Abstract
Vibrio cholerae is a facultative human pathogen. The ability of V. cholerae to form biofilms is crucial for its survival in aquatic habitats between epidemics and is advantageous for host-to-host transmission during epidemics. Formation of mature biofilms requires the production of extracellular matrix components, including Vibrio polysaccharide (VPS) and matrix proteins. Biofilm formation is positively controlled by the transcriptional regulators VpsR and VpsT and is negatively regulated by the quorum-sensing transcriptional regulator HapR, as well as the cyclic AMP (cAMP)-cAMP receptor protein (CRP) regulatory complex. Transcriptome analysis of cyaA (encoding adenylate cyclase) and crp (encoding cAMP receptor protein) deletion mutants revealed that cAMP-CRP negatively regulates transcription of both VPS biosynthesis genes and genes encoding biofilm matrix proteins. Further mutational and expression analysis revealed that cAMP-CRP negatively regulates transcription of vps genes indirectly through its action on vpsR transcription. However, negative regulation of the genes encoding biofilm matrix proteins by cAMP-CRP can also occur independent of VpsR. Transcriptome analysis also revealed that cAMP-CRP regulates the expression of a set of genes encoding diguanylate cyclases (DGCs) and phosphodiesterases. Mutational and phenotypic analysis of the differentially regulated DGCs revealed that a DGC, CdgA, is responsible for the increase in biofilm formation in the Δcrp mutant, showing the connection between of cyclic di-GMP and cAMP signaling in V. cholerae.
Vibrio cholerae, the causative agent of cholera (26), is a natural inhabitant of aquatic environments (14). Seasonal cholera outbreaks occur where the disease is endemic and can spread worldwide (14, 34). The ability of V. cholerae to cause epidemics is linked to its ability to survive in natural aquatic ecosystems. One important factor for environmental survival and transmission of V. cholerae is its ability to form biofilms (14, 60, 63). Biofilms are surface-attached microbial communities composed of microorganisms and the extrapolymeric substances that they produce (9). The biofilm mode of growth is the preferred lifestyle in the microbial world, as it enhances survival in natural settings. In addition, biofilms protect the constituent microbes from predators, such as protozoa and viruses, and from toxic compounds, such as antimicrobial agents (4, 10, 13, 36, 41, 63).
The process of biofilm development in V. cholerae can be divided into distinct stages: transport and attachment of bacteria to the surface, colonization of the attached surface, formation of a monolayer of cells, and synthesis of the extracellular matrix, leading to formation of a mature biofilm with a characteristic three-dimensional (3D) architecture. The Vibrio polysaccharide (VPS), encoded by the vps genes, is essential for the development of 3D biofilm structures (63). The vps genes are clustered in two regions on the large chromosome of V. cholerae O1 El Tor; the vps-I cluster consists of vpsU (VC0916) and vpsA to vpsK (VC0917 to VC0927), and the vps-II cluster consists of vpsL to vpsQ (VC0934 to VC0939). Recently, we identified protein components of the biofilm matrix of V. cholerae and showed that the RbmA, RbmC, and Bap1 proteins are also required for the formation of a wild-type biofilm. Mutants that are not able to produce these matrix proteins form biofilms that are structurally unstable (15, 16).
The regulation of biofilm formation in V. cholerae is complex and involves several transcriptional regulators. Two proteins that positively regulate VPS production and biofilm formation have been identified, VpsR and VpsT. Disruption of vpsR prevents expression of the vps genes and production of VPS, and it eliminates formation of typical 3D biofilm structures (61). A vpsT mutant exhibits reduced vps gene expression and biofilm-forming capacity (7). A population-density-dependent regulatory system, known as the quorum-sensing system, negatively regulates biofilm formation in V. cholerae. HapR is the master regulator of the quorum-sensing regulatory system, and a hapR mutant has increased biofilm-forming capacity (19, 62, 64). Consistent with this observation, expression of the vps genes, including vpsR and vpsT, is increased in the hapR mutant (62). In addition, a second messenger, cyclic di-GMP (c-di-GMP), which is produced by diguanylate cyclases (DGCs) containing a GGDEF amino acid motif and is degraded by phosphodiesterases (PDEs) that have EAL or HD-GYP domains (8, 46, 49), positively regulates biofilm formation in V. cholerae (3, 31, 56). We recently determined that cdgA (encoding a DGC), whose transcription is positively regulated by VpsR and negatively regulated by HapR, positively regulates biofilm formation in V. cholerae (2).
The cyclic AMP (cAMP)-cAMP receptor protein (CRP) regulatory complex was recently identified as a negative regulator of biofilm formation in V. cholerae (29, 30). These studies showed that cAMP-CRP negatively regulates vpsL and vpsT expression and positively regulates vpsR expression. Additional study showed that growth in the presence of glucose, which leads to a decrease in cellular cAMP levels, induces biofilm formation, while addition of cAMP to a growth medium leads to a decrease in biofilm formation in wild-type V. cholerae (23). To further evaluate the mechanism by which cAMP-CRP negatively regulates biofilm formation in V. cholerae, we determined whole-genome expression profiles of cyaA (encoding adenylate cyclase) and crp (encoding CRP) deletion mutants. Our analysis revealed that cAMP-CRP negatively regulates transcription of VPS biosynthesis genes and genes encoding biofilm matrix proteins. cAMP-CRP negatively regulates transcription of both vps genes and the genes encoding biofilm matrix proteins indirectly, through its action on vpsR transcription. In addition, cAMP-CRP can also negatively regulate transcription of the genes encoding biofilm matrix proteins independent of VpsR. We also determined that cAMP-CRP regulates the expression of a set of genes encoding DGCs and PDEs. Through mutational and phenotypic analysis, we showed that CdgA is largely responsible for the increased transcription of vps and biofilm matrix protein genes, as well as enhanced biofilm formation in a Δcrp mutant, revealing the connection between c-di-GMP and cAMP-CRP signaling in V. cholerae.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. All V. cholerae and Escherichia coli strains were routinely grown aerobically in Luria-Bertani (LB) medium (1% tryptone, 0.5% yeast extract, 1% NaCl; pH 7.5) at 30 and 37°C, respectively, unless otherwise noted. The E. coli DH10B and CC118λpir strains were used for DNA manipulation, while the E. coli S17-1λpir strain was used for conjugation with V. cholerae A1552. Conjugation with other V. cholerae strains (C6706, N16961, and MO10) was carried out using the E. coli SM10λpir strain. Agar medium contained 1.5% granulated agar (Difco), unless otherwise noted. Ampicillin, rifampin, and streptomycin were used at a concentration of 100 μg/ml, while gentamicin was used at a concentration of 50 μg/ml. We observed that ΔcyaA and Δcrp mutants had increased doubling times in LB medium at 30°C compared to the wild type (data not shown). This finding is similar to the growth defect reported for a crp mutant grown in LB medium at 37°C (52). To minimize the effect of reduced growth rates in our experiments, expression profiling and β-galactosidase assays were carried out with cultures grown to stationary phase.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and phenotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH10B | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 endA1 araΔ139 Δ(ara leu) 7697 galU galK λ-rpsL (Smr) nupG | Invitrogen |
| CC118λpir | Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE(Am) recA1 λpir | 21 |
| S17-1λpir | Tpr SmrrecA thi pro rK− mK+ RP4::2-Tc::MuKm Tn7 λpir | 11 |
| SM10λpir | thi thr leu tonA lacY supE recA (RP4-2-Tc::Mu) λpirR6K Kmr π+ | 54 |
| V. cholerae strains | ||
| FY_Vc_1 | V. cholerae O1 El Tor A1552, wild-type variant, Rifr | 63 |
