Abstract
The phoPR gene locus of Clostridium acetobutylicum ATCC 824 comprises two genes, phoP and phoR. Deduced proteins are predicted to represent a response regulator and sensor kinase of a phosphate-dependent two-component regulatory system. We analyzed the expression patterns of phoPR in Pi-limited chemostat cultures and in response to Pi pulses. A basic transcription level under high-phosphate conditions was shown, and a significant increase in mRNA transcript levels was found when external Pi concentrations dropped below 0.3 mM. In two-dimensional gel electrophoresis experiments, a 2.5-fold increase in PhoP was observed under Pi-limiting growth conditions compared to growth with an excess of Pi. At least three different transcription start points for phoP were determined by primer extension analyses. Proteins PhoP and an N-terminally truncated *PhoR were individually expressed heterologously in Escherichia coli and purified. Autophosphorylation of *PhoR and phosphorylation of PhoP were shown in vitro. Electromobility shift assays proved that there was a specific binding of PhoP to the promoter region of the phosphate-regulated pst operon of C. acetobutylicum.
In soil, one of the natural habitats of Clostridium acetobutylicum, phosphate is usually present in low concentrations and therefore represents a major growth-limiting factor for soil-borne bacteria (8, 20, 55). In C. acetobutylicum, the limitation of phosphate in a chemostat culture in combination with an excess of glucose and a pH below 5 leads to the so-called solvent shift, a change in metabolism from the production of organic acids (acetate and butyrate) to solvents (butanol and acetone) as the main fermentation products (7, 11). In a batch culture, this metabolic switch is linked to other particular features of the clostridial cell cycle, like the formation of endospores (35), morphological changes (motility, cell shape), and the synthesis of granulose (47). The limitation of phosphate seems to be at least one important factor in this complex regulation network (33).
Up to now, only a little about the phosphate-dependent gene regulation in C. acetobutylicum has been determined, e.g., the existence of a clostridial phosphate (Pho) regulon, a set of genes for which transcription is controlled by PhoP (20, 55). The Pho regulon genes in several bacteria are described as being controlled by a two-component regulatory system, usually consisting of a membrane-associated sensor kinase and a cytosolic response regulator (16, 20, 26, 55, 56). The sensor kinases are supposed to detect the extracellular phosphate concentration via an N-terminal sensor domain situated between two transmembrane domains. When the phosphate concentration in the environment drops below a threshold concentration, conformational changes activate the C-terminal autokinase domain, leading to autophosphorylation of a specific conserved histidine residue. The phosphate group is then further transferred to the N-terminal receiver domain of the response regulator (24). Phosphorylation results in a changed binding affinity of the C-terminal domain of the response regulator to conserved DNA motifs, so-called Pho boxes which have been found for several bacteria, e.g., Escherichia coli and Bacillus subtilis (25, 29). Binding of the phosphorylated response regulator normally enhances the induction of transcription of genes but can also cause downregulation of transcription when Pho boxes are encoded on the noncoding DNA strand of B. subtilis (28).
In E. coli, the Pho regulon is under the control of the two-component system PhoBR and comprises at least 38 different genes (55), whereas in B. subtilis, the 34 genes of the Pho regulon are regulated mainly by the homologous two-component system PhoPR (3, 5, 20, 53). Interestingly, many of these genes, e.g., alkaline phosphatase genes (9, 12, 19, 49) and the genes which are responsible for teichuronic acid synthesis or teichoic acid synthesis (except for tagA), are not annotated for C. acetobutylicum (5, 29, 45). An exception is the pst operon, coding for a high-affinity Pi uptake system. This is essentially composed of an ATP-binding cassette (ABC) transporter and usually belongs to the Pho regulon (2, 44, 46). For C. acetobutylicum, we recently described the pst operon as a potential first member of a Pho regulon and suggested a putative Pho box motif (14).
Here, we report on the transcriptional analysis of the phoPR gene locus and the characterization of encoded proteins as a two-component regulatory system which seems to be involved in the transcriptional regulation of the pst operon and therefore might be a key regulator for phosphate-dependent gene regulation in C. acetobutylicum.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. acetobutylicum ATCC 824 (laboratory collection) was grown under anaerobic conditions at 37°C in a chemostat culture with a surplus of glucose (4%, wt/vol) as the carbon source and a growth-limiting Pi concentration of 0.5 mM K2HPO4 in the supplying medium (14). Phosphate surplus was generated by the addition of 1 M KH2PO4 to a final concentration of 10 mM in the culture vessel. Sampling and measurement of phosphate concentration (59) were performed as described previously over a period of at least 36 h, unless Pi could no longer be detected in the chemostat (14).
Isolation of DNA and preparation of total RNA.
Chromosomal DNA of C. acetobutylicum from batch cultures grown in 2× YTG medium (10 g/liter yeast extract, 16 g/liter tryptone, 4 g/liter glucose, 4 g/liter Nacl) (41) was isolated by following the protocol described by Fischer et al. (14). Total RNA was purified using a modified hot phenol protocol as described by Oelmüller et al. (40).
RT-PCR and primer extension.
Reverse transcriptase PCR (RT-PCR) and primer extension methods were as reported elsewhere (14). Oligonucleotides used in this work are given in Table 1. In parallel to the primer extension experiments, sequencing reactions were carried out using the same IRD800-labeled oligonucleotide and plasmid DNA (pSN1−) as the template. Labeled products were separated and analyzed together by using a denaturing sequencing polyacrylamide gel with a LI-COR 4200 sequencer according to the instructions of the manufacturer (MWG-Biotech, Ebersberg, Germany). Plasmid pSN1− is a derivative of pBluescript II SK(+) (Stratagene, Amsterdam, The Netherlands) containing a EcoRI-trimmed 3,241-bp PCR fragment (primers 2032/1 and phoR2/3′) (Table 1) spanning the open reading frames cac1699, cac1700 (phoP), and cac1701 (phoR).
TABLE 1.
