOI] [PubMed] [Google Scholar]
- 33.Neve, H., K. I. Zenz, F. Desiere, A. Koch, K. J. Heller, and H. Brussow. 1998. Comparison of the lysogeny modules from the temperate Streptococcus thermophilus bacteriophages TP-J34 and Sfi21: implications for the modular theory of phage evolution. Virology 24161-72. [DOI] [PubMed] [Google Scholar]
- 34.Okuma, K., K. Iwakawa, J. D. Turnidge, W. B. Grubb, J. M. Bell, F. G. O'Brien, G. W. Coombs, J. W. Pearman, F. C. Tenover, M. Kapi, C. Tiensasitorn, T. Ito, and K. Hiramatsu. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 404289-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panton, P. N., M. C. Camb, F. C. O. Valentine, and M. R. C. P. Lond. 1932. Staphylococcal toxin. Lancet i506-508. [Google Scholar]
- 36.Prevost, G., B. Cribier, P. Couppie, P. Petiau, G. Supersac, V. Finck- Barbancon, H. Monteil, and Y. Piemont. 1995. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 634121-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenover, F. C., L. K. McDougal, R. V. Goering, G. Killgore, S. J. Projan, J. B. Patel, and P. M. Dunman. 2006. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J. Clin. Microbiol. 44108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ushioda, H., T. Terayama, S. Sakai, H. Zen-Yoji, M. Nishiwaki, and A. Hidano. 1981. Coagulase typing of Staphylococcus aureus and its application in routine work, p. 77-83. In J. Jaljaszewicz (ed.), Staphylococci and staphylococcus infections. Zentbl. Bakteriol., suppl. 10. Gustav Fischer Verlag, Stuttgart, Germany.
- 39.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi, K., and A. Ohno. 2005. Investigation of the susceptibility trends in Japan to fluoroquinolones and other antimicrobial agents in a nationwide collection of clinical isolates: a longitudinal analysis from 1994 to 2002. Diagn. Microbiol. Infect. Dis. 52135-143. [DOI] [PubMed] [Google Scholar]
- 41.Yoshizawa, Y. 1985. Isolation and characterization of restriction negative mutants of Staphylococcus aureus. Jikeikai Med. J. 32415-421. [Google Scholar]