| C6706 | V. cholerae O1 El Tor C6706, wild-type variant, Smr | 35 |
| N16961 | V. cholerae O1 El Tor N16961, wild-type variant, Smr | 20 |
| MO10 | V. cholerae O139 MO10, wild-type variant, Smr | P. Watnick |
| FY_Vc_2322 | ΔcyaA, Rifr | This study |
| FY_Vc_2326 | Δcrp, Rifr | This study |
| FY_Vc_237 | FY_Vc_1 mTn7-gfp, Rifr Gmr | 3 |
| FY_Vc_2448 | ΔcyaA mTn7-gfp, Rifr Gmr | This study |
| FY_Vc_2451 | Δcrp mTn7-gfp, Rifr Gmr | This study |
| FY_Vc_3 | FY_Vc_1 ΔlacZ, Rifr | 7 |
| FY_Vc_3756 | C6706 ΔlacZ | This study |
| FY_Vc_3748 | N16961 ΔlacZ | This study |
| FY_Vc_3763 | MO10 ΔlacZ | This study |
| FY_Vc_2456 | FY_Vc_1 ΔcyaA ΔlacZ, Rifr | This study |
| FY_Vc_2459 | FY_Vc_1 Δcrp ΔlacZ, Rifr | This study |
| FY_Vc_3759 | C6706 Δcrp ΔlacZ | This study |
| FY_Vc_3751 | N16961 Δcrp ΔlacZ | This study |
| FY_Vc_3766 | MO10 Δcrp ΔlacZ | This study |
| FY_Vc_2919 | FY_Vc_1 ΔhapR ΔlacZ, Rifr | This study |
| FY_Vc_2922 | FY_Vc_1 ΔvpsT ΔlacZ, Rifr | This study |
| FY_Vc_2874 | FY_Vc_1 ΔvpsR ΔlacZ, Rifr | This study |
| FY_Vc_344 | FY_Vc_1 ΔcdgA, Rifr | 31 |
| FY_Vc_360 | FY_Vc_1 ΔcdgA mTn7-gfp, Rifr Gmr | 31 |
| FY_Vc_3296 | FY_Vc_1 ΔcdgA ΔlacZ, Rifr | This study |
| FY_Vc_350 | FY_Vc_1 ΔcdgB, Rifr | 31 |
| FY_Vc_987 | FY_Vc_1 ΔcdgF, Rifr | 3 |
| FY_Vc_1592 | FY_Vc_1 ΔcdgH, Rifr | Beyhan et. al., submitted |
| FY_Vc_956 | FY_Vc_1 ΔcdgI, Rifr | This study |
| FY_Vc_354 | FY_Vc_1 ΔrocS, Rifr | 31 |
| FY_Vc_152 | FY_Vc_1 ΔVC0072, Rifr | This study |
| FY_Vc_869 | FY_Vc_1 ΔVC1376, Rifr | This study |
| FY_Vc_158 | FY_Vc_1 ΔVC2750, Rifr | This study |
| FY_Vc_154 | FY_Vc_1 ΔVCA0217, Rifr | This study |
| FY_Vc_2779 | FY_Vc_1 Δcrp ΔhapR ΔlacZ, Rifr | This study |
| FY_Vc_2781 | FY_Vc_1 Δcrp ΔvpsT ΔlacZ, Rifr | This study |
| FY_Vc_2916 | FY_Vc_1 Δcrp ΔvpsR ΔlacZ, Rifr | This study |
| FY_Vc_3299 | FY_Vc_1 Δcrp ΔcdgA ΔlacZ, Rifr | This study |
| FY_Vc_3712 | FY_Vc_1 Δcrp ΔcdgA ΔlacZ mTn7-gfp, Rifr Gmr | This study |
| FY_Vc_3311 | FY_Vc_1 Δcrp ΔcdgB ΔlacZ | This study |
| FY_Vc_3323 | FY_Vc_1 Δcrp ΔcdgF ΔlacZ | This study |
| FY_Vc_3317 | FY_Vc_1 Δcrp ΔcdgH ΔlacZ | This study |
| FY_Vc_3308 | FY_Vc_1 Δcrp ΔcdgI ΔlacZ | This study |
| FY_Vc_3314 | FY_Vc_1 Δcrp ΔrocS ΔlacZ | This study |
| FY_Vc_3305 | FY_Vc_1 Δcrp ΔVC0072 ΔlacZ | This study |
| FY_Vc_3318 | FY_Vc_1 Δcrp ΔVC1376 ΔlacZ | This study |
| FY_Vc_3320 | FY_Vc_1 Δcrp ΔVC2750 ΔlacZ | This study |
| FY_Vc_3322 | FY_Vc_1 Δcrp ΔVCA0217 ΔlacZ | This study |
| FY_Vc_3302 | FY_Vc_1 ΔhapR ΔcdgA ΔlacZ, Rifr | This study |
| FY_Vc_337 | FY_Vc_1 ΔflaA, Rifr | Beyhan et. al., submitted |
| FY_Vc_231 | FY_Vc_1 Δvps-I, Rifr | 3 |
| FY_Vc_3787 | FY_Vc_1 Δcrp Δvps-I, Rifr | This study |
| FY_Vc_3411 | FY-Vc_1 Δvps-I Δvps-II, Rifr | This study |
| FY_Vc_3788 | FY-Vc_1 Δcrp Δvps-I Δvps-II, Rifr | This study |
| FY_Vc_102 | FY_Vc_1 ΔrbmA, Rifr | 15 |
| FY_Vc_3789 | FY_Vc_1 Δcrp ΔrbmA, Rifr | This study |
| FY_Vc_3790 | FY_Vc_1 Δbap1, Rifr | This study |
| FY_Vc_3791 | FY_Vc_1 Δcrp Δbap1, Rifr | This study |
| Plasmids | ||
| pGP704-sacB28 | pGP704 derivative, mob/oriT sacB, Apr | G. Schoolnik |
| pFY-659 | pGP704-sacB28::Δvps-II operon, Apr | This study |
| pFY-308 | pGP704-sacB28::ΔcyaA, Apr | This study |
| pFY-333 | pGP704-sacB28::Δcrp, Apr | This study |
| pFY-149 | pGP704-sacB28::ΔcdgA, Apr | 31 |
| pFY-447 | pGP704-sacB28::ΔcdgI, Apr | This study |
| pCC27 | pGP704-sacB28::ΔvpsR, Apr | 7 |
| pCC2 | pGP704-sacB28::ΔlacZ, Apr | 7 |
| pFY-252 | pGP704-sacB28::ΔVC0072, Apr | This study |
| pFY-384 | pGP704-sacB28::ΔVC1376, Apr | This study |
| pFY-237 | pGP704-sacB28::ΔVC2750, Apr | This study |
| pFY-250 | pGP704-sacB28::ΔVCA0217, Apr | This study |
| pRS415 | Promoterless lacZ cloning vector for transcriptional fusion studies, Apr | 51 |
| pCC12 | pRS415 vpsL promoter, Apr | 7 |
| pCC25 | pRS415 vpsT promoter, Apr | 7 |
| pCC10 | pRS415 vpsR promoter, Apr | 7 |
| pFY-169 | pRS415 rbmA promoter, Apr | 15 |
| pFY-578 | pRS415 rbmC promoter, Apr | This study |
| pFY-581 | pRS415 bap1 promoter, Apr | This study |
| pFY-150 | pACYC177::cdgA operon (includes VCA0074 and VCA0075), Apr | 31 |
| pMCM11 | pGP704::mTn7-gfp, Gmr Apr | M. Miller and G. Schoolnik |
| pUX-BF13 | oriR6K helper plasmid, mob/oriT, provides the Tn7 transposition function in trans, Apr | 1 |
Recombinant DNA techniques.
DNA manipulations were carried out by using standard molecular techniques (47). Restriction and DNA modification enzymes were purchased from New England Biolabs. PCRs were carried out using primers purchased from Operon Technologies (Table 2) and a high-fidelity PCR kit (Roche). DNA sequencing was carried out by the UC Berkeley DNA Sequencing Facility.
TABLE 2.
Sequences of oligonucleotides used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| vps-II_del_A | CATGCCATGGCATGCGGCTGGTCTATGTGGCTTG |
| vps-II_del_B | CGAGCATAGTCCCTAGCAAGGCAACCGAAA |
| vps-II_del_C | GTCTTGCTAGGGACTATGCTCGCGGGTTTACTGC |
| vps-II_del_D | CGAGCTCGCTCGATCTTTGCCGATCACC |
| cyaA_del_A | GATCCCATGGGTTTTCCCGCTTGATTGTGT |
| cyaA_del_B | CGCGGTTGGCCTGCTGGATAGGATTGGCTTC |
| cyaA_del_C | TATCCAGCAGGCCAACCGCGTTGAAGTCTAT |
| cyaA_del_D | GATCTCTAGACAAATCGATTGATGGCGAAT |
| crp_del_A | GATCTCTAGATGAGTTTTGCGATGGATTTG |
| crp_del_B | TGATCTTGATCGCAACTGAACCTTTTACG |
| crp_del_C | TTCAGTTGCGATCAAGATCACTCGCCAAGAG |
| crp_del_D | GATCGAGCTCCCAACATGGCTTTAGCATCA |
| cdgI_del_A | CTAGCCATGGCCTCTTCGTGTCCCGGAGTATC |
| cdgI_del_B | CAACGGGTAAGGCCAAAGAATGAATCTTGC |
| cdgI_del_C | TTCTTTGGCCTTACCCGTTGCGACAAACAGT |
| cdgI_del_D | CTAGTCTAGACTCATCAAGGAATCGCATCA |
| VC0072_del_A | GATCTCTAGACTAGGCTAATCGAACGCTCATTCC |
| VC0072_del_B | GCTGCAAAGCTAACGGTTGGCTCGATAAGG |
| VC0072_del_C | CCAACCGTTAGCTTTGCAGCAGATTGGTGTG |
| VC0072_del_D | GGAGCTCGAGACTGATGCGCTCACTGAC |
| VC1376_del_A | CGAGCTCCAGCCCAGCATGGAGCATATC |
| VC1376_del_B | ACCTTTCGATACCAGCAAGGGCACAATCAC |
| VC1376_del_C | CCTTGCTGGTATCGAAAGGTCGAAATCGTGT |
| VC1376_del_D | CATGCCATGGCATGCATTCACCAGCCAACAGACG |
| VC2750_del_A | CATGCCATGGCAGCCAAAGAGCTCGGAG |
| VC2750_del_B | GCAACCGACAAACGGCAGTATGATGGC |
| VC2750_del_C | TTTGTCGGTTGCCCACGCGGGCAAGGC |
| VC2750_del_D | CGATTCTAGAGTACCAAAGGTGCGGCTC |
| VCA0217_del_A | GATCTCTAGACTAGTGCGCCATGTAACCAATAGA |
| VCA0217_del_B | GCTTTTACCGATGCGCTATTGGGTTCAACT |
| VCA0217_del_C | AATAGCGCATCGGTAAAAGCAGGAGAGTGA |
| VCA0217_del_D | GGAGCTCGGTCTTATTGATGCGGGAGCA |
| rbmC_pro F | GATCGAATTCCTAGAAAATGCTTCTTGA |
| rbmC_pro R | GATCGGATCCTTGTAAGACTCCCTTTACCT |
| bap1_pro F | GAGAGAATTCCGCCGCGTTGCTGAG |
| bap1_pro R | GAGAGGATCCGGCTTGACCTTCATCT |
| RecA578 | GTGCTGTGGATGTCATCGTTGTTG |
| RecA863 | CCACCACTTCTTCGCCTTCTTTGA |
| cdgA_rt F | CAAGCGATCTGGTTCTTATTCC |
| cdgA_rt R | AAAACGGCTCCAAGTCAGC |
| cdgI_rt F | GATGTGGAAAGGCAAAGAGC |
| cdgI_rt R | CTGTGCTATGCGAGTTTTGC |
| rocS_rt F | CAGGTTGCACCTCTTTACTCG |
| rocS_rt R | ACCCGTGTCGGTTATACAGC |
Generation of in-frame deletion mutants.
Deletion mutants of V. cholerae strains were generated by using a previously described protocol (16). The DNA sequences of the constructed deletion plasmids were verified by DNA sequencing. Primers used in the construction of the deletion plasmids are shown in Table 2.
Pellicle formation and motility assays.
Pellicle formation experiments were carried out using glass culture tubes (18 by 150 mm) containing 5 ml of medium. The medium was inoculated with overnight cultures, resulting in 200-fold dilution. The tubes were incubated at 30°C without shaking for 2 days. LB soft agar plates (0.3% agar) were used to determine the motility of the bacterial strains. The diameter of each migration zone (in cm) was measured after 18 h of incubation at 30°C. Assays were repeated with at least two biological replicates.
Generation of lacZ transcriptional fusion constructs.
lacZ transcriptional fusions with promoters of rbmC and bap1 were constructed by cloning the PCR-amplified ∼300-bp promoter regions immediately upstream of the start codons of rbmC and bap1 into pRS415 (51) as described previously (15). The resulting transcriptional fusion plasmids were sequenced. The plasmids were electroporated into V. cholerae strains containing a lacZ in-frame deletion. The primers used for amplification of the promoter regions are shown in Table 2.
β-Galactosidase assays.