Oligonucleotides
| Primer name | Oligonucleotide sequence (5′→3′)a | Use |
|---|---|---|
| phoP-neu-a | TTCAAATTCCTTAAGGGTAAGC | phoP cDNA |
| phoP-neu-1 | ATGGATATAAGGTTATTACGGC | 5′ phoP RT-PCR |
| phoP-neu-2 | TTGAAATTGGTTAAGGGTAAGC | 3′ phoP RT-PCR |
| phoR-neu-a | ATTCAACATTAACCCTTGTTTC | phoR cDNA |
| phoR-neu-1 | TATTTCAAGTGCTCTTAAAGTC | 5′ phoR RT-PCR |
| phoR-neu-2 | AGAGCTTGTATTTTGAAGTTCC | 3′ phoR RT-PCR |
| phoP-neu-PE2 | IRD800-ATCTAGAAGAACCAGTTGAGGC | phoP primer extension and sequencing reactions |
| phoP-neu-PE3 | IRD800-TCAACAAACATCGTAGCCATCC | |
| phoP-neu-PE4 | IRD800-TTGAAATGGATTGATCCCTTCG | |
| 2032/1 | ACTTAGAATTCAAATGTTAATTTCTGG | Cloning of cac1699, cac1700, and cac1701 in pBluescript II SK(+) |
| phoR2/3′ | CTCTTGAATTCTATTTATAAGGTATTGT | |
| RT-ptb-a | GTCTTATACATTACATTTCCAG | ptb cDNA |
| RT-ptb-1 | TTTGGCATTAAGAAGATATCAG | 5′ ptb RT-PCR |
| RT-ptb-2 | GCATATTAAACAAAGAAGTTGG | 3′ ptb RT-PCR |
| PhoR5′2b | AAAAAGggatccGCTATACTTGTTAGTAT | Cloning of *phoR into pMAL-c2X |
| PhoR3′1b | CTCTTTTctgcagTTTATAAGGTATTGTGAT | |
| PhoP2-5BamHI-B | GGAGGggatccATGGCAGGCGAAAAA | Cloning of phoP into pASK-IBA2 |
| PhoP2-3PstI-A | TCTTTTTCctgcagATCACCACTATAGTTAAAT | |
| pstS-Bam-P4 | TTAAAAATggatccTGTTAACTGTAAAGAGGA | 5′ pst promoter fragment PF1 |
| pstS-Bam-P1 | ACATAggatccTTTAGATTGTAAAGATCAATG | 5′ pst promoter fragment PF2 |
| pstS-Bam-P3 | GATTAAggatccGCTGTTAAAAATACTAAAAC | 5′ pst promoter fragment PF3 |
| pstS-Sal-P | TGATTTTgtcgacTTTCATTTAAAATACCTCC | 3′ pst promoter fragments |
Lowercase letters indicate nucleotide substitutions used to generate restriction sites in the PCR products.
2-DE.
Two-dimensional gel electrophoresis (2-DE) was performed following the standard operating procedure described by Schwarz et al. (48). Gels were stained with colloidal Coomassie blue (37), destained in distilled water for 12 to 20 h, and scanned using a Umax Mirage II scanner (Biostep GmbH, Jahnsdorf/Erzgebirge, Germany) with a resolution of 300 dots per inch. Spot detection in gel images and quantitative analyses were done densitometrically with Delta2D software (version 3.3; DECODON, Greifswald, Germany).
Peptide mass fingerprinting by MALDI-TOF MS.
Protein spots were picked from colloidal Coomassie blue-stained 2-DE gels (37) automatically using a Flexys Proteomics picker (Genomic Solutions, Ann Arbor, MI) (23) or manually with a cut pipette tip. The gel plugs were destained, and proteins were digested with trypsin (sequencing-grade trypsin, 10 ng/μl in 3 mM Tris-HCl [pH 8.5]; Promega, Madison, WI) or endoproteinase AspN (5.0 ng/μl in 3 mM Tris-HCl [pH 8.5]; Roche, Mannheim, Germany). The resulting peptide-containing solutions were prepared on matrix-assisted laser desorption ionization (MALDI) targets (384/600-μm AnchorChip; Bruker Daltonik, Bremen, Germany) (39), and peptide masses were measured by MALDI-time of flight mass spectrometry (MALDI-TOF MS) using a Reflex III mass spectrometer (Bruker Daltonik) (15).
A mass tolerance of 100 ppm and one missing cleavage site were allowed. Oxidation of methionine residues was considered a variable modification, and carboxyamidomethylation of cysteines a fixed modification. Searches were restricted to C. acetobutylicum proteins.
Construction of plasmids expressing PhoP N-terminally fused to a streptavidin tag (PhoP Strep-Tag) or truncated PhoR C-terminally fused to maltose binding protein (MBP-*PhoR).
Primers PhoR5′2b and PhoR3′1b (Table 1) were used for PCR amplification of the 1,005-bp fragment of phoR lacking the first 720 nucleotides (nt) of this open reading frame (*phoR). Primers PhoP2-5BamHI-B and PhoP2-3PstI-A (Table 1) were used for PCR amplification of phoP. PCRs were carried out with Pwo DNA polymerase (PeqLab Biotechnologie GmbH, Erlangen, Germany) and chromosomal DNA of C. acetobutylicum ATCC 824 as the template. After PstI (MBI Fermentas GmbH, St. Leon-Rot, Germany) and BamHI (Invitrogen Life Technologies GmbH, Karlsruhe, Germany) digestion of the amplificates, the *phoR fragment was ligated into PstI- and BamHI-digested, dephosphorylated pMAL-c2X (NEB, Frankfurt/Main, Germany) and the phoP fragment was ligated into the equally treated vector pASK-IBA2 (IBA GmbH, Göttingen, Germany), resulting in plasmids pTF5 and pMM15, respectively.
Overexpression and purification of MBP-*PhoR and PhoP Strep-Tag.
A preculture of E. coli BL21CodonPlus(DE3)-RIL (pTF5) was grown in LB medium enriched with 2% (wt/vol) glucose overnight at 37°C with shaking and then inoculated into 200 ml of the same medium at a ratio of 1:100. The cells were further incubated at 30°C until the optical density at 600 nm reached about 0.5. Specific expression of the MBP-*PhoR fusion protein was then induced by the addition of 24 μg/ml isopropyl-β-d-thiogalactopyranoside, and incubation was continued for another 2 to 3 h. Cells were harvested by centrifugation (7,000 × g at 4°C for 10 min) and stored at −20°C overnight. After suspension in 5 ml of washing buffer (20 mM Tris-HCl [pH 7.5], 200 mM NaCl, 1 mM EDTA, 1 mM dithioerythritol, 0.02% [wt/vol] NaN3), cells were disrupted by sonication three times for 1 min each time (20 W at 20 kHz, Ultraschall Desintegrator Sonopuls HD60; Medizin- und Labortechnik KG, Hamburg, Germany), and lysis was microscopically confirmed. After centrifugation (15,000 × g at 4°C for 30 min), the clear supernatant was diluted 1:5 with washing buffer and applied to a 1-ml amylose resin affinity column (NEB) followed by a washing step using 12 ml of the same buffer. Bound proteins were eluted in ten 200-μl fractions using buffer E (washing buffer plus 10 mM maltose) and, after supplementation with glycerin (10%, vol/vol), could be stored at −20°C for a maximum of 30 days.
PhoP Strep-Tag was heterologously synthesized in E. coli DH5α harboring pMM15. Cells grown in LB medium overnight with agitation at 37°C were used to inoculate 800 ml of fresh LB at a ratio of 1:100. The main culture was grown at 30°C until the optical density at 600 nm reached 0.4. For the expression of PhoP Strep-Tag, anhydrotetracycline was added (0.2 μg/ml), and the incubation was resumed; after 3 to 5 h of incubation, the cells were harvested as described above. Frozen cell pellets were thawed, suspended in 1 ml of buffer W (100 mM Tris-HCl [pH 8.0], 1 mM EDTA, 150 mM NaCl), and subjected to sonication. Cell debris was sedimented by centrifugation (see above), and the clear supernatant was pipetted onto a 1-ml StrepTactin-Sepharose affinity column (IBA). The column was washed with 5 ml buffer W. The protein bound to the column was eluted in six fractions with 0.5 ml buffer E (buffer W plus 2.5 mM desthiobiotin). Protein fractions were stored at −20°C with 10% (vol/vol) glycerin for up to 30 days.
Determination of protein concentration.
Protein concentration was determined using the method described by Bradford (10).
Phosphorylation assays.
For phosphorylation assays, 17 μM PhoP Strep-Tag and 2 μM MBP-*PhoR were incubated together or separately in phosphorylation buffer (10 mM Tris-HCl [pH 7.0], 5 mM MgCl2, 4 mM dithiothreitol) (27) with 10 μCi [γ-32P]ATP for 5 to 30 min at room temperature in a total volume of 20 μl. Phosphorylation was stopped by the addition of 5 μl loading buffer (40% [vol/vol] glycerin, 40 mM dithioerythritol, 10% [wt/vol] sodium dodecyl sulfate [SDS], 0.4% [wt/vol] bromphenol blue, 250 mM Tris-HCl [pH 6.8]) and cooling on ice. Phosphorylated proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (12.5%). Gels were dried, and radioactivity was detected by a 30- to 60-min exposure of dried gels to BAS III imaging plates and visualized using a BAS2000 bioimaging analyzer (Fuji, Tokyo, Japan).
Electromobility shift assays.
Fragments spanning 120 nt (PF1), 190 nt (PF2), and 389 nt (PF3) of the promoter region of the pst operon of C. acetobutylicum were PCR amplified using the primers pstS-Bam-P4, pstS-Bam-P1, pst-Bam-P3, and pstS-Sal-P (Table 1). The PCRs were carried out with AccuTherm DNA polymerase (GeneCraft GmbH, Lüdinghausen, Germany) with chromosomal DNA of C. acetobutylicum ATCC 824 as the template. PCR products were purified with a Nucleospin Extract II kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany) and 3′ digoxigenin (DIG) labeled using a DIG gel shift kit, second generation (Roche Applied Science, Mannheim, Germany), following the manufacturer's instructions. Labeled promoter fragments were incubated for 15 to 30 min at room temperature with 1.5 to 5 μM MBP-*PhoR and/or 1 to 25 μM PhoP Strep-Tag in the absence or presence of 5 to 7.5 mM ATP and binding buffer, poly-l-lysine, and poly[d(I-C)] provided with the DIG gel shift kit. Gel shift reactions were applied to 6% (wt/vol) native polyacrylamide gels with 0.5× Tris-borate-EDTA buffer (44.5 mM Tris-HCl [pH 8.0], 44.5 mM boric acid, 5 mM EDTA) as the running buffer. Electrophoreses were run for 1.5 to 2.5 h at 80 V at room temperature. DNA blotting on nylon membranes (Nytran SuPerCharge nylon transfer membrane, 0.45-μm pore size; Schleicher & Schuell Bioscience GmbH, Dassel, Germany) and DIG detection were performed as described previously (32).
RESULTS
phoPR gene locus of C. acetobutylicum.
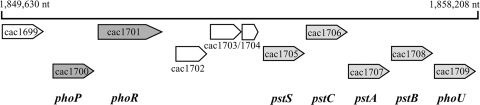
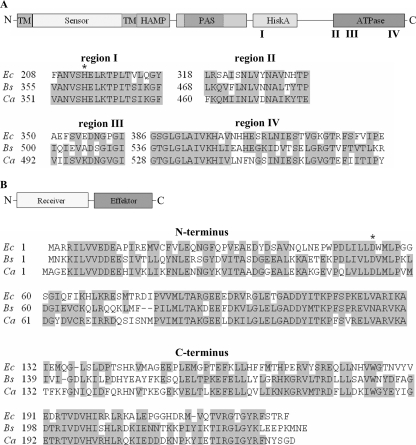
In order to identify a potential regulatory system involved in the phosphate-dependent gene regulation of C. acetobutylicum, we used the polypeptide sequences of the members of the two-component systems PhoPR of B. subtilis (49, 50) and PhoBR of E. coli (30, 31), representing the main regulators of the Pho regulons in these bacteria (20, 55), for BLAST searches (4) against the genome of C. acetobutylicum (38). The results strongly recommended the gene products of the open reading frames cac1700 and cac1701 as the most promising candidates. Cac1700 revealed amino acid residues that were 52% and 40% identical and 74% and 62% similar to those of PhoP and PhoB, respectively. Thus, the gene cac1700, which is located on the leading strand of the chromosome starting at position 1850304 (Fig. 1), could be expected to code for a PhoP homologous protein in C. acetobutylicum. Furthermore, the deduced PhoP protein (232 aa) possesses an N-terminal CheY-like receiver domain (aa 7 to 121) with a conserved aspartate (residue 54) as a phosphoacceptor site and a C-terminal DNA binding effector domain (aa 133 to 227) (Fig. 2B); both are typical features of two-component response regulators.
FIG. 1.
Gene architecture and localization of the phoPR operon of C. acetobutylicum. Numbers above the black line indicate the localization of the gene region on the chromosome. Arrows symbolize the orientation of transcription of the annotated open reading frames on the chromosome of C. acetobutylicum, and known gene names are given below in boldface letters.
FIG. 2.
PhoR proteins (A) and PhoP proteins (B) of E. coli (Ec), B. subtilis (Bs), and C. acetobutylicum (Ca). Schemes with the domain architectures of the polypeptides are presented at the tops of panels A and B. TM, transmembrane domain; HiskA, histidine kinase A dimerization/phosphoacceptor domain. Below the schemes, alignments of selected protein regions (I to IV in the case of PhoP and the N and C termini in the case of PhoR) are shown. Letters represent the amino acid residues, gray boxes highlight homologous or identical residues (accepted as homologous substitutions are A and G; R and K; F and Y; S and T; D and E; N and Q; and I, L, V, and M), and asterisks indicate conserved phosphorylation sites.
Not surprisingly, the deduced gene product (566 aa) of the open reading frame directly downstream of phoP (cac1701) shows 32% identity (52% and 50% similarity, respectively) with phosphate-dependent sensor histidine kinases PhoR of B. subtilis and PhoR of E. coli and therefore was named phoR. The PhoR protein of C. acetobutylicum contains common functional regions of histidine kinases (24), like an N-terminal transmembrane domain (aa 5 to 27), an extracellular sensing domain (aa 28 to 156), a histidine kinase, adenylyl cyclase, methyl binding protein, and phosphatase (HAMP) domain (aa 159 to 227) (6) that includes a transmembrane part (aa 157 to 179), a histidine kinase A (phosphoacceptor) domain (aa 344 to 406), and a histidine kinase-like ATPase domain (aa 460 to 563). Additionally, there are two possible PAS domains (aa 238 to 293 or aa 246 to 337) located between the HAMP and phosphoacceptor domains. PAS domains are supposed to function as dimerization sites (58), while HAMP domains mediate the signal transduction between sensor and effector domains of sensor kinases (21). The four conserved C-terminal regions (36) in the phosphoacceptor and ATPase domains show striking similarities between PhoR of B. subtilis and that of C. acetobutylicum (Fig. 2A). In region I, carrying the histidine residue necessary for autophosphorylation, the two proteins show 100% homologous amino acid residues.