β-Galactosidase assays were carried out by using a protocol similar to that described by Miller (38). Briefly, overnight cultures were diluted 200-fold in LB medium supplemented with ampicillin and incubated at 30°C for 10 h with shaking (200 rpm). The optical densities at 600 nm (OD600) of the stationary-phase cultures were determined, and 1-ml portions of the cultures were harvested and washed with 1 ml of buffer Z (16.1 g/liter Na2HPO4·7H2O, 5.5 g/liter NaH2PO4·H2O, 0.75 g/liter KCl, 0.246 g/liter MgSO4·7H2O; pH 7.0). Cells were lysed by resuspending a cell pellet in 1 ml of buffer Z containing 0.69% β-mercaptoethanol, 0.02% cetyltrimethylammonium bromide, and 0.01% deoxycholic acid (sodium salt), followed by incubation at room temperature for 5 min. Cell lysates (100 μl) of different dilutions were pipetted into flat-bottom 96-well microtiter plates, and 20-μl portions of an o-nitrophenyl-β-d-galactopyranoside solution (4 mg/ml) were added, followed by incubation at 30°C until sufficient color development was observed. The reactions were stopped by adding 50 μl of 1 M Na2CO3, and the color intensities were measured at OD420 and OD550. The duration of color development was noted, and the β-galactosidase activity (expressed in Miller units) was calculated as previously described (15, 38). The assays were repeated with at least two different biological replicates and eight technical replicates.
Generation of gfp-tagged strains and confocal laser scanning microscopy (CLSM).
V. cholerae strains were chromosomally tagged with the gene encoding green fluorescent protein (gfp), using a previously described procedure (2, 15). Non-flow-cell experiments were carried out using Lab-Tek II chambered coverglass systems (Nalge Nunc) and a previously described protocol (2). Briefly, overnight cultures were diluted to obtain an OD600 of 0.2, and 3-ml portions of the diluted cultures were placed into chambers and incubated at 30°C for 8 h. The chambers were then washed twice with 1 ml of LB medium. Biofilms formed by non-gfp-tagged strains were stained for 15 min at room temperature in the dark with 1 ml of 5 μM SYTO9 (Molecular Probes). Images of the biofilms formed in the chambers were acquired using a Zeiss Axiovert 200 M laser scanning microscope. 3D images of the biofilms were reconstructed using IMARIS software (Bitplane) and were quantified using the COMSTAT program (22). Non-flow-cell experiments were carried out with at least two different biological replicates.
Biofilm formation assays.
Biofilms were formed in 96-well polyvinyl chloride microtiter plates by using 100-μl portions of overnight cultures diluted to obtain an OD600 of 0.2. The microtiter plates were incubated at 30°C for 8 h. Crystal violet staining and ethanol solubilization were carried out as previously described (15, 63). The assays were repeated with two different biological replicates and eight technical replicates.
RNA isolation.
Total RNA was isolated from V. cholerae strains in stationary growth phase by using a previously described protocol (62). Briefly, overnight cultures of V. cholerae grown in LB medium at 30°C with shaking (200 rpm) were diluted 200-fold in LB medium and incubated at 30°C for 10 h. Aliquots (2 ml) of the cultures were collected and centrifuged for 2 min at room temperature. The cell pellets were immediately resuspended in 1 ml of TRIzol (Invitrogen) and stored at −80°C. Total RNA was isolated according to the manufacturer's instructions. To remove contaminating DNA, total RNA was incubated with RNase-free DNase I (Ambion), and an RNeasy mini kit (Qiagen) was used to clean up RNA after DNase digestion.
Whole-genome expression profiling.
Whole-genome expression profiling was performed by using a previously described procedure (3). A common reference RNA was used, which contained equal amounts of total RNA isolated from V. cholerae cells grown to stationary phase in LB medium. Normalized signal ratios were obtained with LOWESS print tip normalization using the Bioconductor packages (http://www.bioconductor.org) in the R environment (18). Differentially regulated genes were determined (with three biological and two technical replicates for each data point) using the Significance Analysis of Microarrays (SAM) software (58) with a ≥2-fold difference in gene expression and a false discovery rate (FDR) of ≤1% as cutoff values, unless otherwise noted.
qPCR.
Quantitative PCR (qPCR) was carried out by first synthesizing cDNA from 1 μg of a total RNA sample using an iScript cDNA synthesis kit (Bio-Rad). The cDNA product was then diluted 1:4 with water, and 4 μl was used as a template with 12 pmol of each qPCR primer (Table 2) in a PCR performed with the Expand high-fidelity PCR system (Roche). The PCR conditions were as follows: 94°C for 2 min and then 25 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s and a final incubation at 72°C for 2 min. The amplified products were analyzed on a 2% agarose gel and were quantified using the ImageQuant 5.2 software (Molecular Dynamics). The intensity of each DNA band was normalized to that of the corresponding recA band amplified with primers RecA578 and RecA863 (29). The data presented below are from three biological replicates, and reaction mixtures containing no template or reverse transcriptase were used as negative controls.
RESULTS
Identification of genes differentially regulated in ΔcyaA and Δcrp mutants.
cAMP-CRP negatively regulates biofilm formation in V. cholerae. To further understand how cAMP-CRP regulates biofilm formation, we generated in-frame cyaA (VC0122) and crp (VC2614) deletion mutants of our prototype V. cholerae O1 El Tor A1552 strain and performed whole-genome expression profiling of these mutants. The gene expression data were analyzed by using the SAM software and the following criteria to define significantly regulated genes, unless otherwise indicated: an FDR of ≤1% and a ≥2-fold transcript abundance difference between samples. This analysis revealed that cAMP-CRP differentially regulates transcription of a large set of genes and that the overall gene expression profiles of the ΔcyaA and Δcrp mutants are similar (see Fig. S1 in the supplemental material). Altogether, 889 genes (22.9% of the genome) and 822 genes (21.2% of the genome) are differentially regulated in the ΔcyaA and Δcrp mutants compared to the wild type, respectively. Of the 889 differentially regulated genes in the ΔcyaA mutant, 431 are upregulated and 458 are downregulated, whereas of the 822 differentially regulated genes in the Δcrp mutant, 386 are upregulated and 436 are downregulated. All the differentially regulated genes are shown in Tables S1 and S2 in the supplemental material.
In this study, our main objective was to determine how cAMP-CRP negatively regulates biofilm formation. Thus, for the genes that are differentially regulated by cAMP-CRP, we focused on two sets of genes: the genes required for biofilm matrix production and its regulation and the genes predicted to be involved in the production and degradation of c-di-GMP, as this second messenger regulates biofilm formation in V. cholerae.
cAMP-CRP negatively regulates transcription of the vps, rbmA, rbmC, and bap1 genes.
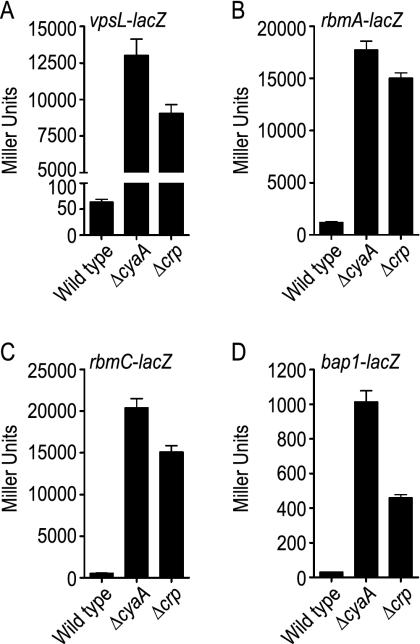
As expected for a negative regulator of biofilm formation, the levels of expression of vps genes and vpsT were higher in both the ΔcyaA and Δcrp mutants than in the wild type (Table 3). Gene expression profiling also revealed that the expression of hapR was decreased in both the ΔcyaA and Δcrp mutants. This finding is consistent with previously described results (29, 50). In addition to these genes, we observed that the levels of expression of the genes encoding the biofilm matrix proteins, rbmA, rbmC, and bap1, were higher in both the ΔcyaA and Δcrp mutants than in the wild type. To verify this finding, we monitored transcription of the genes encoding biofilm matrix proteins by using rbmA-lacZ, rbmC-lacZ, and bap1-lacZ fusion constructs and measuring β-galactosidase activities. In parallel, we also monitored transcription of the genes involved in VPS biosynthesis using a vpsL-lacZ fusion construct. As expected, vpsL transcription was increased in both the ΔcyaA mutant (205-fold) and the Δcrp mutant (142-fold) compared to the wild type (Fig. 1A). Transcription of the genes encoding biofilm matrix proteins was also increased in the ΔcyaA mutant (15-fold for rbmA, 36-fold for rbmC, and 32-fold for bap1) and the Δcrp mutant (13-fold for rbmA, 27-fold for rbmC, and 15-fold for bap1) compared to the wild type (Fig. 1B to D). This indicates that cAMP-CRP negatively regulates transcription of genes required for the production of both VPS and biofilm matrix proteins.
TABLE 3.