Interestingly, the pst operon starts about 1.2 kb downstream of these two genes, at position 1853987. This operon comprises five genes encoding the components of a high-affinity phosphate uptake system (Pst) and a PhoU-like protein. It is the only known phosphate-regulated operon in C. acetobutylicum so far (14). Only three short open reading frames, cac1702, cac1703, and cac1704, are located in between the two operons (Fig. 1). The deduced polypeptides encoded by cac1702 (126 aa) and cac1704 (71 aa) show no significant similarities to known proteins, while the 116-aa polypeptide encoded by cac1703 is listed as a methyl-accepting chemotaxis protein (fragment) in the annotated NCBI database (http://www.ncbi.nlm.nih.gov/).
Expression analysis of the phoPR gene locus of C. acetobutylicum.
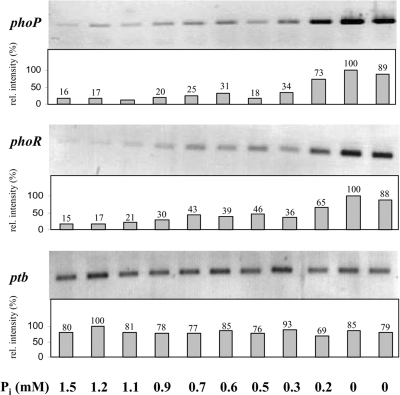
To evaluate whether the transcription of phoPR is restricted to phosphate depletion, Northern blotting and RT-PCR experiments were carried out. Thus, after a Pi pulse of 10 mM KH2PO4, cell aliquots were harvested and total RNA was isolated. Samples represented the decreasing phosphate concentrations due to the Pi consumption by the bacteria and the dilution rate. Northern blots with DIG-labeled DNA or even RNA probes against phoP or phoR did not reveal specific signals even if up to 20 μg total RNA was applied per lane (data not shown). However, RT-PCR experiments clearly revealed low basal transcript levels of phoP and phoR under conditions of phosphate excess in the culture supernatant. Going along with the decrease in phosphate levels, the first marginal increase of phoP and phoR mRNA transcripts was detected when the external Pi dropped below 0.7 mM. A strong, at least threefold increase of phoPR-specific transcripts was found below concentrations of 0.3 mM Pi in the cell-free supernatant as shown in Fig. 3.
FIG. 3.
Transcription of the phoPR operon of C. acetobutylicum in response to phosphate depletion. Individual RT-PCR analyses of genes phoP, phoR, and ptb with total RNA of cells after a phosphate pulse are shown. External phosphate concentrations are given below the lanes (0 mM Pi symbolizes steady-state conditions). The ptb gene encoding phosphotransbutyrylase, which is expected to be constitutively expressed under experimental conditions, was used as the positive control. Bars represent relative signal intensity (rel. intensity), and the maximum signal intensity was set as 100%.
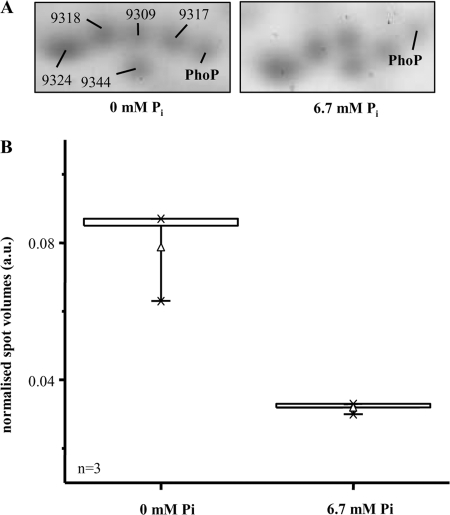
In a manner nearly directly proportional to this induction of transcription of phoPR in cells growing under phosphate limitation, an increase of PhoP in the protein level in 2-DE gels could be verified. Therein, soluble cytosolic proteins (300 μg on each gel) from cells kept under phosphate limitation conditions in the chemostat culture were compared with protein preparations of cells grown at surplus of Pi (6.5 mM Pi in the cell-free supernatant, 6 h after the application of a phosphate pulse). Protein spots of these gels (Fig. 4A) were subjected to peptide mass fingerprinting using MALDI-TOF MS. One single spot represented PhoP, which was identified with a high level of confidence upon trypsin digestion and AspN digestion. The probability score for identification was 179 in the case of tryptic digestion and 171 for digestion of PhoP with AspN. Probability scores above 65 are considered statistically significant. As scores greater than 170 were obtained, PhoP was reliably identified from the 2-DE gel samples. A combination of the results yielded a sequence coverage of 91% for the amino acid sequence of PhoP (UniProtKB/TrEMBL accession no. Q97IE8). This high sequence coverage significantly enhances the reliability of the identification results. To estimate the changes in PhoP amounts, spot volumes were compared. Distributions of normalized spot volumes are shown in Fig. 4B as box-and-whisker plots. Therein, a 2.5-fold-larger amount of PhoP in phosphate-limited cells was revealed.
FIG. 4.
Expression of PhoP under different phosphate concentrations. (A) Shown are sections of 2-DE gels with PhoP proteins with a surplus of Pi (6.7 mM) or Pi limitation (0 mM). The lines mark the spots of PhoP. The other spots serve orientation purposes (9309, dihydrodipicolinate reductase [dapB, cac2379]; 9317, acetoacetate decarboxylase [adc, cap0165]; 9324, triosephosphate isomerase [tpi, cac0711]; 9344, adenylate kinase [adk, cac3112]; 9318, not identified [score below 65]). (B) Distribution of normalized spot volumes (arbitrary units [a.u.]; n = 3) for PhoP (see Fig. 4A) are shown as box-and-whisker plots. The boxes represent the upper and lower quartiles. The separation line in the box marks the 50% value (mean; not visible). The whiskers indicate the 5th and 95th percentiles, respectively. Average values are depicted as little triangles. Upper and lower extreme values are indicated with a minus sign. The × symbol shows the 99% and 1% range of the individual values, respectively.
However, the analysis of the 2-DE gels further shows that PhoP was migrating to slightly different locations depending on the growth conditions. The spot for PhoP derived from the cultures grown with a surplus of Pi is found higher up than the spot for PhoP derived from the cultures grown with Pi limitation. This result indicates that in addition to upregulation of the protein amount, a not-yet-analyzed structural difference may also be induced. The vertical shift of the positions of the spots in the 2-DE gels indicates protein mass differences. The suspected alteration of the PhoP structure has no detectable effect on the pI of the protein; otherwise, migration would also be affected in the horizontal direction. PhoR was not detected in the protein fraction by 2-DE gel analysis, as it is expected to be a membrane-associated protein (51).