Differentially expressed genes involved in biofilm matrix production and c-di-GMP signaling in ΔcyaA and Δcrp mutants compared to the wild typea
| Gene no. | Designation | Change (fold)
|
|
|---|---|---|---|
| ΔcyaA/wild type | Δcrp/wild type | ||
| Biofilm matrix production genes | |||
| VC0916 | vpsU | 3.76 | 3.35 |
| VC0917 | vpsA | 1.51 | |
| VC0918 | vpsB | 2.93 | 2.16 |
| VC0919 | vpsC | 2.36 | 2.37 |
| VC0922 | vpsF | 2.04 | |
| VC0928 | rbmA | 5.89 | 5.45 |
| VC0929 | rbmB | 2.83 | 3.57 |
| VC0930 | rbmC | 21.04 | 8.01 |
| VC0931 | rbmD | 2.30 | |
| VC0932 | rbmE | 7.46 | 4.27 |
| VC0933 | rbmF | 5.89 | 5.99 |
| VC0935 | vpsM | 12.75 | 8.06 |
| VC0936 | vpsN | 2.63 | 2.32 |
| VC0937 | vpsO | 1.61 | 1.99 |
| VC0939 | vpsQ | 2.73 | 2.84 |
| VC0583 | hapR | 0.11 | 0.48 |
| VC1888 | bap1 | 7.84 | 6.28 |
| VCA0952 | vpsT | 74.62 | 48.70 |
| c-di-GMP signaling genes: GGDEF and EAL | |||
| VC0072 | 1.91 | 2.53 | |
| VC0653 | rocS | 1.81 | |
| VC0658 | cdgI | 2.95 | 3.34 |
| VC0703 | mbaA | 1.78 | 1.77 |
| VC1934 | 0.42 | 0.44 | |
| VC2750 | 1.70 | ||
| VCA0785 | cdgC | 1.53 | |
| c-di-GMP signaling genes: only GGDEF | |||
| VC1029 | cdgB | 1.88 | 2.17 |
| VC1067 | cdgH | 1.58 | |
| VC1216 | 0.56 | 0.45 | |
| VC1353 | 0.53 | ||
| VC1367 | cdgE | 0.64 | |
| VC1370 | 0.37 | ||
| VC1376 | 1.99 | ||
| VC1599 | 0.44 | 0.40 | |
| VCA0049 | 0.58 | ||
| VCA0074 | cdgA | 1.61 | |
| VCA0165 | 0.56 | ||
| VCA0217 | 1.94 | ||
| VCA0697 | cdgD | 0.09 | 0.11 |
| VCA0956 | cgdF | 1.90 | 1.87 |
| VCA0965 | 0.31 | 0.50 | |
| c-di-GMP signaling genes: only EAL | |||
| VC0137 | 1.69 | 1.93 | |
| VC1086 | 0.38 | 0.40 | |
| VC1211 | 1.58 | ||
| VC1641 | 1.66 | 2.56 | |
| VC1710 | 0.21 | 0.29 | |
| c-di-GMP signaling genes: HD-GYP | |||
| VC1087 | 0.43 | 0.55 | |
| VC1348 | 0.62 | ||
| VC2340 | 2.57 | ||
| VC2497 | 0.54 | 0.47 | |
| VCA0210 | 1.76 | ||
| VCA0895 | 0.46 | 0.61 | |
| VCA0931 | 0.48 | ||
Differentially expressed genes were determined using the SAM software with a ≥1.5-fold change in gene expression and an FDR of ≤3% as the criteria.
FIG. 1.
cAMP-CRP negatively regulates expression of genes involved in VPS biosynthesis and biofilm matrix protein production. β-Galactosidase assays of wild-type, ΔcyaA, and Δcrp strains harboring (A) vpsL-lacZ, (B) rbmA-lacZ, (C) rbmC-lacZ, and (D) bap1-lacZ fusion constructs were performed. The data are representative of at least two independent experiments. The error bars indicate standard deviations.
cAMP-CRP negatively regulates transcription of vpsT and vpsR in V. cholerae O1 El Tor A1552.
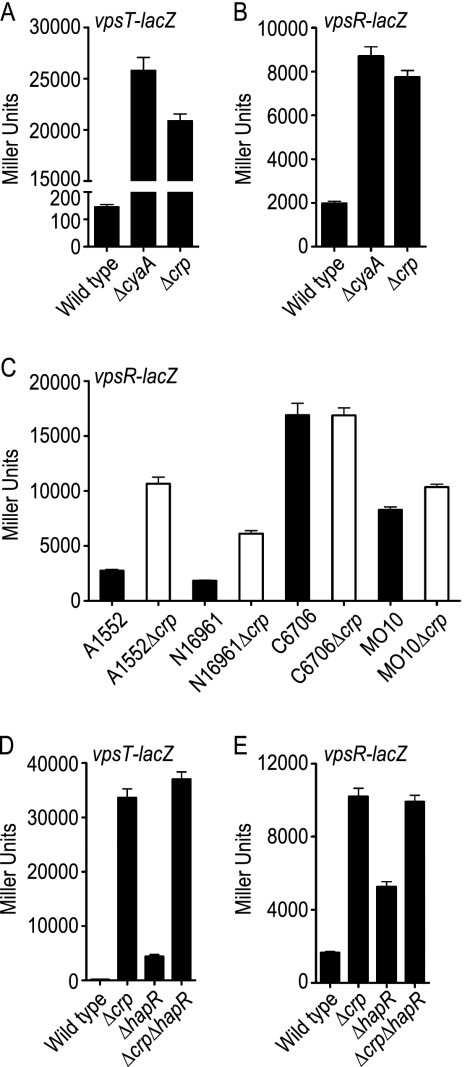
To understand how cAMP-CRP negatively regulates transcription of vps genes and biofilm matrix protein genes, we analyzed how transcription of vpsR and vpsT is altered in ΔcyaA and Δcrp mutants. Using vpsT-lacZ and vpsR-lacZ fusion constructs, we determined that vpsT and vpsR transcription was increased in the ΔcyaA mutant (178-fold for vpsT and 4-fold for vpsR) and the Δcrp mutant (144-fold for vpsT and 4-fold for vpsR) compared to the wild type, indicating that cAMP-CRP also negatively regulates transcription of vpsT and vpsR (Fig. 2A and B). Interestingly, transcription of vpsR was shown to be positively regulated by cAMP-CRP in the V. cholerae O1 El Tor C7258 strain (30). Differences in the regulation of vpsR by cAMP-CRP in our prototype strain, V. cholerae O1 El Tor A1552, and V. cholerae O1 El Tor C7258 prompted us to look at vpsR regulation in other commonly used V. cholerae strains. To this end, we generated in-frame crp deletion mutants of V. cholerae strains N16961, C6706, and MO10 and introduced a reporter plasmid harboring a vpsR-lacZ fusion construct into these strains. We then monitored transcription of vpsR by measuring β-galactosidase activity (Fig. 2C). In our prototype A1552Δcrp strain, vpsR-lacZ transcription was increased 3.9-fold compared to the wild-type transcription. N16961Δcrp also exhibited a similar increase in transcription of vpsR-lacZ (3.4-fold), while MO10Δcrp exhibited a slight increase (1.3-fold) in vpsR-lacZ transcription compared to the corresponding wild-type strains. On the other hand, in C6706 and transcription of vpsR-lacZ in C6706Δcrp did not differ significantly. These results indicate that while cAMP-CRP negatively regulates vpsR transcription in the A1552, N16961, and MO10 (albeit slightly) strains of V. cholerae, there is no such regulation in strain C6706. Hence, the results are consistent with the idea that vpsR regulation by cAMP-CRP varies in strains of V. cholerae. Together, our results indicate that in our prototype strain, cAMP-CRP represses biofilm formation in V. cholerae by negatively regulating transcription of the genes required for VPS biosynthesis and matrix protein production, as well as the genes encoding the positive transcriptional regulators VpsT and VpsR.
FIG. 2.
cAMP-CRP negatively regulates vpsT and vpsR expression. (A and B) β-Galactosidase assays of wild-type strain A1552 and ΔcyaA and Δcrp mutants harboring (A) vpsT-lacZ and (B) vpsR-lacZ fusion constructs. (C) β-Galactosidase assays of different V. cholerae strains (A1552, N16961, C6706, and MO10) and Δcrp deletion strains harboring the vpsR-lacZ fusion construct. (D and E) β-Galactosidase assays of wild-type, Δcrp, ΔhapR, and Δcrp ΔhapR strains harboring (D) vpsT-lacZ and (E) vpsR-lacZ fusion constructs. The data are representative of at least two independent experiments. The error bars indicate standard deviations.
crp is epistatic to hapR in the regulation of vpsT and vpsR transcription.
Biofilm formation in V. cholerae is negatively regulated by both HapR (19, 62, 64) and cAMP-CRP (29, 30). Since cAMP-CRP positively regulates hapR transcription, repression of biofilm formation by cAMP-CRP could be mediated through HapR. Thus, to better evaluate the mechanism by which cAMP-CRP and HapR negatively regulate biofilm formation, we analyzed vpsT and vpsR transcription in wild-type, Δcrp, ΔhapR, and Δcrp ΔhapR strains harboring vpsT-lacZ and vpsR-lacZ fusion plasmids (Fig. 2D and E). We observed 27- and 3.2-fold increases in β-galactosidase activities in the ΔhapR mutants harboring vpsT-lacZ and vpsR-lacZ fusion plasmids, respectively, compared to the wild type. These results are congruent with the results of our previous studies showing that HapR negatively regulates the expression of vpsT and vpsR in strain A1552 (2, 62). It is noteworthy that in V. cholerae strains C6706 and C7258 negative regulation of vpsR by HapR has not been observed (19, 30, 59), indicating that, similar to vpsR repression by cAMP-CRP, vpsR repression by HapR also varies in strains of V. cholerae. Interestingly, similar increases in the transcriptional levels of vpsT (210-fold in the Δcrp mutant and 231-fold in the Δcrp ΔhapR mutant) and vpsR (6.2-fold in the Δcrp and mutant 6.0-fold in the Δcrp ΔhapR mutant) were observed for the Δcrp and Δcrp ΔhapR mutants, indicating that crp is epistatic to hapR in regulating vpsT and vpsR transcription.
cAMP-CRP regulates rbmC and bap1 expression both through and independent of VpsR.
Formation of mature biofilms in V. cholerae requires production of VPS and the matrix proteins RbmA, RbmC, and Bap1. As discussed above, cAMP-CRP negatively regulates the expression of both vps genes and genes encoding matrix proteins. However, we have a limited understanding of the mechanism by which cAMP-CRP regulates transcription of these genes and do not know whether vps and matrix protein genes are regulated differently by cAMP-CRP.
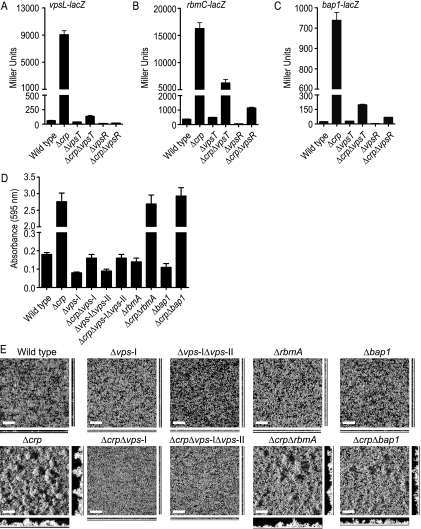
We previously reported that VpsR is the most downstream regulator of vps gene transcription and genes encoding matrix proteins in the VpsT, VpsR, and HapR regulatory circuitry (2). We wanted to determine how cAMP-CRP contributes to this regulatory circuitry. To this end, we monitored transcription of vpsL, rbmC, and bap1 using lacZ transcriptional fusion constructs in the wild-type strain and Δcrp, ΔvpsT, ΔvpsR, Δcrp ΔvpsT, and Δcrp ΔvpsR mutants (Fig. 3). As discussed above, in the Δcrp strain harboring the fusion construct vpsL-lacZ, transcription of vpsL was markedly increased compared to that in the wild type. Transcription of vpsL-lacZ was 1.7- and 4.6-fold lower in the ΔvpsT and ΔvpsR mutants, respectively, than in the wild type (Fig. 3A). This finding is consistent with our previous report that the magnitude of regulation of vps gene expression by VpsR is greater than that by VpsT (2). In the Δcrp ΔvpsT double-deletion mutant, a 2.1-fold increase in vpsL transcription was observed compared to the wild type. This slight increase in vpsL transcription was likely due to decreased expression of hapR in the Δcrp genetic background. cAMP-CRP positively regulates hapR expression, and a decrease in hapR message abundance, in turn, leads to an increase in vpsL and vpsR expression (2, 62). Unlike the findings for the Δcrp ΔvpsT mutant, the vpsL transcription in the Δcrp ΔvpsR mutant was 3.3-fold lower than that in the wild type (similar to the ΔvpsR mutant), indicating that VpsR acts downstream of cAMP-CRP.
FIG. 3.
Analysis of the cAMP-CRP contribution to VpsR regulation of rbmC and bap1 expression. (A to C) β-Galactosidase assays of wild-type, Δcrp, ΔvpsT, Δcrp ΔvpsT, ΔvpsR, and Δcrp ΔvpsR strains harboring (A) vpsL-lacZ, (B) rbmC-lacZ, and (C) bap1-lacZ fusion constructs. The data are representative of at least two independent experiments. The error bars indicate standard deviations. (D) Quantitative comparison of biofilm formation by wild-type, Δcrp, Δvps-I, Δcrp Δvps-I, Δvps-I Δvps-II, Δcrp Δvps-I Δvps-II, ΔrbmA, Δcrp ΔrbmA, Δbap1, and Δcrp Δbap1 strains. The data are representative of two independent experiments. The error bars indicate standard deviations. (E) Biofilms formed after 8 h of incubation at 30°C in a non-flow-cell system by the wild-type, Δcrp, Δvps-I, Δcrp Δvps-I, Δvps-I Δvps-II, Δcrp Δvps-I Δvps-II, ΔrbmA, Δcrp ΔrbmA, Δbap1, and Δcrp Δbap1 strains. Biofilms were stained with SYTO9, and images were acquired by CLSM. The large images are images of the upper surfaces of biofilms, and the images below and to right of the large images are orthogonal views. Bars = 40 μm.
Transcription of rbmC and bap1 was increased in the Δcrp mutant compared to the wild type. Although deletion of vpsR resulted in decreases in transcription of rbmC (7.6-fold) and bap1 (6.8-fold), deletion of vpsT did not significantly alter rbmC and bap1 transcription compared to the wild type (Fig. 3B and C). This result suggests that, like the findings for vpsL transcription, the magnitude of transcriptional regulation by VpsR is greater than the magnitude of transcriptional regulation by VpsT for rbmC and bap1. Intriguingly, unlike the results for vpsL transcription, deletion of crp in both the ΔvpsT and ΔvpsR genetic backgrounds resulted in increased transcription of rbmC (16.4-fold in the Δcrp ΔvpsT mutant and 3.0-fold in the Δcrp ΔvpsR mutant) and bap1 (8.1-fold in the Δcrp ΔvpsT mutant and 2.8-fold in the Δcrp ΔvpsR mutant) compared to the wild type. Together, these findings indicate that cAMP-CRP negatively regulates the transcription of vps genes and genes encoding matrix proteins in a different manner. While VpsR is the most downstream positive transcriptional regulator of vps gene expression, cAMP-CRP negatively regulates transcription of the genes encoding matrix proteins both through and independent of VpsR. Whether the action of cAMP-CRP is mediated by direct binding to the rbmC and bap1 promoter regions or indirectly through another regulatory protein(s) remains unknown.
Since we observed that VPS biosynthesis genes and genes encoding matrix proteins are regulated differently by cAMP-CRP, we wanted to determine the contribution of VPS and biofilm matrix proteins to biofilm formation in the Δcrp genetic background. To this end, we generated Δcrp Δvps-I (vps-I cluster deletion), Δcrp Δvps-I Δvps-II (vps-I and vps-II cluster deletion), Δcrp ΔrbmA, and Δcrp Δbap1 mutants and compared their biofilm-forming capacities to those of the wild type and Δcrp, Δvps-I, Δvps-I Δvps-II, ΔrbmA, and Δbap1 single mutants. Deletion of the vps-I cluster or the vps-I and vps-II clusters in the Δcrp genetic background eliminated formation of a pellicle (biofilm formed at the air-liquid interface) (data not shown) and drastically reduced biofilm formation in both 96-well microtiter plate and non-flow-cell systems (Fig. 3D and E). Deletion of rbmA or bap1 in the Δcrp genetic background decreased, but did not eliminate, biofilm formation. Compared to the Δcrp mutant biofilm, the biofilms formed by the Δcrp ΔrbmA and Δcrp Δbap1 mutants were less structured, and there were fewer mature pillars in the biofilms formed by the double-deletion mutants (Fig. 3E). Although the total biomasses and average biofilm thicknesses were not significantly different for the Δcrp, Δcrp ΔrbmA, and Δcrp Δbap1 mutants, the maximum biofilm thickness was greater for the Δcrp mutant than for the Δcrp ΔrbmA and Δcrp Δbap1 mutants (data not shown). Together, these results indicate that under the experimental conditions that we utilized, VPS production is essential for biofilm formation in a Δcrp genetic background, while biofilm matrix proteins have an accessory role. We do not know yet how biofilm matrix proteins function, whether they bind VPS carbohydrates or mediate cell-cell or cell-surface interactions. It is possible that the relative contributions of VPS and biofilm matrix proteins to biofilm formation are different when the organisms are tested under different environmental conditions using different surfaces.
cAMP-CRP differentially regulates the expression of a set of genes encoding proteins harboring GGDEF, EAL, and HD-GYP domains.
Biofilm formation in V. cholerae is positively regulated by c-di-GMP (3, 31, 32, 56). Since cAMP-CRP negatively regulates biofilm formation in V. cholerae, we wanted to investigate if there is a connection between cAMP-CRP regulatory circuitry and c-di-GMP signaling in regulation of biofilm formation. We hypothesized that c-di-GMP levels may be increased in ΔcyaA and Δcrp mutants due to increased expression of a gene(s) encoding a key DGC or due to decreased expression of a gene(s) encoding a key PDE. Indeed, whole-genome expression profiling of ΔcyaA and Δcrp mutants revealed that genes encoding proteins predicted to exhibit DGC and PDE activities were differentially regulated in ΔcyaA and/or Δcrp mutants compared to the wild type (Table 3).
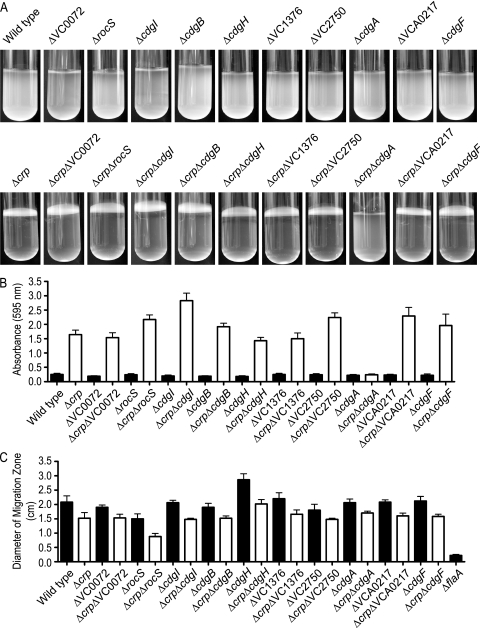
In this set of differentially regulated genes, we focused on 10 genes that encode proteins with a conserved GGDEF domain which is predicted to act as a DGC: VC0072, VC0653 (rocS), VC0658, VC1029 (cdgB), VC1067 (cdgH), VC1376, VC2750, VCA0074 (cdgA), VCA0217, and VCA0956 (cdgF) (referred to below as the GGDEF genes). To examine the involvement of the GGDEF genes in the regulation of biofilm formation in V. cholerae, we generated mutants with in-frame deletion mutations in these genes in the wild-type background, as well as in the Δcrp genetic background. We then analyzed the biofilm-forming capacity of each of these mutants using pellicle formation in glass culture tubes and biofilm formation in 96-well microtiter plates with a crystal violet staining assay. As shown in Fig. 4A and B, deletion of GGDEF genes in the wild type did not alter the pellicle and biofilm formation phenotypes. However, when the genes were deleted in the enhanced biofilm-forming Δcrp genetic background, only the Δcrp ΔcdgA double-deletion mutant failed to form pellicles and exhibited a reduced biofilm-forming capacity. This finding indicates that the increased pellicle- and biofilm-forming capacities of a Δcrp mutant are due to increased expression of cdgA. Interestingly, we recently reported that cdgA positively regulates colony rugosity and biofilm formation in V. cholerae (2, 31). The crystal violet staining assays also revealed that deletion of rocS and VC0658 (designated cdgI [cyclic di-guanylate I]) in the Δcrp genetic background further enhanced biofilm formation. Both RocS and CdgI contain conserved GGDEF-EAL domains. Such proteins can function as either DGCs or PDEs, and the enzymatic functions are commonly regulated by environmental stimuli. Although the enzymatic activities of these proteins have not been determined yet, based on biofilm-forming phenotypes, RocS and CdgI appear to function as a PDE under the experimental conditions that we utilized.
FIG. 4.
Phenotypic characterization of GGDEF deletion mutants and GGDEF crp double-deletion mutants. (A) Pellicle formation, (B) quantitative comparison of biofilm formation, and (C) motility assays for the wild type, for Δcrp and GGDEF single-deletion mutants, and for mutants with GGDEF deletions generated in the Δcrp genetic background. The data are representative of two independent experiments. The error bars indicate standard deviations.
Flagellar motility is negatively regulated by c-di-GMP in V. cholerae. Therefore, we sought to understand the effect of deletion of GGDEF genes on motility. To this end, we measured the migration zone formed by each single-deletion mutant, as well as the migration zones formed by mutants generated in the Δcrp genetic background, when the organisms were grown on LB soft agar plates (Fig. 4C). We also used ΔflaA as a control. Of the GGDEF single-deletion mutants tested, the ΔrocS mutant exhibited a decrease in motility and the ΔcdgH mutant exhibited an increase in motility compared to the wild type. These results are consistent with our previous report (31) and recent findings of Beyhan et al. (S. Beyhan et al., submitted for publication). Deletion of crp led to a decrease in motility, consistent with the expression profiling data showing downregulation of genes involved in flagellar biosynthesis and chemotaxis in ΔcyaA and Δcrp mutants compared to the wild type (see Fig. S1 in the supplemental material). Deletion of crp in individual GGDEF deletion mutants also led to further decreases in motility compared to the corresponding GGDEF single-deletion mutants. One of the mutants tested, the Δcrp ΔrocS double-deletion mutant, exhibited a further reduction in motility compared to both the Δcrp and ΔrocS single-deletion mutants, suggesting that cAMP-CRP and RocS have additive effects on motility regulation.