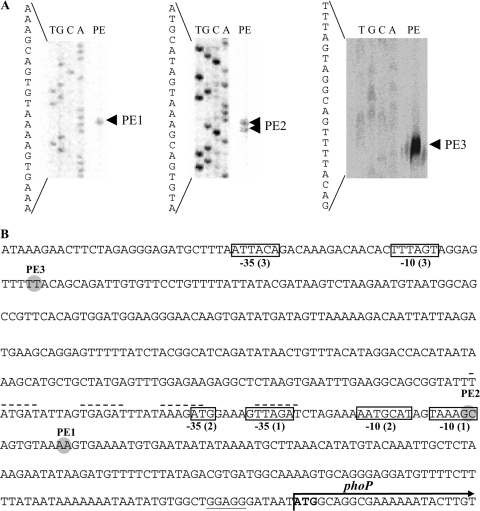
For determination of the transcription start point of phoP, primer extension analyses were performed and at least three different signals (PE1, PE2, and PE3) were obtained (Fig. 5A), located 147 nt (PE1), 156 nt, and 453 nt upstream of the phoP start codon (Fig. 5B). Thus, three possible 5′ ends of the mRNA must be assumed.
FIG. 5.
Transcription start points of phoP of C. acetobutylicum. The products of primer extension reactions using IRD800-labeled oligonucleotides were run on polyacrylamide gels alongside the corresponding sequencing reactions (T, G, C, A) generated with the same primer (A). (B) Locations of the identified transcription start points, PE1 (primer, phoP-neu-PE2), PE2 (phoP-neu-PE3), and PE3 (phoP-neu-PE3) are highlighted (gray circles) within the DNA sequence of the promoter region upstream of the phoP gene. The boldface ATG symbolizes the start codon of the phoP gene, and the arrow indicates its orientation; its dedicated ribosome binding site is underlined. Putative regulatory transcriptional elements of the transcription start points are boxed with a −10 or −35 (see text for details). Numbers in parentheses indicate their assignment to the corresponding transcription start points. Broken lines appear above possible regulatory repeats.
Overproduction, purification, and characterization of PhoP and PhoR.
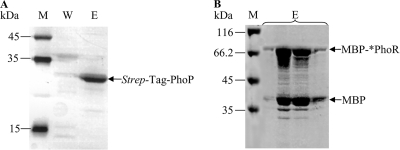
For functional characterization of the activity of PhoPR as a two-component system, the two proteins were individually expressed in E. coli and purified as described in Materials and Methods. The purified recombinant PhoP protein with a Strep-Tag II fused to its C terminus revealed a single signal of approximately 28 kDa after SDS-polyacrylamide gel electrophoresis (PAGE) (Fig. 6).
FIG. 6.
Purification of heterologously expressed PhoP Strep-Tag (A) and MBP-*PhoR (B). Coomassie-stained SDS-PAGE (12.5%). M, protein molecular weight marker; W, washing fractions; E, elution fractions.
As described by Shi and Hulett (51), PhoR was expressed in a truncated form without its 240 N-terminal amino acid (aa) residues harboring two hydrophobic N-terminal transmembrane domains (data not shown). Shortened *PhoR starting with an alanine residue was C-terminally fused to the soluble MBP of E. coli. The purified fusion protein MBP-*PhoR with an apparent molecular weight of approximately 79.5 kDa could be visualized in SDS-polyacrylamide gels together with a second signal (42 kDa) representing MBP only (Fig. 6).
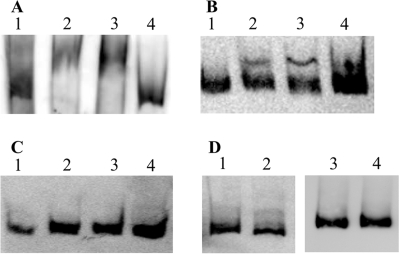
The recombinant proteins PhoP Strep-Tag and MBP-*PhoR were used to affirm in vitro characteristic biochemical features of two-component systems for PhoPR of C. acetobutylicum. Phosphorylation assays revealed a strong autophosphorylation of MBP-*PhoR in the presence of [γ-32P]ATP (Fig. 7), whereas autophosphorylation of Strep-Tag-PhoP could not be detected (data not shown). Furthermore, when MBP-*PhoR and Strep-Tag-PhoP were incubated together under the same conditions, phosphate transfer from phosphorylated MBP-*PhoR to Strep-Tag-PhoP could be detected. In the presence of Strep-Tag-PhoP, the autophosphorylation signal of MBP-*PhoR almost completely disappeared, and instead, a second signal referring to phosphorylated Strep-Tag-PhoP appeared (Fig. 7).
FIG. 7.
Autophosphorylation of MBP-*PhoR and phosphate transfer to PhoP Strep-Tag. Ten μCi of [γ-32P]ATP was incubated with 2 μM MBP-*PhoR (lane 1) or with 2 μM MBP-*PhoR and 17 μM Strep-Tag-PhoP (lane 2) for 15 min at room temperature and was separated by SDS-PAGE (12.5%). Shown is an autoradiogram; relative signal intensities referring to the certain proteins are given below the lanes.
To study the possible regulatory function of the PhoPR two-component system in transcriptional control of the cellular response to depletion of phosphate, we analyzed the ability of the predicted response regulator PhoP to bind to the promoter region of the pst operon in vitro. This promoter region was chosen since the pst operon was the only known phosphate-regulated operon in C. acetobutylicum to date (14). For this purpose, three PCR DNA fragments (PF1, PF2, PF3) of the pst promoter region were generated. They had in common that they exhibited identical 3′ ends (representing the nineteenth nucleotide of the pst gene) but showed different 5′ extensions in that the largest fragment (PF3) covered 371 nt upstream of the pstS start codon, PF2 covered 172 nt, and the fragment PF1 covered only 102 nt. Thus, the middle-length amplificate, PF2, comprises the “core region” with all predicted regulatory sites of the pst promoter, including the potential Pho boxes (14), whereas PF1 covers only the −10 promoter sequence without any further signal motifs, and PF3 extends PF2 by about 200 bp. Fragments were DIG labeled and used for electromobility shift assays with different concentrations of Strep-Tag-PhoP and MBP-*PhoR in the absence and presence of ATP. These assays showed a specific binding of PhoP to the fragments PF2 and PF3, but not to PF1 (Fig. 8A to C). While PF3 was totally shifted (Fig. 8A), PF2 partly remained unbound by PhoP (Fig. 8B). As the negative control, PhoP was incubated with an unspecific DNA fragment and promoter fragment PF3 was incubated with MBP-*PhoR only (Fig. 8D). Neither approach led to a shift of the particular fragment, proving the specificity of the binding of PhoP to the pst promoter fragments.
FIG. 8.
Binding of PhoP to the pst promoter. Electromobility shift assays were carried out with 0.5 ng 3′ DIG-labeled pst promoter fragment P3 (A), fragment P1 (B), and fragment P4 (C) in the presence of 5 mM ATP. Fragments were incubated without protein (lane 1), with 5 μM Strep-Tag-PhoP (lane 2), with 5 μM Strep-Tag-PhoP and 1.5 μM MBP-*PhoR (lane 3), or with 5 μM Strep-Tag-PhoP, 1.5 μM MBP-*PhoR, and 100 ng unlabeled fragment. (D) As negative controls, unspecific DIG-labeled DNA fragments were incubated under the same conditions without protein (lane 1) and with 5 μM Strep-Tag-PhoP and 1.5 μM MBP-*PhoR (lane 2). Additionally DIG-labeled fragment P3 was incubated without protein (lane 3) and with 3 μM MBP-*PhoR (lane 4).