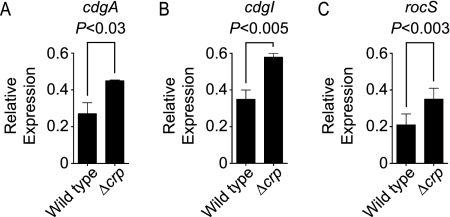
In summary, for the 10 GGDEF genes tested, deletion of cdgA, rocS, and cdgI in the Δcrp genetic background altered biofilm-forming phenotypes. Since we chose these genes based on their increased expression in the ΔcyaA and/or Δcrp mutant in whole-genome expression profiling experiments, we further confirmed their message abundance using qPCR. In agreement with microarray data, the message levels of cdgA, rocS, and cdgI were greater in the Δcrp mutant than in the wild type (Fig. 5).
FIG. 5.
qPCR analysis of cdgA, cdgI, and rocS message levels in the Δcrp mutant: quantification of relative repression of (A) cdgA, (B) cdgI, and (C) rocS in the wild type and the Δcrp mutant, normalized using recA. The results are from three independent biological replicates. The error bars indicate standard deviations. P values (two-tailed t test) are indicated at the top.
Expression of VC1934, which encodes a protein containing an EAL domain and a nonconserved GGDEF domain, expression of VC1086 and VC1710, which encode proteins containing an EAL domain, and expression of VC1087, VC1348, VC2497, VCA0895, and VCA0931, which encode proteins containing a HD-GYP domain, were downregulated, with ≥1.5-fold changes and FDR of ≤3% in the ΔcyaA and/or Δcrp mutant (Table 3).
It is possible that a decrease in the expression of these genes encoding proteins predicted to have PDE activity (leading to a decrease in the cellular c-di-GMP level) (45, 49) may also contribute to an overall increase in the c-di-GMP level, thus giving rise to the increased biofilm-forming capacities observed for the ΔcyaA and Δcrp mutants. Regulation of the expression of these genes by cAMP-CRP in V. cholerae is currently under investigation.
CdgA is required for the enhanced biofilm-forming phenotype in the Δcrp mutant and negative regulation of vpsL and rbmC expression by cAMP-CRP.
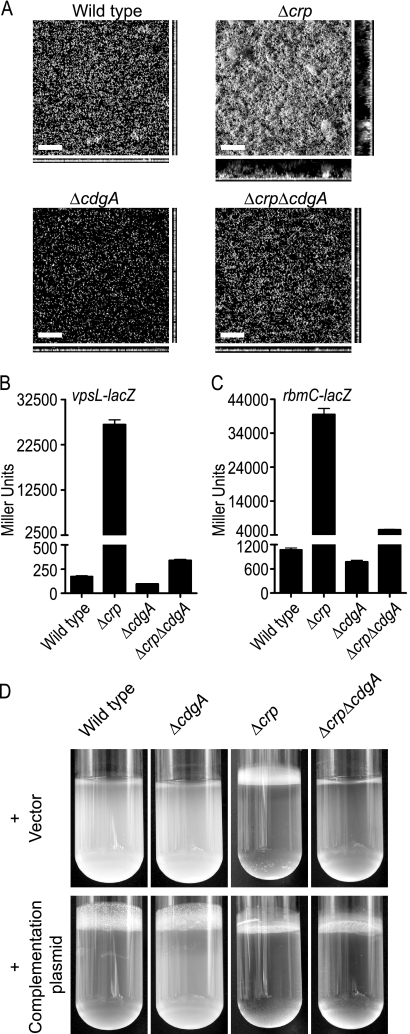
In the experiments described above we showed that while the Δcrp single-deletion mutant exhibits enhanced pellicle- and biofilm-forming capacities (as determined by the crystal violet staining assay), the crp and cdgA double-deletion mutant (Δcrp ΔcdgA mutant) exhibited a decrease in biofilm formation. We confirmed this finding by carrying out a CLSM analysis of biofilms formed by the wild type and the Δcrp, ΔcdgA, and Δcrp ΔcdgA mutants in a non-flow-cell system (Fig. 6A). After 8 h of biofilm development, the Δcrp mutant formed a thicker and more-structured biofilm, and the total biomass, average and maximum biofilm thicknesses, and substratum coverage were greater for the Δcrp mutant than for the wild type (Table 4). The ΔcdgA mutant formed biofilms with reduced total biomass, average biofilm thickness, and substratum coverage compared to the wild-type biofilms. Although deletion of cdgA in the Δcrp mutant significantly reduced biofilm formation, the Δcrp ΔcdgA double mutant formed biofilms whose total biomass, average biofilm thickness, and substratum coverage were greater than those of the biofilms formed by the ΔcdgA mutant (Fig. 6A and Table 4). These results indicate that the negative regulation of biofilm formation by cAMP-CRP is largely mediated by CdgA, but additional factors may also contribute to an increase in biofilm formation in the Δcrp mutant.
FIG. 6.
Phenotypic characterization of Δcrp, ΔcdgA, and Δcrp ΔcdgA mutants. (A) Biofilms of wild-type, Δcrp, ΔcdgA, and Δcrp ΔcdgA strains formed after 8 h of incubation at 30°C in a non-flow-cell system. Images were acquired by CLSM. The large images are images of the upper surfaces of biofilms, and the images below and to right of the large images are orthogonal views. Bars = 40 μm. (B and C) β-Galactosidase assays for the wild-type, Δcrp, ΔcdgA, and Δcrp ΔcdgA strains harboring (B) vpsL-lacZ and (C) rbmC-lacZ fusion constructs. The data are representative of two independent experiments. The error bars indicate standard deviations. (D) Pellicle formation in the wild-type, Δcrp, ΔcdgA, and Δcrp ΔcdgA strains harboring the vector or the cdgA complementation plasmid.
TABLE 4.
COMSTAT analysis for biofilms of the wild-type, Δcrp, ΔcdgA, and Δcrp ΔcdgA strainsa
| Strain | Total biomass (μm3/μm2) | Thickness (μm)
|
Substratum coverage | |
|---|---|---|---|---|
| Avg | Maximum | |||
| Wild type | 2.1 (0.4) | 1.9 (0.4) | 8.7 (2.8) | 0.4 (0.1) |
| Δcrp | 6.6 (1.6) | 9.0 (1.8) | 40.4 (4.4) | 0.7 (0.1) |
| ΔcdgA | 1.0 (0.1) | 0.9 (0.1) | 8.0 (1.7) | 0.2 (0.02) |
| Δcrp ΔcdgA | 1.6 (0.3) | 1.3 (0.2) | 9.3 (0.9) | 0.4 (0.1) |
The values are means of data from at least six z-series image stacks. The numbers in parentheses are standard deviations.
To determine if the altered pellicle- and biofilm-forming phenotypes of the Δcrp ΔcdgA double-deletion mutant were due to a reduction in the transcription of genes involved in biofilm matrix production, we carried out β-galactosidase assays with the wild-type, Δcrp, ΔcdgA, and Δcrp ΔcdgA strains harboring vpsL-lacZ and rbmC-lacZ transcriptional fusion constructs (Fig. 6B and C). While the Δcrp mutant exhibited increased transcription of vpsL-lacZ (154-fold) and rbmC-lacZ (37-fold), the ΔcdgA mutant exhibited decreases in the transcription of vpsL-lacZ (1.8-fold) and rbmC-lacZ (1.3-fold) compared to the wild type, consistent with our previous finding (obtained using gene expression profiling) that CdgA positively regulates vps and rbm gene expression (2). The Δcrp ΔcdgA double-deletion mutant exhibited a decrease in the transcription of vpsL-lacZ (79-fold) and rbmC-lacZ (7.1-fold) compared to the Δcrp single-deletion mutant. These results indicate that the decreased biofilm-forming capacities of the Δcrp ΔcdgA double mutant compared to the Δcrp mutant were due to decreased expression of genes involved in VPS biosynthesis and matrix protein production. Interestingly, in the Δcrp ΔcdgA double-deletion mutant the levels of transcription of vpsL-lacZ and rbmC-lacZ were higher than those in the wild type (1.9-fold higher for vpsL-lacZ and 5.1-fold higher for rbmC-lacZ) and in the ΔcdgA mutant (3.5-fold higher for vpsL-lacZ and 6.5-fold higher for rbmC-lacZ). These findings suggest that besides CdgA, other factors and processes also contribute to an increase in biofilm formation in the Δcrp mutant.
We also carried out a complementation assay by introducing cdgA, in which transcription is driven from its own promoter, in a multicopy number plasmid into the wild type and into the ΔcdgA, Δcrp, and Δcrp ΔcdgA mutants. The ΔcdgA and Δcrp ΔcdgA mutants harboring the complementation plasmid exhibited increased pellicle-forming capacities compared to the same strains carrying only the vector (Fig. 6D), further confirming that CdgA is a key DGC that positively regulates biofilm formation. Although we now know many of the molecular players, it is still unclear how c-di-GMP regulates transcription of the genes involved in biofilm matrix production.
DISCUSSION
Environmental cues, such as nutrient availability, affect biofilm formation and/or dispersal (6, 9), linking carbon metabolism and biofilm formation. In Pseudomonas aeruginosa, the catabolite repression control protein (Crc), which regulates carbon metabolism, positively regulates biofilm formation (40), and a sudden increase in carbon substrate availability (including glucose availability) induces biofilm dispersion (48). In addition, glucose represses biofilm formation in Bacillus subtilis (53), as well as in E. coli, Citrobacter freundii, Klebsiella pneumoniae, and Salmonella enterica serovar Typhimurium (24). In contrast, V. cholerae exhibits an increase in biofilm-forming capacity when it is grown in minimal medium supplemented with 0.5% (wt/vol) glucose (23). The cAMP-CRP global transcriptional regulatory complex, which regulates carbon metabolism, positively regulates biofilm formation in E. coli, in which a crp mutation results in decreased biofilm formation (24), and in Shewanella oneidensis, in which a crp mutation results in a defect in biofilm detachment in the stop-of-flow-induced detachment response compared to the wild type (55). In contrast, a V. cholerae Δcrp deletion mutant exhibits increased biofilm formation (29), indicating that cAMP-CRP can act as either a positive regulator or a negative regulator of biofilm formation. We were, therefore, interested in further elucidating the molecular mechanism by which cAMP-CRP regulates biofilm formation in V. cholerae.
Using whole-genome transcriptional profiles of ΔcyaA and Δcrp mutants, we showed that cAMP-CRP regulates the expression of a large set of genes in V. cholerae (see Fig. S1 and Tables S1 and S2 in the supplemental material), consistent with its role as a global transcriptional regulatory complex (5, 12). It is also noteworthy that the overall transcriptional profiles of the ΔcyaA and Δcrp mutants were similar (see Fig. S1 in the supplemental material), consistent with the requirement for both cAMP and CRP in transcriptional regulation (5). More than 20% of the predicted genes in the genome of V. cholerae (as annotated by The Institute for Genomic Research) are differentially regulated in both ΔcyaA and Δcrp mutants (≥2-fold change and FDR of ≤1%) compared to the wild type, and approximately equal numbers of genes are positively and negatively regulated. Consistent with the negative role of cAMP-CRP in biofilm formation, many of the genes involved in biofilm matrix production are upregulated in ΔcyaA and Δcrp mutants (Table 3). Several genes involved in pathogenesis are also upregulated, while genes involved in flagellum biosynthesis and chemotaxis are downregulated in the deletion mutants (see Fig. S1 and Tables S1 and S2 in the supplemental material).
V. cholerae wild-type biofilm formation requires the production of both VPS and matrix proteins, including RbmA, RbmC, and Bap1 (15, 16). These matrix proteins play a crucial role in maintaining the structural integrity of the biofilm. They may function as an agglutinin/adhesion in binding cells together and/or binding to VPS. They may also act as anchors for biofilm and/or cells to attach to surfaces that contain carbohydrates. As a consequence of environmental signals, V. cholerae may modulate the ratio of matrix proteins to VPS within a biofilm, giving rise to different degrees of biofilm rigidity and stability, which may promote detachment of single cells or cell aggregates for dispersal. It is therefore intriguing but not surprising that, although VpsR is the most downstream regulator of vps gene expression, cAMP-CRP can also regulate rbmC and bap1 expression independent of VpsR regulation (Fig. 3).
Putative cAMP-CRP binding sites were predicted upstream of the rbmA, rbmC, and bap1 coding regions. We are currently investigating if cAMP-CRP binds directly to these predicted binding sites. It is very intriguing that a predicted VpsR binding site (GTCTCATTACTGAGGCGT) overlaps by 11 bp the putative cAMP-CRP binding site (AACTTTGAGATGTCTCATTACT) upstream of rbmC. A very plausible hypothesis is that cAMP-CRP negatively regulates rbmC expression by directly binding to its promoter region and, in doing so, interferes with the binding of VpsR to the upstream regulatory region of rbmC and thus prevents VpsR from positively regulating rbmC expression. Similar antagonistic functions have been reported for cAMP-CRP and AphA/AphB for tcpPH expression (28), as well as for HapR and VpsR for aphA expression (33). We are currently examining the possible antagonistic role of cAMP-CRP and VpsR in binding the rbmC promoter region. Using Virtual Footprint software (http://www.prodoric.de/vfp/index.php), a putative cAMP-CRP binding site was also predicted upstream of the coding region of vpsR, but not upstream of the coding region of vpsT. It is therefore possible that cAMP-CRP negatively regulates vpsR expression by direct binding to the upstream promoter region of vpsR. Since no cAMP-CRP binding sites upstream of vpsT have been predicted, the cAMP-CRP negative regulation of vpsT may be via VpsR (in addition to HapR) or by a so-far-unidentified repressor protein(s). We are currently investigating if cAMP-CRP binds directly upstream of the vpsR promoter region.
A glucose effect or catabolite repression has been observed in many microorganisms (5) and is a likely environmental nutritional cue for regulating biofilm formation. It has been reported that vpsL expression is regulated by one of the components of the phosphoenolpyruvate phosphotransferase (PTS) system, such that the accumulation of the phosphorylated form of enzyme I (EI∼P) due to the absence of a PTS sugar, such as glucose, represses vps gene expression and leads to a reduction in biofilm formation (23). Furthermore, addition of cAMP to the growth medium, mimicking activation of adenylate cyclase (CyaA) by EIIAGlu∼P (12), represses biofilm accumulation in V. cholerae (23). It is noteworthy that addition of exogenous cAMP eliminated the enhanced pellicle-forming capacity of the ΔcyaA mutant (see Fig. S2 in the supplemental material). Consistent with this model, ΔcyaA and Δcrp mutants both exhibit increased biofilm-forming capacities. We also observed increased biofilm formation in wild-type V. cholerae grown in LB medium supplemented with 0.2 and 0.5% (wt/vol) glucose (data not shown). Interestingly, mannose (another PTS sugar) has also been reported to enhance biofilm formation in V. cholerae, likely through the PTS system (23, 27, 39). Similarly, growth on N-acetylglucosamine (also a PTS sugar) has been shown to promote attachment of V. cholerae and subsequent colonization of chitinous surfaces (37, 42). V. cholerae can colonize surfaces of phytoplankton and zooplankton (14, 34). Nutrients provided by mucous secretions of phytoplankton and zooplankton, as well as chitinous surfaces of zooplankton, could then facilitate or enhance biofilm formation by V. cholerae by providing environmental stimuli that lead to enhanced biofilm formation, thereby facilitating environmental persistence and growth of the pathogen.
The V. cholerae genome contains 62 genes coding for proteins containing GGDEF, EAL, and HD-GYP domains (17, 20). These proteins modulate cellular c-di-GMP levels (8, 45, 46, 49), which in turn regulate several phenotypic characteristics, including colony morphology, pellicle- and biofilm-forming capacities, and motility (3, 31, 32, 56, 57). c-di-GMP also regulates similar biological processes in several other microorganisms, including P. aeruginosa, E. coli, and S. enterica serovar Typhimurium (25, 43, 44). Using whole-genome expression profiles of ΔcyaA and Δcrp mutants, we identified 10 genes encoding proteins that contain a conserved GGDEF domain and are upregulated in the ΔcyaA and/or Δcrp mutant compared to the wild type (Table 3). For these genes, only deletion of cdgA in the Δcrp genetic background eliminated pellicle formation and reduced the biofilm-forming capacity (Fig. 4 and 6), indicating that the increased biofilm-forming capacity of the Δcrp mutant was due in part to increased expression of cdgA, which in turn modulated the expression of vps genes and genes encoding matrix proteins (Fig. 6B and C). Interestingly, we also observed increases in biofilm formation for the Δcrp ΔrocS and ΔcrpΔcdgI mutants compared to the Δcrp, ΔrocS, and ΔcdgI single-deletion mutants (Fig. 4B). Although both RocS and CdgI contain conserved GGDEF-EAL domains, the results of biofilm formation assays suggest that, like RocS, CdgI functions mainly as a PDE under the conditions that we utilized. The contributions of other PDEs (Table 3) to the negative regulation of biofilm formation by cAMP-CRP remain to be investigated.
Regulation of biofilm formation in V. cholerae is complex. We previously described the regulatory network controlling vps gene expression, where vps genes are positively regulated by VpsR, VpsT, and CdgA and negatively regulated by HapR (2). HapR also negatively regulates the expression of vpsT, vpsR, and cdgA. Interestingly, a recent study reported that HapR binds directly to the promoter regions of vpsT and cdgA (59). We also previously described the positive regulation of cdgA by VpsR and VpsT. Using Virtual Footprint software, two putative cAMP-CRP binding sites were predicted upstream of the cdgA coding region, suggesting that cAMP-CRP can regulate cdgA expression both directly by binding to the cdgA promoter region and indirectly through HapR.
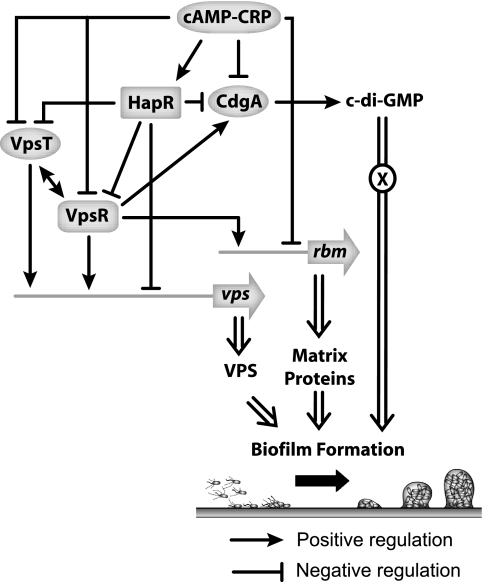
Data obtained in this study, together with results described in other reports, indicate that cAMP-CRP regulates biofilm formation at multiple levels (Fig. 7). First, cAMP-CRP negatively regulates biofilm formation in V. cholerae through positive regulation of hapR expression, which in turn negatively regulates expression of vps and rbm (and bap1) genes, as well as cdgA, vpsT, and, in our strain, vpsR. VpsR, VpsT, and CdgA, in turn, positively regulate expression of vps and rbm (as well as bap1) genes. Second, cAMP-CRP may also negatively regulate expression of vpsR, cdgA, and rbmC expression by directly binding to their promoter regions. The connection between cAMP-CRP regulatory circuitry and c-di-GMP signaling reported here is particularly intriguing. Further characterization of this connection should provide additional insight into the wiring of the regulatory circuitry controlling biofilm formation in V. cholerae.
FIG. 7.
Model of cAMP-CRP regulation of biofilm formation in V. cholerae. cAMP-CRP regulates biofilm formation at multiple levels. Expression of vps and rbm genes is negatively regulated by cAMP-CRP through positive regulation of hapR expression and through negative regulation of vpsR and vpsT expression. In turn, VpsR and VpsT positively regulate vps and rbm gene expression, while HapR negatively regulates vps and rbm gene expression. VpsR, VpsT, and HapR regulation of vps and rbm gene expression also involves c-di-GMP signaling, where cdgA expression is negatively regulated by HapR and positively regulated by VpsR and VpsT. An increase in cdgA transcription leads to an increase in the c-di-GMP level, which in turn could interact with an effector protein(s) to positively regulate biofilm formation. We have a very limited understanding of the link between c-di-GMP pools and the signaling that leads to biofilm formation in V. cholerae. In addition, cAMP-CRP may also directly regulate vpsR, cdgA, and rbmC expression and indirectly regulate vpsT expression.