DISCUSSION
Two-component systems are commonly utilized for the sensing of certain environmental conditions, especially in bacteria and archaea, but can also be found in some eukaryotes, like plants, yeasts, and other fungi. Normally, in all microorganisms investigated so far, one two-component system is responsible mainly for the regulation of the phosphate starvation response. Most prominent are PhoPR-like proteins (16, 20, 26, 55, 56).
For clostridia, almost nothing is known aside from in silico alignment data, which led to annotations of putative PhoPR two-component systems in the sequenced genomes of Clostridium tetani and Clostridium perfringens (13). Interestingly, even within this group of bacteria, genetic organizations differ and seem not to be highly conserved. For example, in C. acetobutylicum, the phoPR operon is separated from the pst operon by three short, seemingly unrelated open reading frames (Fig. 1), whereas in C. tetani E88, the putative phoPR operon directly precedes the pst operon. A different architecture is postulated for C. perfringens, where the phoR gene product is predicted as an orphan sensor histidine kinase which is located far from the pst operon on the opposite strand (13).
Although they are referred to as phosphate-dependent regulatory systems, none of the clostridial PhoPR systems has been characterized in detail so far. Since we had started to analyze molecular aspects of Pi depletion in C. acetobutylicum (14), an obvious step was to focus on the search for a PhoPR system in this bacterium. As shown here, based on similarity searches, the most likely candidates are the gene products of the chromosomal open reading frames cac1700 and cac1701, which were named PhoP and PhoR, respectively.
Indirect evidence for the involvement of PhoP and PhoR in processes restricted to phosphate limitation arises from the fact that a specific induction of the transcription of these genes is coupled to the drop in external Pi concentration to below 0.3 mM. Obviously, this behavior corresponds with the induction of the pst operon, which is expected to encode a high-affinity phosphate-specific ABC transport system in C. acetobutylicum (14). Here, under phosphate limitation, we demonstrate a clear increase in the phoP and phoR mRNA levels. A nearly directly proportional, 2.5-fold-higher PhoP protein level was detected for 2-DE gels. These findings prove a specific need for PhoP under phosphate limitation and, in combination with the in silico alignment data, support its proposed function as a key regulator of the phosphate starvation response in C. acetobutylicum.
Interestingly, PhoP proteins derived from cultures grown with Pi limitation migrated to different locations, indicating at least slightly modified proteins. A similar behavior is not known for PhoP proteins of other organisms so far. The reason for this behavior remains speculative, as by means of MALDI-TOF MS, neither a phosphorylation of the protein nor an elongation of the N-terminal peptide was seen (data not shown). Actually, we cannot exclude the possibility that the different mRNA molecules discussed below might yield different N termini of the respective proteins, resulting in the migration differences shown in the 2-DE gels. It might be important to note that no other protein spot in these 2-DE gels was identified as PhoP.
For B. subtilis, σA-driven transcription of the phoPR operon starts at two different promoters (43) and, additionally, σE- and σB-dependent separate promoters exist, resulting in at least four different mRNA 5′ ends (42). Our primer extension results also indicate multiple transcription start points which might suggest a similar situation for C. acetobutylicum. Possible −10 and −35 promoter sequences only weakly match the consensus sequences (Fig. 5A). An involvement of different sigma factors as described for B. subtilis seems likely not only because of the close relatedness of these bacteria. The sequence 5′-ATG-N18-AATGCAT-3′ can be found 6 nt upstream of the PE2 signal (Fig. 5A). This element resembles the consensus −10 and −35 sequences for σE-dependent transcription (ATA-N16-18-CATAcaT) of B. subtilis (18) and the described σE promoter of the phoPR operon of B. subtilis (ATG-N17-CATAAAAT) (42). Thus, a σE-dependent transcription might be speculated for phoPR starting at the PE2 signal. Possible promoter elements for both of the other transcription start points at PE1 and PE3 (Fig. 5A) show only small similarities to the consensus TTGACA (−35) and TATAAT (−10) sequences for σA-dependent transcription (52) and none to the postulated Pho boxes in C. acetobutylicum (14). But another 6-bp element, separated by 4 to 6 bp, is repeated four times within the region upstream of the PE1 and PE2 signals (TXT/AGAT). However, the significance of these elements remains unclear.
Although we were not able to show an operon structure of phoPR experimentally because Northern blot experiments did not reveal specific signals, we are convinced that a monocistronic transcription of phoR is unlikely. The stop codon of the annotated phoP gene and the start codon of annotated phoR gene are separated by 1 nt only, and no putative Shine-Dalgarno sequences are located in the upstream region of the phoR gene. These facts may indicate a translational coupling as has been described for several other two-component systems (17, 22, 34, 54, 57). In this context, the possibility of different initiation sites of translation of PhoR has to be discussed. Within the same reading frame, two GTG codons are located two and three codons upstream of the annotated start codon of phoR, probably representing alternative initiation translation sites (data not shown). In consequence, translation overlaps with the end of phoP, and the resulting proteins would show N-terminal extensions of 3 or 4 aa.
The crucial features of a two-component system in their domain architecture for the deduced gene products PhoP and PhoR were demonstrated. The autophosphorylation of PhoR in the presence of ATP as well as the phosphate transfer to PhoP confirmed these characteristics. PhoP of C. acetobutylicum was able to bind to the promoter region of the pst operon in the absence and presence of ATP. A similar situation was found for B. subtilis, where the binding affinity of PhoP∼P to Pho box promoters is not significantly higher than that of PhoP, but transcription is affected only by PhoP∼P, not by PhoP (43). The specific binding of PhoP to the promoter region of the phosphate-dependent transcribed pst operon (14) indicates a role of the PhoPR two-component system as a regulator in the phosphate limitation response of C. acetobutylicum. Further work has to focus on the characterization of the specific function of the putative Pho box motifs.
For B. subtilis, a cross-link between the PhoPR regulatory system and sporulation was shown (1). The possibility of a similar cross-regulatory mechanism in C. acetobutylicum would fit with the finding that phosphate limitation seems to be involved in sporulation and solvent production in this bacterium (7). Nevertheless, the involvement of further regulatory mechanisms remains speculative.
Acknowledgments
We thank Michael Kreutzer, from Proteome Center Rostock, for excellent assistance in 2-DE gel analysis and protein spot picking.
This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft (DFG) and by fellowships of the Graduiertenförderung of the federal state of Mecklenburg-Vorpommern to T.F. and M.M.
Footnotes
Published ahead of print on 8 August 2008.
REFERENCES
- 1.Abdel-Fattah, W. R., Y. Chen, A. Eldakak, and F. M. Hulett. 2005. Bacillus subtilis Phosphorylated PhoP: direct activation of the EσA- and repression of the EσE-responsive phoB-PS+V promoters during Pho response. J. Bacteriol. 1875166-5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguena, M., E. Yagil, and B. Spira. 2002. Transcriptional analysis of the pst operon of Escherichia coli. Mol. Genet. Genomics 268518-524. [DOI] [PubMed] [Google Scholar]
- 3.Allenby, N. E. E., N. O'Connor, Z. Prágai, A. C. Ward, A. Wipat, and C. R. Harwood. 2005. Genome-wide transcriptional analysis of the phosphate starvation stimulon of Bacillus subtilis. J. Bacteriol. 1878063-8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antelmann, H., C. Scharf, and M. Hecker. 2000. Phosphate starvation-inducible proteins of Bacillus subtilis: proteomics and transcriptional analysis. J. Bacteriol. 1824478-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aravind, L., and C. P. Ponting. 1999. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol. Lett. 176111-116. [DOI] [PubMed] [Google Scholar]
- 7.Bahl, H., W. Andersch, and G. Gottschalk. 1982. Continuous production of acetone and butanol by Clostridium acetobutylicum in a two-stage phosphate limited chemostat. Eur. J. Appl. Microbiol. Biotechnol. 15201-205 (Erratum, 17:73, 1983.) [Google Scholar]
- 8.Bieleski, R. L. 1973. Phosphate pools, phosphate transport, and phosphate availability. Annu. Rev. Plant Physiol. 24225-252. [Google Scholar]
- 9.Bookstein, C., C. W. Edwards, N. V. Kapp, and F. M. Hulett. 1990. The Bacillus subtilis 168 alkaline phosphatase III gene: impact of a phoAIII mutation on total alkaline phosphatase synthesis. J. Bacteriol. 1723730-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradford, M. M. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 11.Dürre, P., and H. Bahl. 1996. Microbial production of acetone/butanol/isopropanol, p. 229-268. In P. Stadler (ed.), Biotechnology: a multi-volume comprehensive treatise, 2nd ed., vol. 1. VCH Verlagsgesellschaft, Weinheim, Germany. [Google Scholar]
- 12.Eder, S., W. Liu, and F. M. Hulett. 1996. A Bacillus subtilis secreted phosphodiesterase/alkaline phosphatase is the product of a Pho regulon gene, phoD. Microbiology 1422041-2047. [DOI] [PubMed] [Google Scholar]
- 13.Fischer, R.-J., and H. Bahl. 2005. Transport of phosphate, p. 287-294. In P. Dürre (ed.), Handbook on clostridia. CRC Press, Taylor and Francis Group, Boca Raton, FL.
- 14.Fischer, R.-J., S. Oehmcke, U. Meyer, K. Schwarz, M. Mix, T. Fiedler, and H. Bahl. 2006. Transcription of the pst operon of Clostridium acetobutylicum is dependent on phosphate concentration and pH. J. Bacteriol. 1885469-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulda, S., S. Mikkat, F. Huang, J. Huckauf, K. Marin, B. Norling, and M. Hagemann. 2006. Proteome analysis of salt stress response in the cyanobacterium Synechocystis sp. strain PCC6803. Proteomics 62733-2745. [DOI] [PubMed] [Google Scholar]
- 16.Ghorbel, S., J. Kormanec, A. Artus, and M.-J. Virolle. 2006. Transcriptional studies and regulatory interactions between the phoR-phoP operon and the phoU, mtpA, and ppk genes of Streptomyces lividans TK24. J. Bacteriol. 188677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorham, H. C., S. J. McGowan, P. R. H. Robson, and D. A. Hodgson. 1996. Light-induced carotenogenesis in Myxococcus xanthus: light-dependent membrane sequestration of ECF sigma factor CarQ by anti-sigma factor CarR. Mol. Microbiol. 19171-186. [DOI] [PubMed] [Google Scholar]
- 18.Helmann, J. D., and C. P. Moran, Jr. 2002. RNA polymerase and sigma factors, p. 289-312. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC.
- 19.Hulett, F. M., E. E. Kim, C. Bookstein, N. V. Kapp, C. W. Edwards, and H. W. Wyckoff. 1991. Bacillus subtilis alkaline phophatases III and IV: cloning, sequencing, and comparisons of deduced amino acid sequence with Escherichia coli alkaline phosphatase three-dimensional structure. J. Biol. Chem. 2661077-1084. [PubMed] [Google Scholar]
- 20.Hulett, F. M. 2002. The Pho regulon, p. 193-201. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC.
- 21.Hulko, M., F. Berndt, M. Gruber, J. U. Linder, V. Truffault, A. Schultz, J. Martin, J. E. Schultz, A. N. Lupas, and M. Coles. 2006. The HAMP domain structure implies helix rotation in transmembrane signaling. Cell 126929-940. [DOI] [PubMed] [Google Scholar]
- 22.Inokuchi, Y. A. Hirashima, Y. Semine, L. Janosi, and A. Kaji. 2000. Role of ribosome recycling factor in translational coupling. EMBO J. 193788-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Just, T., E. Gafumbegete, J. Gramberg, I. Prüfer, B. Ringel, S. Mikkat, H. W. Pau, and M. O. Glocker. 2006. Differential proteome analysis of tonsils from children with chronic tonsillitis in comparison with hyperplasia reveals disease-associated protein expression differences. Anal. Bioanal. Chem. 3841134-1144. [DOI] [PubMed] [Google Scholar]
- 24.Khorchid, A., and M. Ikura. 2006. Bacterial histidine kinase as signal sensor and transducer. Int. J. Biochem. Cell. Biol. 38307-312. [DOI] [PubMed] [Google Scholar]
- 25.Kimura, S., K. Makino, H. Shinagawa, M. Amemura, and A. Nakata. 1989. Regulation of the phosphate regulon of Escherichia coli: characterization of the promoter of the pstS gene. Mol. Gen. Genet. 215374-380. [DOI] [PubMed] [Google Scholar]
- 26.Kočan, M., S. Schaffer, T. Ishige, U. Sorger-Herrmann, V. F. Wendisch, and M. Bott. 2006. Two-component systems of Corynebacterium glutamicum: deletion analysis and involvement of the PhoS-PhoR system in the phosphate starvation response. J. Bacteriol. 188724-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, W., and F. M. Hulett. 1997. Bacillus subtilis PhoP binds to the phoB tandem promoter exclusively within the phosphate starvation-inducible promoter. J. Bacteriol. 1796302-6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, W., S. Eder, and F. M. Hulett. 1998. Analysis of Bacillus subtilis tagAB and tagDEF expression during phosphate starvation identifies a repressor role of PhoP∼P. J. Bacteriol. 180753-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, W., and F. M. Hulett. 1998. Comparison of PhoP binding to the tuaA promoter with PhoP binding to other Pho-regulon promoters establishes a Bacillus subtilis Pho core binding site. Microbiology 1441443-1450. [DOI] [PubMed] [Google Scholar]
- 30.Makino, K., H. Shinagawa, M. Amemura, and A. Nakata. 1986. Nucleotide sequence of the phoB gene, the positive regulatory gene for the phosphate regulon of Escherichia coli K-12. J. Mol. Biol. 19037-44. [DOI] [PubMed] [Google Scholar]
- 31.Makino, K., H. Shinagawa, M. Amemura, and A. Nakata. 1986. Nucleotide sequence of the phoR gene, a regulatory gene for the phosphate regulon of Escherichia coli. J. Mol. Biol. 192549-556. [DOI] [PubMed] [Google Scholar]
- 32.May, A., F. Hillmann, O. Riebe, R.-J. Fischer, and H. Bahl. 2004. A rubrerythrin-like oxidative stress protein of Clostridium acetobutylicum is encoded by a duplicated gene and identical to the heat shock protein Hsp21. FEMS Microbiol. Lett. 238249-254. [DOI] [PubMed] [Google Scholar]
- 33.Meinecke, B., H. Bahl, and G. Gottschalk. 1984. Selection of an asporogenous strain of Clostridium acetobutylicum in continuous culture under phosphate limitation. Appl. Environm. Microbiol. 481064-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merighi, M., A. Carroll-Portillo, A. N. Septer, A. Bhatiya, and J. S. Gunn. 2006. Role of Salmonella enterica serovar Typhimurium two-component system PreA/PreB in modulatine PmrA-regulated gene transcription. J. Bacteriol. 188141-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell, W. J. 2001. Spores and sporulation, p. 72-83. In P. Dürre (ed.), Clostridia: biotechnology and medical applications. Wiley-VCH Verlag GmbH, Weinheim, Germany.