Supplementary Material
Acknowledgments
We thank Chad Saltikov, Manel Camps, and members of the Yildiz laboratory for their valuable comments on the manuscript, as well as Sinem Beyhan for generating FY_Vc_956, Nicholas Shikuma for generating FY_Vc_3411, and James Meir for generating FY_Vc_152, FY_Vc_869, FY_Vc_154, and FY_Vc_158.
This work was supported by grant AI055987 from NIH.
Footnotes
Published ahead of print on 15 August 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Bao, Y., D. P. Lies, H. Fu, and G. P. Roberts. 1991. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene 109167-168. [DOI] [PubMed] [Google Scholar]
- 2.Beyhan, S., K. Bilecen, S. R. Salama, C. Casper-Lindley, and F. H. Yildiz. 2007. Regulation of rugosity and biofilm formation in Vibrio cholerae: comparison of VpsT and VpsR regulons and epistasis analysis of vpsT, vpsR, and hapR. J. Bacteriol. 189388-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyhan, S., A. D. Tischler, A. Camilli, and F. H. Yildiz. 2006. Transcriptome and phenotypic responses of Vibrio cholerae to increased cyclic di-GMP level. J. Bacteriol. 1883600-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyhan, S., and F. H. Yildiz. 2007. Smooth to rugose phase variation in Vibrio cholerae can be mediated by a single nucleotide change that targets c-di-GMP signalling pathway. Mol. Microbiol. 63995-1007. [DOI] [PubMed] [Google Scholar]
- 5.Botsford, J. L., and J. G. Harman. 1992. Cyclic AMP in prokaryotes. Microbiol. Rev. 56100-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowden, G. H., and Y. H. Li. 1997. Nutritional influences on biofilm development. Adv. Dent. Res. 1181-99. [DOI] [PubMed] [Google Scholar]
- 7.Casper-Lindley, C., and F. H. Yildiz. 2004. VpsT is a transcriptional regulator required for expression of vps biosynthesis genes and the development of rugose colonial morphology in Vibrio cholerae O1 El Tor. J. Bacteriol. 1861574-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christen, M., B. Christen, M. Folcher, A. Schauerte, and U. Jenal. 2005. Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 28030829-30837. [DOI] [PubMed] [Google Scholar]
- 9.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49711-745. [DOI] [PubMed] [Google Scholar]
- 10.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235386-405. [DOI] [PubMed] [Google Scholar]
- 12.Deutscher, J., C. Francke, and P. W. Postma. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70939-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faruque, S. M., M. J. Albert, and J. J. Mekalanos. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 621301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fong, J. C., K. Karplus, G. K. Schoolnik, and F. H. Yildiz. 2006. Identification and characterization of RbmA, a novel protein required for the development of rugose colony morphology and biofilm structure in Vibrio cholerae. J. Bacteriol. 1881049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fong, J. C., and F. H. Yildiz. 2007. The rbmBCDEF gene cluster modulates development of rugose colony morphology and biofilm formation in Vibrio cholerae. J. Bacteriol. 1892319-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galperin, M. Y., A. N. Nikolskaya, and E. V. Koonin. 2001. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol. Lett. 20311-21. [DOI] [PubMed] [Google Scholar]
- 18.Gentleman, R. C., V. J. Carey, D. M. Bates, B. Bolstad, M. Dettling, S. Dudoit, B. Ellis, L. Gautier, Y. Ge, J. Gentry, K. Hornik, T. Hothorn, W. Huber, S. Iacus, R. Irizarry, F. Leisch, C. Li, M. Maechler, A. J. Rossini, G. Sawitzki, C. Smith, G. Smyth, L. Tierney, J. Y. Yang, and J. Zhang. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammer, B. K., and B. L. Bassler. 2003. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50101-104. [DOI] [PubMed] [Google Scholar]
- 20.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, O. White, S. L. Salzberg, H. O. Smith, R. R. Colwell, J. J. Mekalanos, J. C. Venter, and C. M. Fraser. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 1726557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 1462395-2407. [DOI] [PubMed] [Google Scholar]
- 23.Houot, L., and P. I. Watnick. 2008. A novel role for enzyme I of the Vibrio cholerae phosphoenolpyruvate phosphotransferase system in regulation of growth in a biofilm. J. Bacteriol. 190311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson, D. W., J. W. Simecka, and T. Romeo. 2002. Catabolite repression of Escherichia coli biofilm formation. J. Bacteriol. 1843406-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenal, U., and J. Malone. 2006. Mechanisms of cyclic-di-GMP signaling in bacteria. Annu. Rev. Genet. 40385-407. [DOI] [PubMed] [Google Scholar]
- 26.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 848-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kierek, K., and P. I. Watnick. 2003. Environmental determinants of Vibrio cholerae biofilm development. Appl. Environ. Microbiol. 695079-5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacikova, G., and K. Skorupski. 2001. Overlapping binding sites for the virulence gene regulators AphA, AphB and cAMP-CRP at the Vibrio cholerae tcpPH promoter. Mol. Microbiol. 41393-407. [DOI] [PubMed] [Google Scholar]
- 29.Liang, W., A. Pascual-Montano, A. J. Silva, and J. A. Benitez. 2007. The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae. Microbiology 1532964-2975. [DOI] [PubMed] [Google Scholar]
- 30.Liang, W., A. J. Silva, and J. A. Benitez. 2007. The cyclic AMP receptor protein modulates colonial morphology in Vibrio cholerae. Appl. Environ. Microbiol. 737482-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim, B., S. Beyhan, J. Meir, and F. H. Yildiz. 2006. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol. Microbiol. 60331-348. [DOI] [PubMed] [Google Scholar]
- 32.Lim, B., S. Beyhan, and F. H. Yildiz. 2007. Regulation of Vibrio polysaccharide synthesis and virulence factor production by CdgC, a GGDEF-EAL domain protein, in Vibrio cholerae. J. Bacteriol. 189717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin, W., G. Kovacikova, and K. Skorupski. 2007. The quorum sensing regulator HapR downregulates the expression of the virulence gene transcription factor AphA in Vibrio cholerae by antagonizing Lrp- and VpsR-mediated activation. Mol. Microbiol. 64953-967. [DOI] [PubMed] [Google Scholar]
- 34.Lipp, E. K., A. Huq, and R. R. Colwell. 2002. Effects of global climate on infectious disease: the cholera model. Clin. Microbiol. Rev. 15757-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh, J. W., D. Sun, and R. K. Taylor. 1996. Physical linkage of the Vibrio cholerae mannose-sensitive hemagglutinin secretory and structural subunit gene loci: identification of the mshG coding sequence. Infect. Immun. 64460-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matz, C., D. McDougald, A. M. Moreno, P. Y. Yung, F. H. Yildiz, and S. Kjelleberg. 2005. Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 10216819-16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meibom, K. L., X. B. Li, A. T. Nielsen, C. Y. Wu, S. Roseman, and G. K. Schoolnik. 2004. The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. USA 1012524-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller, J. H. 1972. Assay of β-galactosidase, p. 352-355. In J. H. Miller (ed.), Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 39.Moorthy, S., and P. I. Watnick. 2004. Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development. Mol. Microbiol. 52573-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Toole, G. A., K. A. Gibbs, P. W. Hager, P. V. Phibbs, Jr., and R. Kolter. 2000. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 182425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57677-701. [DOI] [PubMed] [Google Scholar]
- 42.Pruzzo, C., L. Vezzulli, and R. R. Colwell. 2008. Global impact of Vibrio cholerae interactions with chitin. Environ. Microbiol. 101400-1410. [DOI] [PubMed] [Google Scholar]
- 43.Romling, U., and D. Amikam. 2006. Cyclic di-GMP as a second messenger. Curr. Opin. Microbiol. 9218-228. [DOI] [PubMed] [Google Scholar]
- 44.Romling, U., M. Gomelsky, and M. Y. Galperin. 2005. C-di-GMP: the dawning of a novel bacterial signalling system. Mol. Microbiol. 57629-639. [DOI] [PubMed] [Google Scholar]
- 45.Ryan, R. P., Y. Fouhy, J. F. Lucey, L. C. Crossman, S. Spiro, Y. W. He, L. H. Zhang, S. Heeb, M. Camara, P. Williams, and J. M. Dow. 2006. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc. Natl. Acad. Sci. USA 1036712-6717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Ryjenkov, D. A., M. Tarutina, O. V. Moskvin, and M. Gomelsky. 2005. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 1871792-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring, NY.
- 48.Sauer, K., M. C. Cullen, A. H. Rickard, L. A. Zeef, D. G. Davies, and P. Gilbert. 2004. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J. Bacteriol. 1867312-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt, A. J., D. A. Ryjenkov, and M. Gomelsky. 2005. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J. Bacteriol. 1874774-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva, A. J., and J. A. Benitez. 2004. Transcriptional regulation of Vibrio cholerae hemagglutinin/protease by the cyclic AMP receptor protein and RpoS. J. Bacteriol. 1866374-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 5385-96. [DOI] [PubMed] [Google Scholar]
- 52.Skorupski, K., and R. K. Taylor. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 94265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanley, N. R., R. A. Britton, A. D. Grossman, and B. A. Lazazzera. 2003. Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J. Bacteriol. 1851951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor, R. K., C. Manoil, and J. J. Mekalanos. 1989. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J. Bacteriol. 1711870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thormann, K. M., R. M. Saville, S. Shukla, and A. M. Spormann. 2005. Induction of rapid detachment in Shewanella oneidensis MR-1 biofilms. J. Bacteriol. 1871014-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tischler, A. D., and A. Camilli. 2004. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 53857-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tischler, A. D., and A. Camilli. 2005. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun. 735873-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 985116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waters, C. M., W. Lu, J. D. Rabinowitz, and B. L. Bassler. 2008. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol. 1902527-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yildiz, F. H., N. A. Dolganov, and G. K. Schoolnik. 2001. VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPSETr-associated phenotypes in Vibrio cholerae O1 El Tor. J. Bacteriol. 1831716-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yildiz, F. H., X. S. Liu, A. Heydorn, and G. K. Schoolnik. 2004. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol. Microbiol. 53497-515. [DOI] [PubMed] [Google Scholar]
- 63.Yildiz, F. H., and G. K. Schoolnik. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. USA 964028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu, J., and J. J. Mekalanos. 2003. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5647-656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.