- 36.Msadek, T., F. Kunst, and G. Rapoport. 1993. Two-component regulatory systems, p. 729-745. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, DC.
- 37.Neuhoff, V., N. Arold, D. Taube, and W. Ehrhardt. 1988. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 9255-262. [DOI] [PubMed] [Google Scholar]
- 38.Nölling, J., G. Breton, M. V. Omelchenko, K. S. Marakowa, Q. Zeng, R. Gibson, H. M. Lee, J. Dubios, D. Qiu, and J. Hitti. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 1834823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nordhoff, E., M. Schurenberg, G. Thiele, C. Lubbert, K. D. Kloeppel, D. Theiss, H. Lehrach, and J. Gobom. 2003. Sample preparation protocols for MALDI-MS of peptides and oligonucleotides using prestructured sample supports. Int. J. Mass Spectrom. 226163-180. [Google Scholar]
- 40.Oelmüller, U., N. Krüger, A. Steinbüchel, and C. G. Freidrich. 2002. Isolation of prokaryotic RNA and detection of specific mRNA with biotinylated probes. J. Microbiol. Methods 1173-81. [Google Scholar]
- 41.Oultram, J. D., M. Loughlin, T.-J. Swinfield, J. K. Brehm, D. E. Thompson, and N. P. Minton. 1988. Introduction of plasmids into whole cells of Clostridium acetobutylicum by electroporation. FEMS Microbiol. Lett. 5683-88. [Google Scholar]
- 42.Paul, S., S. Birkey, W. Liu, and F. M. Hulett. 2004. Autoinduction of Bacillus subtilis phoPR operon transcription results from enhanced transcription from EσA- and EσE-responsive promoters by phosphorylated PhoP. J. Bacteriol. 1864262-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prágai, Z., N. N. E. Allenby, N. O'Connor, S. Dubrac, G. Rapoport, T. Msadek, C., and R. Harwood. 2004. Transcriptional regulation of the phoPR operon in Bacillus subtilis. J. Bacteriol. 1861182-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi, Y., Y. Kobayashi, and F. M. Hulett. 1997. The pst operon of Bacillus subtilis has a phosphate-regulated promoter and is involved in phosphate transport but not in regulation of the Pho regulon. J. Bacteriol. 1792534-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi, Y., and F. M. Hulett. 1998. Role of PhoP∼P in transcriptional regulation of genes involved in cell wall anionic polymer biosynthesis in Bacillus subtilis. J. Bacteriol. 1804007-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao, N. N., and A. Torriani. 1990. Molecular aspects of phosphate transport in Escherichia coli. Mol. Microbiol. 41083-1090. [DOI] [PubMed] [Google Scholar]
- 47.Rogers, P. G., and G. Gottschalk. 1993. Biochemistry and regulation of acid and solvent formation in clostridia, p. 25-50. In D. R. Woods (ed.), The clostridia and biotechnology. Butterworth-Heinemann, London, United Kingdom.
- 48.Schwarz, K., T. Fiedler, R.-J. Fischer, and H. Bahl. 2007. A standard operating procedure (SOP) for preparation of intra- and extracellular proteins of Clostridium acetobutylicum for proteome analysis. J. Microbiol. Methods 68396-402. [DOI] [PubMed] [Google Scholar]
- 49.Seki, T., H. Yoshikawa, H. Takahashi, and H. Saito. 1987. Cloning and nucleotide sequence of phoP, the regulatory gene for alkaline phosphatase and phosphodiesterase in Bacillus subtilis. J. Bacteriol. 1692913-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seki, T., H. Yoshikawa, H. Takahashi, and H. Saito. 1988. Nucleotide sequence of the Bacillus subtilis phoR gene. J. Bacteriol. 1705935-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi, L., and F. M. Hulett. 1999. The cytoplasmic kinase domain of PhoR is sufficient for the low phosphate-inducible expression of Pho regulon genes in Bacillus subtilis. Mol. Microbiol. 31211-222. [DOI] [PubMed] [Google Scholar]
- 52.Sonenshein, A. L., J. D. Haraldsen, and B. Dupuy. 2005. RNA polymerase and alternative σ factors, p. 607-629. In P. Dürre (ed.), Handbook on clostridia. CRC Press, Taylor and Francis Group, Boca Raton, FL.
- 53.Sun, G., S. M. Birkey, and F. M. Hulett. 1996. Three two-component signal-transduction systems interact for Pho regulation in Bacillus subtilis. Mol. Microbiol. 19941-948. [DOI] [PubMed] [Google Scholar]
- 54.Supply, P., J. Magdalena, S. Himpens, and C. Locht. 1997. Identification of novel intergenic repetitive units in a mycobacterial two-component system operon. Mol. Microbiol. 26991-1003. [DOI] [PubMed] [Google Scholar]
- 55.Wanner, B. L. 1996. Phosphorus Assimilation and Control of the Phosphate Regulon, p. 1357-1381. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 56.Watson, G. M. F., D. J. Scanlan, and N. H. Mann. 1996. Characterization of the genes encoding a phosphate-regulated two-component sensory system in the marine cyanobacterium Synechococcus sp. WH7803. FEMS Microbiol. Lett. 142105-109. [DOI] [PubMed] [Google Scholar]
- 57.Yarwood, J. M., J. K. McCormick, and P. M. Schlievert. 2001. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J. Bacteriol. 1831113-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhulin, I. B., B. L. Taylor, and R. Dixon. 1997. PAS-domain S-boxes in archaea, bacteria and sensors for oxygen and redox. Trends Biochem. Sci. 22331-333. [DOI] [PubMed] [Google Scholar]
- 59.Zilversmit, D. B., and A. K. Davis. 1950. Microdetermination of plasma phospholipids by trichloroacetic acid precipitation. J. Lab. Clin. Med. 35155-160. [PubMed] [Google Scholar]